Abstract
Cotton fabric is usually dyed with reactive dyes. During the dyeing process, a large amount of salt is required to achieve higher exhaustion of the dye from the dyebath onto the fiber. Dyeing of cotton with reactive dyes has a substantial environmental impact due to the discharge of a large volume of highly colored and saline effluents. Chemical cationization allows cotton fibers to be dyed without salt by chemically modifying cellulosic macromolecules to introduce positively charged sites. In this study, cotton fabric was cationized using (3-chloro-2-hydroxylpropyl) trimethyl-ammonium chloride (CHPTAC). Dye uptake was assessed using two reactive dyes, CI Reactive Blue 235 and CI Reactive Blue 19. Dye exhaustion kinetics were determined using a Datacolor-HueMetrix Monitor system. Analysis of variance demonstrated significant effects of CHPTAC concentration and exhaustion time on the percent exhaustion. Color strength at the end of the dyeing cycle was significantly higher for cationized fabrics compared to the control fabric. This work shows that treatment of cotton with CHPTAC enhanced dye uptake properties due to the introduction of cationic sites and resulted in superior dyeing without the addition of salt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The predominant commercial cellulosic fiber and the backbone of the world’s textile trade is cotton (Blackburn and Burkinshaw 2003; Plastina 2009). Cotton has several advantageous characteristics for use in the textile industry, such as hydrophilicity, air permeability, biodegradability, and a lack of static electricity (neutrality towards charge build-up) (Wang et al. 2009b). Cotton is almost 100 % cellulose after scouring and bleaching. Cellulose is a linear polymer consisting of β-d glucopyranose units covalently linked by 1,4-glycosidic bonds (Klemm et al. 2005; Park et al. 2010). The hydroxyl groups present on glucose units are the main sites of dye fixation as well as chemical modification.
Dyeing is one of the most important steps in the textile processing of cotton in terms of associated costs of water, energy, and chemical use (Karahan et al. 2008). Furthermore, dyeing of cotton leads to substantial environmental impacts. Cotton fabrics are primarily dyed with reactive dyes because of the brilliancy, varieties of hue, and high wash-fastness of reactive dyes, which are anionic in nature (Kannan et al. 2006; Chattopadhyay et al. 2007; Lewis and Vo 2007; Montazer et al. 2007; Teng et al. 2010). Upon contact with water, cellulosic fibers generate slightly negative charges due to the ionization of the hydroxyl groups (Kannan et al. 2006; Chattopadhyay et al. 2007; Lewis and Vo 2007; Montazer et al. 2007; Teng et al. 2010). Therefore, a large amount of electrolytes, such as Glauber’s salt or sodium chloride, is required to neutralize the repulsive forces between negative charges of the dye and negative charges produced on the fiber surface (Montazer et al. 2007; Khatri 2011). When Glauber’s salt is used, approximately 60 % of the dye is exhausted in conventional dyeing systems. The electrolytes remain in the dyebath after dyeing because they are not exhausted nor destroyed. In addition, an alkali is required in reactive dyeing to generate sufficient cellulosate anions for dye molecule fixation. However, the abundant hydroxyl ions cause significant hydrolysis of the reactive dyes. Therefore, reactive dyeing pollutes the environment via the discharge of a highly colored and saline dyebath (Blackburn and Burkinshaw 2002, 2003; Kannan et al. 2006).
While the direct cost associated with the use of salt in cotton fabrics dyeing might be low, the cost associated with the dyeing process is significantly higher due to the low exhaustion and dye fixation. In addition, the water and energy required to remove the hydrolyzed dye from the fabric and clean the dyebath increase the cost substantially (Shore 1995; Blackburn and Burkinshaw 2002). Moreover, environmental impacts of the highly colored and saline effluents released from the dyeing process cannot be overlooked (Acuner and Dilek 2004; Kannan et al. 2006; Aksu et al. 2007; Wang et al. 2009a). Therefore, methods that eliminate or reduce the salt requirement during dyeing are of utmost importance.
Numerous studies have sought to improve the affinity of anionic dyes toward cotton fabric by introducing positively charged sites on cotton (Hauser 2000; Kanik and Hauser 2004; Tutak 2011). This process is called cationization. The introduction of positively charged sites enables the formation of an electrostatic attraction between the fiber and the negatively charged dye molecules, thus eliminating the need for electrolytes in the cotton dyeing process and increasing the dye exhaustion and color yield of the fabric. Complete exhaustion can be achieved on cationized cotton without the addition of salt (Frazer 2002). Cationized cotton can also be dyed with reactive dyes at neutral pH, resulting in improved dyeing compared to conventional dyeing methods (Blackburn and Burkinshaw 2003). Cationic cotton offers a significant advantage to the textile industry because it can be dyed in less time using less energy and with reduced impact on the environment, while achieving higher color yields and fastness properties compared to those of conventionally dyed cotton (Hauser 2000). The cationization process can also be exploited in the printing of cotton fabrics (Kanik and Hauser 2004).
Cationization of cotton surfaces was performed to enhance silver nanoparticles adsorption (Khalil-Abad et al. 2009). The authors reported that cationization of cotton surface strongly increases the amount of adsorbed nanoparticles. Tarburk et al. (2014) cationized cotton fibers during the mercerization process. The results showed that cationization of cotton resulted in novel cotton cellulose which may lead to a new application in cotton pre-treatment and chemical finishing. Ramie fibers were also cationized to improve dyeuptake (Liu et al. 2007). The authors reported 3 times increase in K/S values for the cationic modified fibers as compared to unmodified fibers.
Cotton is cationized through the reaction of various types of substituted amino compounds (Montazer et al. 2007). The introduction of amino groups renders cellulose fibers cationic, increasing their affinity for anionic dyes due to the electrostatic interaction between positive charges on the fiber and negative charges on anionic dyes (Hauser and Tabba 2001). 3-(Chloro-2-hydroxypropyl) trimethylammonium chloride (CHPTAC) has been widely used to cationize cotton and effectively improves dye uptake properties of cotton fabric (Hashem et al. 2003; Kanik and Hauser 2004; Montazer et al. 2007; Wang et al. 2009b; Ferrero and Periolatto 2011; Patiño et al. 2011). However, studies of the effect of the level of cationizatiottin of cotton fabric (the concentration of CHPTAC) on the % exhaustion kinetics of the dye have been limited.
In this study, cotton fabric was cationized using five different concentrations of CHPTAC, and the real-time exhaustion of two reactive dyes on cationized fabrics dyed without salt was monitored. The main focus of the study was to assess the effect of the cationization level on the exhaustion kinetics of reactive dyes on cationized cotton fabrics.
Experimental
Materials
Fabric
Desized, scoured and bleached 100 % cotton fabric was used in this study. This fabric has the following characteristics: weight 229.06 g/m2, yarn size 21.9 × 13 Ne, 99.4 picks per in. and 55.4 ends per in.
Chemicals
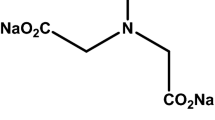
The quaternary ammonium compound CHPTAC (Fig. 1), which has a molecular weight of 188.1 g/mol, was used to cationize the cotton fabrics. This chemical compound is commercially available under the name CR-2000, which is a 65 % aqueous solution of CHPTAC.
Dyes
Two reactive dyes were used in this study: CI Reactive Blue 235 (CI RB 235) and CI Reactive Blue 19 (CI RB 19). CI RB 19 (Fig. 2) contains anthraquinone groups as chromophores and vinyl sulfone as the reactive group (Asgher and Bhatti 2012). However, the detailed chemical composition of CI RB 235 is not available (trade secret). CI RB 235 is available under the commercial name of Novacron Blue FN-R and is a product of the Huntsman Corporation.
Molecular structure of CI RB 19 (Siddique et al. 2011)
Fluid B
Fluid B, which has a lower surface tension than water, was prepared and used as a carrier fluid for the HueMetrix. Fluid B was composed of 16.14 % anhydrous sodium acetate, 12 % acetic acid, 0.1 % Tomadol 1–7 and 0.1 % Triton X-100 (w/w basis). It was used as a carrier fluid for each sample during the calibration and dyeing. Fluid B conditions the sample under analysis to ensure consistent sample analysis. It is also used to prevent crystallization of auxiliary agents (salt and alkali) in the small hoses of the HueMetrix unit.
Other chemicals
Sodium hydroxide (NaOH) was used as a catalyst for the reaction of CHPTAC and cotton fabric. Other chemicals used include surfactants (Tomadol 1–7 and Triton X-100), anhydrous sodium acetate, acetic acid, sodium sulfate (Na2SO4), and sodium carbonate (Na2CO3). The surfactants were used in the Fluid B to reduce the surface tension. Anhydrous sodium acetate and acetic acid were added to Fluid B as a buffer to maintain a constant pH in the dyebath during the measurement of the absorbance and in the HueMetrix system. Sodium sulfate was used as a salt to neutralize negative repulsive forces between the fabric surface and dye molecules. Sodium carbonate was used to facilitate the fixation of dye molecules on the fabric.
Methods
Cationization of the fabric
The fabrics were cationized using the exhaust method at room temperature in a liquor ratio of 17:1. Cationization was performed using one of five different concentrations of CHPTAC (35, 17.5, 13.1, 8.8 and 4.4 g/l). Each 60 g fabric sample was first immersed in a solution of CHPTAC at one of the specified concentrations. Then, NaOH was added to the solution to achieve a CHPTAC/NaOH ratio of 2.33. The fabric was gently stirred and left for 20 min, then removed and hand squeezed to remove excess water. The wet pick-up was approximately 100 %. The treated fabric was then placed in a plastic bag to prevent chemical migration and water evaporation and stored at room temperature for approximately 24 h (Montazer et al. 2007). After rinsing 5 times with tap water, the treated fabric was immersed in an acetic acid solution (1 g/l) for 3–5 min to neutralize the alkalinity. The fabric was again rinsed in tap water 5 times and oven dried overnight at 40 °C.
Dyeing
The control (not cationized) and cationized fabrics were dyed with reactive dyes CI RB 235 and CI RB 19. CI RB 235 was used to dye the control fabric and fabrics cationized with the five different concentrations of CHPTAC. However, only the control and fabrics cationized with 17.5 g/l CHPTAC were dyed with CI RB 19. All fabrics were dyed using the exhaustion method in the Datacolor Dyeing Machine (Lawrenceville, NJ, USA) at a liquor ratio of 20:1 with 1.75 % dye per weight of fabric (1.05 g dye on 60 g fabric). Dye exhaustion isotherms were determined using the HueMetrix monitoring system.
Before the start of dyeing, the dyebath solution was added to the Datacolor Dyeing Machine and circulated for 30 min through the loop connecting the dyeing machine and the HueMetrix to distribute the dye homogeneously in the dyebath. The dyeing process started at room temperature, and the temperature of the dyebath was raised at a rate of 2 °C/min until a temperature of 60 °C was achieved; the temperature was then kept constant. The dyeing procedure was the same for all fabrics with the exception of the addition of salt. Control fabrics were dyed with and without the addition of salt. However, the cationized fabrics were dyed without salt. When dyeing the control fabric with salt, 50 g of Na2SO4 was divided into 4 equal parts. The first 12.5 g was added to the dyebath at 38 min after the start of the dyeing process, and the remaining parts were added every 5 min thereafter. For the control and cationized fabrics, 25 g of Na2CO3, divided into 4 equal parts, was added to the dyebath. The first portion of the base was added at 68 min after the start of the dyeing process, and the remaining parts were added every 5 min thereafter. When the HueMetrix Monitor system recorded the last reading of the dye concentration of the dyebath at the set time of 130 min, the Datacolor program was ended manually. At the end of the dyeing process, the dyebath was flushed from the Datacolor, and the fabric was removed and rinsed with cold tap water 5 times until the wash water became clear. After rinsing, the dyed fabric was oven dried overnight at 40 °C. Three replicates were performed from each treatment. The charts shown in Figs. 3 and 4 summarize the dyeing profile.
Real-time dye exhaustion monitoring
HueMetrix Monitor system
The HueMetrix monitor system is connected to the Datacolor dyeing machine by a small circulation loop. During the dyeing process, the HueMetrix automatically collects a microsample (0.1 ml) equivalent to a fraction of a drop from the dyebath every 90 s. The HueMetrix system conditions the sample both physically and chemically to ensure consistent measurement. The absorbance of the sample is measured with an integrated UV–Vis spectrophotometer. Then, using the calibration curve of the dye, the resulting dye concentration is determined in real-time based on the absorbance of the dyebath sample. In addition to the dye concentration, data on the temperature, pH and conductivity of the dyebath are also collected in real-time.
The HueMetrix monitor system was calibrated for each dye used (one for CI RB 235 and one for CI RB 19). To this end, dye solutions (0.05, 0.1, 0.25, 0.5, 0.75 and 1 g/l) were injected and their absorbance values at the wavelength corresponding to the maximum absorbance (610 nm for CI RB 235 and 590 nm for CI RB 19) were determined using the integrated UV–Vis spectrophotometer. Each concentration was injected twice. The absorbance values were plotted against the concentration to establish a calibration curve for each dye, and each calibration curve was validated by the HueMetrix software.
The dyebath exhaustion percentage (% E) was calculated using the following equation:
where C0 and Ct are the concentrations of the dye in the dyebath at the beginning of dyeing process and at time t, respectively.
The exhaustion isotherms of the control fabric and the fabrics cationized with 17.5 and 35 g/l were fitted to the following equation:
The first derivative of the Eq. (2) is given by the following equation:
where x represents the time in min and y represents the exhaustion expressed in percent.
Exhaustion isotherms of fabrics cationized with 4.4, 8.8 and 13.1 g/l CHPTAC were fitted to the following equation:
The first derivative of Eq. (4) is given by the following equation:
where x represents the time in min and y represents the exhaustion percent.
Colorimetry
The color of each dyed fabric was determined using a Macbeth Color Eye 3000 Colorimeter. The instrument was first calibrated using a standard white tile as specified by the manufacturer. The relative color strength (K/S) of each dyed fabric was measured from the reflectance value at the wavelength of the maximum absorbance (λmax) of the dye. For each sample, K/S was measured randomly at 3 different locations along the front side of the length of the fabric and at three different locations along the back side of the length of the fabric. The 6 measurements were then averaged to obtain a single value for each sample. K/S values were determined using the Kubelka–Munk equation (Kannan et al. 2006):
where R is the reflectance at λmax.
Results and discussion
Calibration of the HueMetrix monitor
Figure 5 shows the absorbance of the CI RB 235 solutions as a function of the concentration. The absorbance increases as the concentration of CI RB 235 increases. The maximum absorbance is observed at 610 nm. The absorbance values at 610 nm were used to establish a calibration curve according to the Beer–Lambert law (Fig. 6). The high correlation indicates that the HueMetrix monitor system can be used to measure the concentration of the dye in the dyebath and monitor the exhaustion of the dye during the dyeing process. Similar results were obtained with CI RB 19 (results not shown).
Effect of salt on the dye exhaustion
Figure 7 shows the evolution of the % exhaustion of CI RB 235 as a function of dyeing time for control fabric dyed with and without salt. Virtually no dye exhaustion occurred until the addition of the alkali at 68 min after the start of the dyeing process. The dye exhaustion started to increase about 2 min after the addition of the alkali. The % exhaustion continued to increase for both fabrics until a plateau was reached before the end of the dyeing cycle at 130 min. The final % exhaustion was low for the fabric dyed with salt (57 %) compared to the fabric dyed without salt (65 %). This behavior is not typical for reactive dyes. In general, the addition of salt increases the % exhaustion (Montazer et al. 2007). Because the chemistry of CI RB 235 is unknown, an explanation of this behavior cannot be made. In contrast to CI RB235, the final % exhaustion of CI RB 19 was significantly higher when the fabric was dyed using salt (89 %) compared to dyeing without salt (63 %) (Fig. 8). Notably, the % exhaustion began to increase immediately upon the addition of salt. When no salt was added, the % exhaustion increased only when alkali was added. This behavior is typical of a reactive dye.
Effect of fabric cationization on % exhaustion
To examine the effect of the cationization level on the % exhaustion of the dye, fabrics cationized with varying concentrations of CHPTAC were dyed with CI RB 235. No salt was used; only alkali was added as indicated by the arrows in Fig. 9. For fabrics cationized with higher concentrations of CHPTAC (35.0 and 17.5 g/l), the % exhaustion increased until the % exhaustion reached a peak of 94 or 99 % after only 30 min. The addition of alkali initially decreased the amount of fixed dye. However, at the end of the dyeing cycle, the % exhaustion reached again 95 %. For fabrics cationized with lower concentrations of CHPTAC (4.4, 8.8 and 13.1 g/l), the % exhaustion reached 14, 43, and 71 %, respectively, after 30 min. The addition of alkali further increased the % exhaustion to 71, 84, and 90 %, respectively. For the control fabric (not cationized), no dye was exhausted until the addition of alkali.
Cotton fabric dyed with CI RB 235. Effect of the concentration of CHPTAC on the % exhaustion. a Control fabric, b fabric cationized with 4.4 g/l, c fabric cationized with 8.8 g/l, d fabric cationized with 13.1 g/l, e fabric cationized with 17.5 g/l, f fabric cationized with 35.0 g/l. The arrows indicate when alkali is added
An analysis of variance was performed with the cationization level as the treatment and the exhaustion times (30 and 130 min) as the factors (Table 1). There was significant effects of the treatment and the exhaustion time, and there was also a significant interaction treatment * exhaustion time. The % exhaustion was significantly higher for each cationized fabric than for the control fabric. The % exhaustion was significantly higher at 130 min than at 30 min for each treatment, with the exception of fabrics cationized with 17.5 and 35 g/l CHPTAC.
The initial exhaustion of fabrics treated with CHPTAC can be attributed solely to the presence of cationic sites on the fabric due to the chemical cationization because early exhaustion before the addition of alkali did not occur with the control fabric. The increase in the % exhaustion at 30 and 130 min with the increase in the CHPTAC concentration (Fig. 10) can be attributed to the increase in the number of cationic sites on the fabric when the concentration of CHPTAC is increased. However, as is evident from Fig. 10, increasing the CHPTAC concentration from 17.5 to 35 g/l had practically no effect on the exhaustion of CI RB 235 at 130 min; therefore, a CHPTAC concentration of 17.5 g/l was concluded to be optimal for the cationization of cotton fabric.
The decrease in the % exhaustion on fabrics cationized with higher concentrations of CHPTAC (17.5 and 35 g/l) after the addition of alkali could be due to the removal of some unfixed or weakly fixed CHPTAC molecules from the fabric, resulting in the release of dye molecules that are electrostatically fixed to CHPTAC into the dyebath. Therefore, the concentration of the dye in the dyebath increases, and a decrease in the % exhaustion is observed. However, the addition of alkali creates nucleophilic sites on the fabric, ultimately increasing the fixation of dye molecules.
To calculate the rate of exhaustion of CI RB 235, the exhaustion isotherms were fitted with an empirical equation, and the first derivative was calculated. The maximum rate of exhaustion (% exhaustion/min) was determined from the peak of the derivative. The exhaustion isotherms of the control and fabric cationized with higher levels of CHPTAC (17.5 and 35 g/l) were fitted using Eq. 2, and the derivative was calculated (Eq. 3). The exhaustion isotherms of the fabrics cationized with lower concentrations of CHPTAC (4.4, 8.8 and 13.1 g/l) were fitted using Eq. 4, and the derivative was calculated (Eq. 5).
The maximum rates of exhaustion as a function of the cationization level are shown in Table 2. For the control fabric dyed without salt, a peak of exhaustion was observed only at a later stage of dyeing. However, for fabrics cationized with 4.4, 8.8, and 13.1 g/l CHPTAC, two peaks were observed. The maximum rate of exhaustion increased for the first peak whereas it decreased for the second peak as the cationization level increased. The second peak completely disappeared for fabrics cationized with higher concentrations of CHPTAC (17.5 and 35 g/l), and there was only one peak in the initial stage with a very high maximum rate of exhaustion compared to fabrics cationized with lower concentrations of CHPTAC. The first peak is the result of dye exhaustion due to the electrostatic attraction between cationic sites on the fabric and anionic dye molecules. The second peak is the result of the nucleophilic reaction of the dye with cellulose after the addition of alkali. The disappearance of the second peak for the fabrics cationized with higher concentrations of CHPTAC indicates the presence of sufficient cationic sites in these fabrics for the complete exhaustion of dye molecules at a faster rate.
Because dyeing experiments of the cationized fabric with CI RB 235 revealed that 17.5 g/l CHPTAC was the optimum concentration to cationize cotton, only the fabric cationized with 17.5 g/l was dyed with CI RB 19. As expected, similar results were observed. Figure 11 shows the % exhaustion of CI RB 19 on the cationized (17.5 g/l) and control fabrics. On the cationized fabric, the % exhaustion increased as the dyeing time increased and reached 80 % after 30 min, when a plateau was reached. The addition of alkali first decreased the % exhaustion, but the % exhaustion further increased to 96 % at the end of the dyeing process (130 min). However, for the control fabric dyed without salt, the % exhaustion increased only after the addition of alkali. The final % exhaustion reached 61 % at the end of the dyeing process (130 min).
The results suggest that because the dye exhaustion isotherms on the cationized fabrics reached plateaus within 30 min of the dyeing process and the % exhaustion (at plateau) increased as the CHPTAC concentration increased, high % exhaustion could be achieved in a short time by optimizing the concentration of the cationizing agent. CI RB 235 was almost entirely exhausted on the fabric when the CHPTAC concentration reached 17.5 g/l without the addition of alkali. This high dye exhaustion could lead to a considerable reduction in the dyeing cycle, resulting in huge savings in the energy required for cotton dyeing.
Effect of alkali on the % exhaustion on cationized fabric
As discussed earlier, dye exhaustion experiments on the cationized fabric demonstrated that a second increase in the % exhaustion occurred after alkali was added to the dyebath of fabrics cationized with lower CHPTAC concentrations (4.4, 8.8 and 13.1 g/l). The fabric cationized with 8.8 g/l CHPTAC was dyed with CI RB 235 both with and without the addition of alkali to confirm the effect of alkali. Figure 12 shows the % exhaustion of CI RB 235 on the fabric cationized with 8.8 g/l CHPTAC and dyed with and without alkali as a function of dyeing time. The exhaustion isotherms of the two fabrics were almost identical until the addition of alkali. Before the addition of alkali, the % exhaustion was approximately 40 %. When no alkali was added, the % dye exhaustion remained the same throughout the dyeing time. However, when alkali was added, the exhaustion increased to 84 %. These results indicate that alkali is necessary to achieve high % exhaustion on fabrics cationized with lower concentrations of CHPTAC.
Effect of the dye on the % exhaustion on the cationized fabric
The effect of the dye on the % exhaustion of the cationized fabric was assessed by comparing the % exhaustion isotherms of the fabrics cationized with the same concentration of CHPTAC and dyed with CI RB 235 and CI RB 19. Figure 13 shows the % exhaustion of CI RB 235 and CI RB 19 on the fabric cationized with 17.5 g/l CHPTAC. The % exhaustion isotherms of CI RB 235 and CI RB 19 are similar. However, the % exhaustion of RB 235 reached 94 % at 30 min, at which time a plateau was reached, while the % exhaustion of RB 19 was only 80 % when the plateau was reached at 30 min. The final % exhaustion of both dyes was identical after the alkali was added. As discussed earlier, the initial exhaustion of the dye on the cationized fabric could be attributed to the cationic sites on the fabric. The % exhaustion of CI RB 19 is significantly lower than that of CI RB 235 at 30 min at the same cationization level (Table 3), suggesting that the fixation mechanism of CI RB 19 differs from that of CI RB 235. These results indicate that the exhaustion behavior of the dye on the cationized fabric is governed not only by the level of cationization but also by the type of dye being used.
Effect of cationization on the color strength of the cationized fabric
The color strength parameter (K/S) has been widely used for color measurement of dyed substrates (Rong and Feng 2006; Lewis and Vo 2007; Montazer et al. 2007). Figure 14 shows K/S values (at λmax = 610 nm) of the control and cationized fabrics dyed with CI RB 235. K/S values increased almost linearly until the CHPTAC concentration reached 17.5 g/l. ANOVA demonstrated that all treatments were significantly different from each other (Table 4). K/S values of fabrics cationized with higher concentrations of CHPTAC (17.5 and 35 g/l) were about double those of the control fabric. For CI RB 19, the K/S value of the cationized fabric was also higher than that of the control fabric (results not shown). The K/S value at λmax is directly proportional to the concentration of the dye on the substrate (Lewis and Vo 2007). Higher K/S values for the cationized fabric compared to the control fabric and the increase in the K/S value with the increase in the concentration of CHPTAC confirm that the cationization has a positive effect on the dye fixation on the cotton fabric.
Conclusions
Cotton fabric was treated with different concentrations of CHPTAC and dyed with two reactive dyes, CI RB 235 and CI RB 19. Dyeing kinetics of treated and control fabrics were assessed by plotting the % exhaustion isotherms as a function of dyeing time. The dye uptake properties were assessed by analyzing the % exhaustion of the dye at 30 min (before adding salt and/or alkali) and 130 min (end of the dyeing cycle) and the color yields of the dyed fabrics. Both dyes exhausted onto cationized fabrics during the initial stage of dyeing with the rate of the initial exhaustion of CI RB 235 increasing as the concentration of CHPTAC increased.
The % exhaustion of CI RB 235 was significantly higher on all cationized fabrics than on the control fabric, and the % exhaustion of CI RB 19 on the cationized fabric was also significantly higher. Almost 100 % of the dye was exhausted within 30 min of dyeing on the fabrics cationized with higher concentrations of CHPTAC without the addition of salt and alkali. Dye exhaustion significantly increased at both 30 and 130 min with increasing CHAPTAC concentration. Unlike fabrics treated with higher concentrations of CHPTAC, the addition of alkali increased the % exhaustion of the dye on the fabrics cationized with lower concentrations of CHPTAC; the addition of alkali is required to achieve higher % exhaustion for these fabrics.
Color measurement results demonstrated that the color strength (K/S) of the cationized fabric (17.5 and 35 g/l) was significantly higher than that of the control fabric for both dyes. For CI RB 235, the K/S increased significantly as the concentration of CHPTAC increased.
In summary, the treatment of cotton with CHPTAC enhanced dye uptake properties of cotton due to the addition of cationic sites. Using cationized cotton, superior dyeing results were obtained without the addition of salt over a short dyeing time. The optimum concentration of CHPTAC for cationization depends upon the dye. Cationization could be a viable option for lowering the quantity of effluent discharged by dyeing operations and reducing the environmental impact of the dyeing process. Cationization can also lower the quantity of water, energy and chemicals required for dyeing cotton fabrics.
References
Acuner E, Dilek FB (2004) Treatment of tectilon yellow 2G Chlorella vulgaris. Process Biochem 39:623–631
Aksu Z, Kılıç NK, Ertuğrul S, Dönmez G (2007) Inhibitory effects of chromium (VI) and Remazol Black B on chromium (VI) and dyestuff removals by Trametes versicolor. Enzyme Microb Technol 40:1167–1174
Asgher M, Bhatti HN (2012) Removal of reactive blue 19 and reactive blue 49 textile dyes by citrus waste biomass from aqueous solution: equilibrium and kinetic study. Can J Chem Eng 90:412–419
Blackburn RS, Burkinshaw SM (2002) A greener approach to cotton dyeings with excellent wash fastness. Green Chem 4:47–52. doi:10.1039/b111026h
Blackburn RS, Burkinshaw SM (2003) Treatment of cellulose with cationic, nucleophilic polymers to enable reactive dyeing at neutral pH without electrolyte addition. J Appl Polym Sci 89:1026–1031
Chattopadhyay DP, Chavan RB, Sharma JK (2007) Salt-free reactive dyeing of cotton. Int J Cloth Sci Technol 19:99–108. doi:10.1108/09556220710725702
Ferrero F, Periolatto M (2011) Dyes adsorption on functionalized fibrous materials. Chem Eng Trans 25:1037–1042
Frazer L (2002) A cleaner way to color cotton. Environ Health Perspect 110:A252–A254
Hashem M, Hauser PJ, Smith B (2003) Reaction efficiency for cellulose cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Text Res J 73:1017–1023. doi:10.1177/004051750307301113
Hauser PJ (2000) Reducing pollution and energy requirements in cotton dyeing. Text Chem Color Am Dyest Rep 32:44–48
Hauser PJ, Tabba AH (2001) Improving the environmental and economic aspects of cotton dyeing using a cationised cotton. Color Technol 117:282–288
Kanik M, Hauser PJ (2004) Printing cationized cotton with direct dyes. Text Res J 74:43–50. doi:10.1177/004051750407400108
Kannan MSS, Gobalakrishnan M, Kumaravel S, Nithyanadan R, Rajashankar KJ, Vadicherala T (2006) Influence of cationization of cotton on reactive dyeing. J Text Appar Technol Manag 5:1–16
Karahan HA, Özdoğan E, Demir A, Ayhan H, Seventekin N (2008) Effects of atmospheric plasma treatment on the dyeability of cotton fabrics by acid dyes. Color Technol 124:106–110. doi:10.1111/j.1478-4408.2008.00129.x
Khalil-Abad MS, Yazdanshenas ME, Nateghi MR (2009) Effect of cationization on adsoprtion of silver nanoparticles on cotton surfaces and its antibacterial activity. Cellulose 16:1147–1157
Khatri A (2011) Use of biodegradable organic salts for pad-steam dyeing of cotton textiles with reactive dyes to improve process sustainability. In: International conference on education research and innovation IPEDR. IACSIT Press, Singapore, pp 84–89
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chemie Int Ed 44:3358–3393
Lewis DM, Vo LTT (2007) Dyeing cotton with reactive dyes under neutral conditions. Color Technol 123:306–311
Liu Z-T, Yang Y, Zhang L, Liu Z-W, Xiong H (2007) Study on the cationic modification and dyeing of ramie fiber. Cellulose 14:337–345
Montazer M, Malek RMA, Rahimi A (2007) Salt free reactive dyeing of cationized cotton. Fibers Polym 8:608–612
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10. doi:10.1186/1754-6834-3-10
Patiño A, Canal C, Rodríguez C, Caballero G, Navarro A, Canal JM (2011) Surface and bulk cotton fibre modifications: plasma and cationization. Influence on dyeing with reactive dye. Cellulose 18:1073–1083. doi:10.1007/s10570-011-9554-7
Plastina A (2009) Cotton’s share of world textile fiber use continues to decline. http://www.icac.org/meetings/plenary/68_cape_town/documents/bo3/bo3_e_icac.pdf. Accessed 27 Apr 2012
Rong L, Feng G (2006) Dyeing properties of PECH-amine cationized cotton with acid dyes. J Appl Polym Sci 100:3302–3306. doi:10.1002/app.23155
Shore J (1995) Cellulosics dyeing. The Society of Dyers and Colourists, West Yorkshire
Siddique M, Farooq R, Shaeen A (2011) Removal of Reactive Blue 19 from wastewaters by physicochemical and biological processes-A Review. J Chem Soc Pak 33:284–293
Tarburk A, Grancaric AM, Leskovac M (2014) Novel cotton cellulose by cationisation during the mercerization process-part I: chemical and morphological changes. Cellulose 21:2167–2179
Teng X, Ma W, Zhang S (2010) Application of tertiary amine cationic polyacrylamide with high cationic degree in salt-free dyeing of reactive dyes. Chin J Chem Eng 18:1023–1028. doi:10.1016/S1004-9541(09)60163-4
Tutak M (2011) Reactive dyeing of cationized cotton: effects on the dyeing yield and the fastness properties. J Appl Polym Sci 119:500–504. doi:10.1002/app
Wang H, Zheng XW, Su JQ, Tian Y, Xiong XJ, Zheng TL (2009a) Biological decolorization of the reactive dyes Reactive Black 5 by a novel isolated bacterial strain Enterobacter sp. EC3. J Hazard Mater 171:654–659
Wang L, Ma W, Zhang S, Teng X, Yang J (2009b) Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr Polym 78:602–608. doi:10.1016/j.carbpol.2009.05.022
Acknowledgments
The authors would like to thank Bayer CropScience for financial support. Texas Tech University manuscript number T-4-659.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acharya, S., Abidi, N., Rajbhandari, R. et al. Chemical cationization of cotton fabric for improved dye uptake. Cellulose 21, 4693–4706 (2014). https://doi.org/10.1007/s10570-014-0457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0457-2