Abstract
Traditional cotton fiber dyeing requires an abundance of salt, which leads to environmental pollution. Consequently, decreasing or eliminating the use of salt has become the primary focus of current research. In this study, Crocein Orange G was used to dye carboxymethyl cotton. Carboxymethyl cotton has better color shades than raw cotton and it is used along with a mordant in a simultaneous-mordant dyeing process, at a pH of 7, Al2(SO4)3·18H2O as the mordant. In addition, it was found that the adsorption kinetics of carboxymethyl cotton followed a pseudo-second-order kinetic model. The equilibrium adsorption capacity increased as the temperature increased from 30 to 50 °C, and the maximum equilibrium adsorption was 8.21 mg g−1 at 50 °C. Furthermore, the adsorption isotherm data exhibited good agreement with the Freundlich isotherm. These results will help achieve salt-free dyeing of cotton fabric in the textile industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fiber has many noteworthy properties as a natural polysaccharide material, such as softness, good breathability, and high hygroscopicity (Wang et al. 2015). The traditional dyeing process of cotton fiber usually involves dyeing with reactive dyes. A large amount of inorganic salt and alkali is required to ensure effective utilization and fixation of reactive dyes. However, dyeing wastewater containing an abundance of electrolytes is a source of environmental pollution that will damage the ecological system. As the living standards of people improve, natural dyes have gradually become the focus of consumers because of their green, natural, nontoxic, and environmental protection characteristics (Sadeghi-Kiakhani and Safapour 2015). During the last few decades, researchers have devoted their attention to the study of natural dyes (Bechtold et al. 2003; Mikropoulou et al. 2009; Zarkogianni et al. 2011). The flavonoids from the aqueous extract of Eupatorium odoratum leaves can be used as dyes for cotton dyeing. The dyed fabric possesses better light fastness and washing fastness in the presence of inorganic mordant alum (Chairat et al. 2011). The natural dye anthocyanin is extracted from the aqueous solution for dyeing silk and cotton using black cowpea seed coat as a raw material. Good washing and heat resistance were obtained under suitable dyeing conditions (Jung and Bae 2014). Microwave treatment of silk fabric and wool fabric, under the same dyeing conditions, the fabric after microwave treatment can get better dyeing performance (Adeel et al. 2018a, b). Silk fabric dyed with the natural pigment extracted from pomegranate peel powder, under gamma rays, has the potential to replace gold synthetic dyes in textile industry (Ajmal et al. 2014). UV radiation treatment on the fabric can improve the color intensity value (Bhatti et al. 2016). Gamma irradiated treated cotton fabric, direct dye can have good dyeing effect and excellent fastness (Adeel et al. 2015). The aqueous solution from madder was used to dye wool fabric, and the effects of different mordants on the dyeing properties of wool fabric were studied (Feiz and Norouzi 2015). The traditional natural dyeing process still has some problems, such as poor washing fastness and poor light fastness. Addition of auxiliary reagents or modifications to fabric has proved to be an effective method to combat these problems. Using rare earths as a mordant greatly improves the washing, rubbing and light fastness of ramie fabrics (Zheng et al. 2011). Alum, copper and tin were used as mordant, and carmine was used to dye the wool, and the adsorption equilibrium and kinetics were studied (Ajmal and Piergiovanni 2018). Chemical and biological mordants are used to improve color shades (Adeel et al. 2019). Pre-mordant dyeing that employs sodium chloride can effectively increase the K/S value of dyed fabric (Chauhan et al. 2015). Three metal mordants and three biological mordants were used to dye the cotton fabric to improve its absorption and dyeing fastness (Souissi et al. 2018). Plant mordant, tannins extracted from Emblica officinalis G, and copper sulphate are used in the pre-dyeing process (Chao et al. 2017). Tannic acid and pomegranate peel extracts are used as natural mordants. An aqueous solution of grape leaves is used to dye silk fabric, thereby improving the fastness of the dyed fabric (Mansour et al. 2016).
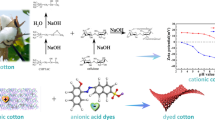
Crocein orange G (COG) is a common natural dye, which is soluble in water and widely used in coloring medicines, cosmetics, and foods (Zhang et al. 2020). The basic properties of cotton fabric are unable to meet the growing environmental requirement. Therefore, the functional modification of cotton fabric is a crucial aspect to consider. In this study, cotton fabric was first modified to produce a large amount of carboxymethyl groups on the fiber, and then dyed with COG according to the complexation mechanism. Complexation reactions can take place under the action of metal mordants, to realize dyeing without the addition of a salt or alkali. Moreover, the kinetics and thermodynamics of COG dyeing on carboxymethyl cotton were also studied. This work explores a novel and effective approach to natural and salt-free dyeing.
Experimental
Material
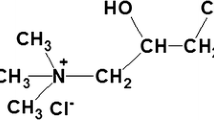
The cotton fabric (High whiteness and wettability after desizing and bleaching) was obtained from Wuhan Rongsheng Printing and Dyeing Co., Ltd., China. Sodium chloroacetate (AR grade) was purchased from Shanghai Macklin Biochemical Co., Ltd., China. COG was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., China. Its chemical structure is illustrated in Fig. 1. All the other reagents, including acetic acid, sodium hydroxide, ethanol, ferric sulfate (Fe2(SO4)3), cupric sulfate (CuSO4), zinc sulfate heptahydrate (ZnSO4·7H2O), and aluminum sulfate octadecahydrate (Al2(SO4)3·18H2O) were supplied by Sinopharm Chemical Reagent Co., Ltd., China, and all reagents are AR grade.
Preparation of carboxymethyl cotton fabric
The preparation process for carboxymethyl cotton is divided into two steps: alkalization and etherification (Liu et al. 2019).
Alkalization: First, the cotton fabric was cut into a piece with a length and width of 30 cm. It was subsequently immersed into the sodium hydroxide aqueous solutions (15 wt%) at 20 °C for 10 min. Finally, it was dried at 60 °C for 10 min.
Etherification: Sodium chloroacetate treatment solution (100 g L−1) was prepared by dissolving sodium chloroacetate in an ethanol/water (v/v = 6/4) solution.
The alkalized cotton fabric obtained in the previous process was dipped in this treatment liquid at 20 °C for 10 min, and then it was placed in a self-sealing bag and kept at 70 °C for 60 min.
After this, the fabric was washed in water thrice, neutralized with acetic acid, washed in water thrice again, and finally dried at ambient temperature. A carboxymethyl cotton fabric was obtained.
Carboxymethyl cotton dyeing with COG
Dyeing parameters, such as dyeing procedures, time, pH value, dye dosage and concentration of mordants were discussed under single-factor conditions. The dyeing time was set at 10–60 min. The pH value was 3–11, and the dye dosage was 1–5% owf. Fe2(SO4)3, CuSO4, ZnSO4·7H2O and Al2(SO4)3·18H2O, this four mordants were used in the experiment at a dosage of 1–5% owf.
Direct dyeing: 0.5 g carboxymethyl cotton was dyed and the liquid ratio was 1:50. The pre-dyeing temperature was 50 °C, and the fabric was placed in stainless steel beakers with dye solution. The heating rate was 2 °C min−1 to 80 °C, and dyeing duration was 30 min. The samples were then washed and dried before further testing.
Pre-mordant dyeing: Before dyeing, the fabric was treated in mordant solution at 50 °C for 30 min. The dyeing process was the same as that of direct dyeing.
Simultaneous mordant dyeing: Initially, both the fabric and mordant were placed together in a beaker at 50 °C for 10 min. The dyeing process was the same as that of direct dyeing.
Post-mordant dyeing: After dyeing by the same process as direct dyeing, the fabric was moved into a mordant solution bath at 50 °C, then heated to 80 °C at a rate of 2 °C min−1, and mordanted for 30 min.
Adsorption kinetics and thermodynamics
A 250 mL flask was filled with the required concentration of the COG solution, the liquor ratio was 1:50, and the pH value was 7.0. The flask was sealed and preheated in a constant-temperature. After 10 min, carboxymethyl cotton fabric was added to the dye solution. The adsorption rate experiments were performed at 30 °C and 50 °C with an initial dye concentration of 2% owf for 1–180 min. Adsorption thermodynamic experiments were performed at 30 °C and 50 °C for 180 min with an initial dye concentration of 0.5–5% owf.
The absorbance of the initial dye before dyeing and the residual dye after dyeing was measured using a V-5600 visible spectrophotometer at the maximum absorption wavelength. The dye percentage is calculated using Eq. (1):
where A0 and A1 are the absorbance of the initial and residual solutions, respectively, and a and b are dilution multiples of the initial and residual solutions, respectively.
K/S values test
The K/S values of unmodified and carboxymethyl cotton fabrics were determined with an x-rite Color i7 computer Color matching apparatus (x-rite, USA). The measurement parameters were set as follows: D65 light source and 10° Standard Observer (D65/10°).
Dyeing fastness test
The dry rubbing and wet rubbing dates were determined using Y571N rubbing color fastness testing instrument according to the ISO 105-X12:2001, MOD test standard. Color fastness to washing with soap and soda testing was carried out according to the ISO 105-C10:2006 standard.
Results and discussion
Effect of carboxymethylation on the K/S value of cotton fabric
As illustrated in Fig. 2, raw cotton fabric and carboxymethyl cotton fabric were dyed at different dosages of COG. During the dyeing process, we used the mordant, and found that the higher the concentration of COG, the higher the K/S value. In addition, the K/S values of carboxymethyl cotton fabric were significantly higher than those of raw cotton fabric.
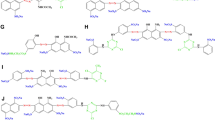
According to Eqs. (2) and (3), under alkaline conditions, the hydroxyl groups on the cotton fabric reacted with alkali to form alkaline cellulose, which was etherified by sodium chloroacetate in the ethanol/water solution to form carboxymethyl cotton. After etherification, some of the hydroxyls of the cotton fiber were replaced by carboxymethyl groups, resulting in the formation of carboxymethyl cotton (Liu et al. 2020).
As depicted in Fig. 3, the carboxymethyl groups were grafted onto the cotton fabric, which made the carboxymethyl cotton fabric more reactive than cotton fabric. As seen in Fig. 4, during this reaction, the mordant acts as a bridge to be able to combine with the fiber group and the dye molecule, where the aluminum ions in the mordant form a stable ring structure with the oxygen atom on the carboxyl group on the fiber, the nitrogen atom on the azo structure of the dye molecule, and the oxygen atom on the terminal hydroxyl group of the dye molecule, with higher binding energy. Therefore, the carboxymethyl cotton fabric possesses a higher adsorption capacity than cotton fabric.
As exhibited in Fig. 5a, of all the 4 mordants, when Al2(SO4)3·18H2O was used, the K/S values were the highest. Aluminum and zinc are located in groups III and II B of the periodic table of elements, respectively, and the ability of these two elements to form complexes is strong. The charge/radius ratio of aluminum is larger than that of zinc, so it is relative to the same ligand. The complexing ability of aluminum should be greater than that of zinc. Copper ions are heavy metal ions, the use of which is banned in textiles. Iron ions can cause fabrics to darken. Therefore, they cannot be widely promoted in industrial applications. Thus, in the following experiment, Al2(SO4)3·18H2O was selected as the mordant.
Four dyeing processes were conducted to explore the dyeing effect in different dyeing procedures. Figure 5b illustrates that under simultaneous mordant dyeing, the K/S value was the highest. In the dyeing process, mordants should be used to promote superior combination with the fiber. With the metal ion mordant, the fabric has a higher color depth than that of the directly dyed fabric.
It can be seen from the Fig. 4b that, except at 1589 cm−1, the curves in the figure all show similar absorption characteristics. The broad absorption band at 3500–3200 cm−1 is the stretching vibration peak of –OH, the peak at 2897 cm−1 is the characteristic peak of C–H bond stretching vibration, and the peak at 1644 cm−1 is the bending of O–H bond vibration characteristic peak, the absorption band at 1160–1000 cm−1 is the tensile vibration characteristic peak of ether bond C–O–C. It can be seen from the figure that the main body of the modified fabric sample is still cellulose. However, on the carboxymethyl cotton spectrum, a new absorption peak was observed at 1589 cm−1, which belongs to the COO− bond in the carboxyl group, which indicates that the cotton fabric was successfully modified by sodium chloroacetate. After mordant dyeing, it is found that the fabric obtained after dyeing, the peak at 1589 cm−1 almost disappeared, and the overall spectrogram was close to that of the unmodified cotton fabric, indicating that the carboxyl group was involved in the coordination and formed coordination bonds with the metal aluminum ions, and the more metal ions in the system, the more carboxyl groups participated in the reaction.
As illustrated in Fig. 5c, addition of a mordant can cause an evident increase in the color depth. On comparison of different dosages of mordants, it was observed that when the concentration was greater than 2% owf, the K/S values of the fabrics did not increase but decreased instead. Therefore, when the mordant exceeds a certain amount, excessive metal ions lead to excessive complexation between metal ions and dye molecules, resulting in agglomeration between molecules, thereby increasing the molecular aggregation of the dye and reducing the color depth. Dyeing took place on carboxymethyl cotton fabric at the selected temperature with 2% owf of dye for 30 min. The K/S values of the dyed fabric are exhibited in Fig. 5d. As observed from the figure, the K/S values of carboxymethyl cotton fabric displayed a trend of first rising and then falling with the increase in pH value and the K/S value of the sample was the highest when the pH value was 7. This is because under low pH conditions, the dye solution is acidic, which inhibits the ionization of carboxyl groups on carboxymethyl cotton fiber. When the pH value was increased, the ionization of carboxymethyl cotton fabric carboxymethyl group continued to increase, resulting in more dye molecules adsorbed on the fabric. Therefore, the K/S value of the dyed fabric sample continued to increase. Nevertheless, when the pH value was too high, the dye solution was alkaline, and the COG was not alkali resistant. Under alkaline conditions, the structure of the dye changes, leading to a decrease in the amount of dyes adsorbed on the fabric, which decreases the K/S value of the dyed fabric sample. Therefore, it is more suitable to dye carboxymethyl cotton fabric with COG dyes with neutral pH values. To study the effect of time on dyeing, the K/S values of the dyed fabric were presented in Fig. 5d. They exhibited a trend of first increasing and then decreasing with increasing dyeing time. The K/S value was the highest when it was dyed for approximately 40 min. Cotton fiber is a natural fiber containing many holes, and it conforms to the channel diffusion model mechanism when dyeing. At the beginning of dyeing, the dye continuously adsorbed on the fabric and gradually diffused into the fabric. At this time, the K/S value of the fabric increases by extending the dyeing time. The dye on the carboxymethyl fabric adsorbs to saturation and the K/S value of the fabric no longer increases with time. On the contrary, the desorption rate of dyes on the fabric is higher than the adsorption rate with the passage of time. This causes a slight decrease in the K/S value of the fabric. Thus, the dyeing time should be controlled and fixed at approximately 40 min.
Figure 6a shows the UV spectrum of COG, it can be seen that the maximum absorption peak is at 442 nm. Figure 6b shows four different mordant dye solutions, the dyeing performance of Al2(SO4)3·18H2O is the best under the same dye concentration and mordant concentration conditions. It is puzzling that the absorption peak of Fe3+ was not observed at the same wavelength, probably because Fe3+ reacted with the dye molecule and changed the molecular structure of the dye, resulting in the inability to observe the corresponding absorption peak at 442 nm and poor dyeing effect.
As shown in Table 1, under the same dye concentration and mordant concentration conditions, for the Df values, Al2(SO4)3·18H2O > CuSO4 > ZnSO4·7H2O, which is consistent with the K/S values obtained from the previous experiments.
Dyeing kinetics
Kinetic rate curves
The dyeing percentage curve is a characteristic of the diffusivity of dyes on fibers. The carboxymethyl cotton fabric was dyed at 30 °C and 50 °C. The dyeing rate curve obtained is depicted in Fig. 7a. The slope of the curve represents the dyeing rate. It can be seen from the figure that the dyeing rate of COG on carboxymethyl cotton fabric increased significantly within a short time and the slope gradually decreased with time and stabilized after 140 min. This indicates that the initial dyeing rate of COG on carboxymethyl cotton fabric was relatively fast, followed by a gradual slowing down before finally attaining dyeing equilibrium. This is due to the treatment of mordant and carboxymethyl cotton fabric with metal cation. The dye in the water contains anions, and the fabric and dye undergo complexation with a strong force, causing the initial dyeing rate to be fast. However, by extending the dyeing time, the dye adsorbed on the carboxymethyl cotton fabric gradually decreases, causing the amount of dye being diffused and adsorbed on the fabric to gradually decrease, thereby decreasing the dyeing rate. When the dye adsorption on the fabric reaches saturation, the dyeing percentage tends to be stable and will not increase with time, having reached dyeing equilibrium.
Pseudo-second-order equation fitting
Dyeing equilibrium refers to a dynamic process in which the amount of adsorbed dyes and desorbed dyes are equal at a moment in the dyeing process. In order to study the mechanism of the adsorption process, a pseudo-second-order kinetic model was adopted to fit the dyeing percentage curve presented in Fig. 7a.
here, k is the dyeing rate constant [kg (g min)−1], C∞ is the amount of dye adsorbed on the fabric when the dyeing reaches equilibrium (g kg−1), and Ct is the amount of dye on the fabric at a specific time (Chairat et al. 2005).
After integrating Eq. (4), it can be converted into:
It can be seen from Eq. (5) that there is a linear correlation between t/Ct and t. The dyeing data is fitted by a pseudo-second-order equation, as shown in Fig. 7b.
C∞ and k were estimated based on the pseudo-second-order plot and the half-adsorption time (t1/2) was estimated according to Eq. (6). These parameters are listed in Table 2.
Half-dyeing time refers to the dyeing time required for the adsorption amount of dyes on the fabric to reach half of its equilibrium adsorption amount. It is observed from Table 2 that both the half-dyeing times are short when dyeing is conducted at different temperatures, which can be attributed to the fact that the carboxymethyl cotton fabric contains a lot of electronegative carboxymethyl and metal ions after the treatment of the mordant. These three kinds of groups combined by complexation make the affinity between carboxymethyl cotton fabrics and COG stronger and lead to faster adsorption. The dyeing rate constant increases and the half-dyeing time decreases with increasing dyeing temperature. This is because the diffusion and adsorption rate of dye molecules on the fabric becomes faster when the temperature increases, leading to a faster dyeing rate. However, the equilibrium adsorption capacity of dyes on the fabric decreases slightly, which may be because the desorption rate of dyes on the fabric is higher than the adsorption rate when the temperature increases.
Diffusion coefficient and activation energy of the diffusion
The diffusion coefficient of COG on carboxymethyl cotton fabrics was calculated using Crank's equation (7) (Yang et al. 2002).
where r is the radius of the carboxymethyl cotton fiber (cm) and r = 6.9625 × 10–4 cm, measured by SEM.
The activation energy of the diffusion was calculated according to Eq. (8):
where Dt is the diffusion coefficient at a particular temperature (cm2 min−1), D0 is a constant, E is the activation energy, and T is the absolute temperature (°C).
As highlighted in Table 3, the diffusion of COG on carboxymethyl cotton fabric increased with an increase in dyeing temperature, which caused the movement of dye molecules to intensify. Therefore, more dye molecules overcame the resistance to the interior of the fiber, thereby increasing the diffusion coefficient (Zhang et al. 2013). The diffusion activation energy of COG on carboxymethyl cotton fabric is 12.9753 KJ mol−1. This represents the energy required to overcome the resistance of COG molecule diffusion and elucidates the relationship between diffusion coefficient and temperature.
Thermodynamic studies
Adsorption isotherms
The dyeing adsorption isotherm refers to the relationship curve between the dye concentration on the fiber ([D]f) and in the dye ([D]s) when the dyeing of the fiber reaches equilibrium at a certain temperature. Dyeing was conducted at 30 °C and 50 °C. Dyeing bath ratio of 1:100, and the adsorption isotherms obtained are portrayed in Fig. 8a.
As depicted in Fig. 8a, the amount of dye on the fiber increases with an increase in dye concentration. This is because the mordant acts as a center ion in dyeing, forming complexes and performing the role of a “bridge” between fiber and dye molecules. When the dosage of mordant was appropriate, along with an increase in the concentration of the dye, more coordination bonds were formed between the dye and fiber by complexation, thereby increasing the adhesion strength of the dye and fiber. In addition, when the temperature is higher, the amount of dye on the fiber also increases. This is because the increase in temperature increases the swelling degree of the fiber and makes the movement of Al3+ in the mordant faster. The more ions effectively bound to the fiber, the more dye molecules were bound and the deeper the color.
Figure 8b presents the batch isothermal data fitted to the linear form of the Freundlich isotherm (Rattanaphani et al. 2007):
where K is constant, and 0 < n < 1.
It illustrates that the correlation coefficients for the linear plots are higher than 0.99 for all experimental data, and indicates that the equilibrium sorption data fits the Freundlich isotherm well when the dye concentration is lower than 0.6 g L−1. Values of n > 1 suggest that the sorption of COG on carboxymethyl cotton fabric is beneficial for sorption in the temperature range 30–50 °C. The Freundlich isotherm is suitable for medium coverage adsorption and multilayer adsorption. After considering the intermolecular interaction in this process, it can be concluded that the adsorption of molecules on the solid surface is completely random, and the adsorption of COG on carboxymethyl cotton fabric displayed good agreement with the Freundlich sorption isotherms.
Standard affinity, dyeing enthalpy and dyeing entropy
Standard affinity is a thermodynamic parameter, which can be calculated by Eq. (10):
where − Δμ° is the affinity of dye molecules to the fabric, R is the gas constant (8.314 J (mol K)−1), and T is the absolute temperature (K), \({\alpha }_{\text{f}}\) is the activity of dye in the fiber and \({\alpha }_{\text{s}}\) is the activity of dye in the solution.
The enthalpy change (ΔH°) in the dyeing system signifies the amount of exothermic energy released from the interactions between the fiber chains and dye molecules. It can be calculated using Eq. (11):
where − Δμ1° and − Δμ2° represent the affinity at T1 and T2 respectively.
The entropy change (ΔS°) indicates an infinite amount of dye in the dye solution from the standard state transferred to the same standard state in the fiber. It was calculated according to Eq. (12):
Dyeing affinity is a characteristic index used to measure the difficulty of dyeing on the fiber. It demonstrates the trend of dye transfer from the dye solution to the fiber in the standard state. The greater the affinity, the greater the tendency of the dye to transfer from the dye solution to the fiber. As depicted in Table 4, the dyeing affinity increases with temperature, that is, the driving force of dye transfer to the fiber increases. The increase in temperature causes the dye molecular kinetic energy to increase, more movement, and a fluffier fiber with larger internal pores. This is advantageous for dye adsorption on the fiber and the internal diffusion of the fiber. The calculated enthalpy change is negative, demonstrating that carboxymethyl cotton fabric dyeing with COG is an exothermic reaction.
As shown in Fig. 9, with the increase of dye concentration, the color depth of dyed fabric are gradually increased, but we can find from the figure that the color depth of dyed fabric under 30 °C is higher than that of 50 °C, this is because COG on dyed carboxymethyl cotton fabric is an exothermic reaction, the increase of temperature is not conducive to the dyeing process, the amount of dye adsorbed by the fabric under 30 °C is higher than that of 50 °C, therefore, its overall K/S values are higher than that of the latter.
Dying fastness properties
Table 5 presents the wet and dry rubbing and washing fastness of the dyed cotton and carboxymethyl cotton fabrics. It is evident that the dyed carboxymethyl cotton fabric has a higher rubbing fastness and washing fastness than the dyed raw cotton fabric. The binding forces between carboxymethyl cotton and COG primarily comprise coordination bonds, van der Waals forces, and hydrogen bonds. In cotton fabrics, only van der Waals forces and hydrogen bonds are present. Under washing conditions, the dye molecules readily desorb from the dyed fabric to water, reducing the color depth and resulting in poor washing fastness. Furthermore, the high solubility of COG in water causes the loss of a large numbers of dye molecules during the washing process, thereby reducing wash fastness.
Conclusions
After the cotton fabric was modified by carboxymethylation, the K/S values and dying fastness of the carboxymethyl cotton fabric dyed with COG were significantly higher than that of unmodified raw cotton fabric. Carboxymethyl cotton fabric dyed with COG conforms to the standard second-order kinetics model and the amount of dye adsorption at the equilibrium capacity of the fabric are 8.15 g kg−1 and 8.21 g kg−1 at 30 °C and 50 °C, respectively. As the temperature increases, the half-dyeing time reduces and the diffusion coefficient and dyeing rate constant increase. This illustrates that the dyeing rate increases with increase in the temperature. The adsorption isotherm data were in good agreement with the Freundlich isotherm. The enthalpy change is negative, indicating that dyeing carboxymethyl cotton fabric on COG is an exothermic reaction, raising the temperature is not conducive to the dyeing process.
References
Adeel S, Usman M, Haider W, Saeed M, Muneer M, Ali M (2015) Dyeing of gamma irradiated cotton using Direct Yellow 12 and Direct Yellow 27: improvement in colour strength and fastness properties. Cellulose 22:2095–2105. https://doi.org/10.1007/s10570-015-0596-0
Adeel S et al (2018a) Microwave-assisted sustainable dyeing of wool fabric using cochineal-based carminic acid as natural colorant. J Nat Fibers 16:1026–1034. https://doi.org/10.1080/15440478.2018.1448317
Adeel S, Rehman F-u, Hameed A, Habib N, Kiran S, Zia KM, Zuber M (2018b) Sustainable extraction and dyeing of microwave-treated silk fabric using arjun bark colorant. J Nat Fibers 17:745–758. https://doi.org/10.1080/15440478.2018.1534182
Adeel S, Zia KM, Abdullah M, Rehman FU, Salman M, Zuber M (2019) Ultrasonic assisted improved extraction and dyeing of mordanted silk fabric using neem bark as source of natural colourant. Nat Prod Res 33:2060–2072. https://doi.org/10.1080/14786419.2018.1484466
Ajmal A, Piergiovanni PR (2018) Effect of mordanting on the adsorption thermodynamics and kinetics of cochineal for wool. Ind Eng Chem Res 57:4462–4469. https://doi.org/10.1021/acs.iecr.7b04915
Ajmal M, Adeel S, Azeem M, Zuber M, Akhtar N, Iqbal N (2014) Modulation of pomegranate peel colourant characteristics for textile dyeing using high energy radiations. Ind Crop Prod 58:188–193. https://doi.org/10.1016/j.indcrop.2014.04.026
Bechtold T, Turcanu A, Ganglberger E, Geissler S (2003) Natural dyes in modern textile dyehouses—how to combine experiences of two centuries to meet the demands of the future? J Clean Prod 11:499–509. https://doi.org/10.1016/s0959-6526(02)00077-x
Bhatti IA, Adeel S, Parveen S, Zuber M (2016) Dyeing of UV irradiated cotton and polyester fabrics with multifunctional reactive and disperse dyes. J Saudi Chem Soc 20:178–184. https://doi.org/10.1016/j.jscs.2012.12.014
Chairat M, Rattanaphani S, Bremner JB, Rattanaphani V (2005) An adsorption and kinetic study of lac dyeing on silk. Dyes Pigments 64:231–241. https://doi.org/10.1016/j.dyepig.2004.06.009
Chairat M, Darumas U, Bremner JB, Bangrak P (2011) Dyeing of cotton yarn with the aqueous extract of the leaves of Eupatorium odoratum L. in Thailand and associated extract toxicity studies. Color Technol 127:346–353. https://doi.org/10.1111/j.1478-4408.2011.00321.x
Chao Y, Ho T, Cheng Z, Kao L, Tsai P (2017) A study on combining natural dyes and environmentally-friendly mordant to improve color strength and ultraviolet protection of textiles. Fiber Polym 18:1523–1530. https://doi.org/10.1007/s12221-017-6964-7
Chauhan K, Dalsaniya P, Pathak H (2015) Optimization of prodigiosin-type biochrome production and effect of mordants on textile dyeing to improve dye fastness. Fibers Polym 16:802–808. https://doi.org/10.1007/s12221-015-0802-6
Feiz M, Norouzi H (2015) Dyeing studies of wool fibers with madder (Rubia tinctorum) and effect of different mordants and mordanting procedures on color characteristics of dyed samples. Fibers Polym 15:2504–2514. https://doi.org/10.1007/s12221-014-2504-x
Jung YS, Bae DG (2014) Natural dyeing with black cowpea seed coat. I. Dyeing properties of cotton and silk fabrics. Fibers Polym 15:138–144. https://doi.org/10.1007/s12221-014-0138-7
Liu Y, Xia L, Zhang Q, Guo H, Wang A, Xu W, Wang Y (2019) Structure and properties of carboxymethyl cotton fabric loaded by reduced graphene oxide. Carbohydr Polym 214:117–123. https://doi.org/10.1016/j.carbpol.2019.03.028
Liu Y et al (2020) Kinetics and thermodynamics studies of cationic dye adsorption onto carboxymethyl cotton fabric. J Nat Fibers. https://doi.org/10.1080/15440478.2020.1731908
Mansour R, Mighri Z, Mhenni F (2016) Exploring the potential uses of Vitis vinifera L. leaves as raw material for textile dyeing without metal mordants. Fibers Polym 17:1621–1626. https://doi.org/10.1007/s12221-016-5033-y
Mikropoulou E, Tsatsaroni E, Varella EA (2009) Revival of traditional European dyeing techniques yellow and red colorants. J Cult Herit 10:447–457. https://doi.org/10.1016/j.culher.2009.02.003
Rattanaphani S, Chairat M, Bremner JB, Rattanaphani V (2007) An adsorption and thermodynamic study of lac dyeing on cotton pretreated with chitosan. Dyes Pigments 72:88–96. https://doi.org/10.1016/j.dyepig.2005.08.002
Sadeghi-Kiakhani M, Safapour S (2015) Salt-free reactive dyeing of the cotton fabric modified with chitosan-poly(propylene imine) dendrimer hybrid. Fibers Polym 16:1075–1081. https://doi.org/10.1007/s12221-015-1075-9
Souissi M, Guesmi A, Moussa A (2018) Valorization of natural dye extracted from date palm pits (Phoenix dactylifera) for dyeing of cotton fabric. Part 2: optimization of dyeing process and improvement of colorfastness with biological mordants. J Clean Prod 204:1143–1153. https://doi.org/10.1016/j.jclepro.2018.08.325
Wang J, Li X, Cai Z, Gu L (2015) Absorption kinetics and thermodynamics of cationic dyeing on easily dyeable copolyester modified by 2-methyl-1,3-propanediol. Fibers Polym 16:2384–2390. https://doi.org/10.1007/s12221-015-5224-y
Yang Y, Brown H, Li S (2002) Some sorption characteristics of poly(trimethylene terephthalate) with disperse dyes. J Appl Polym Sci 86:223–229. https://doi.org/10.1002/app.10953
Zarkogianni M, Mikropoulou E, Varella E, Tsatsaroni E (2011) Colour and fastness of natural dyes: revival of traditional dyeing techniques. Color Technol 127:18–27. https://doi.org/10.1111/j.1478-4408.2010.00273.x
Zhang Y, Chen J, Shen J, Zhong J, Ye R, Liang B (2013) Apparent diffusion coefficient values of necrotic and solid portion of lymph nodes: Differential diagnostic value in cervical lymphadenopathy. Clin Radiol 68:224–231. https://doi.org/10.1016/j.crad.2011.04.002
Zhang L, Lian W, Li P, Ma H, Han X, Zhao B, Chen Z (2020) Crocein Orange G mediated detection and modulation of amyloid fibrillation revealed by surface-enhanced Raman spectroscopy. Biosens Bioelectron 148:111816. https://doi.org/10.1016/j.bios.2019.111816
Zheng GH, Fu HB, Liu GP (2011) Application of rare earth as mordant for the dyeing of ramie fabrics with natural dyes. Korean J Chem Eng 28:2148–2155. https://doi.org/10.1007/s11814-011-0090-9
Acknowledgments
We are very grateful for the financial support from the Hubei Province Central Leading Local Science and Technology Development Special Foundation (2020ZYYD038), National Natural Science Foundation of China (51325306) and the China Chemical Fibers Association, Lv Yu Foundation (CCFALY2018-2-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Liu, Y., Song, D. et al. Dyeing behavior and mechanism of Crocein Orange G on carboxymethyl cotton fabric. Cellulose 28, 5911–5922 (2021). https://doi.org/10.1007/s10570-021-03880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03880-0