Abstract
A new and simple method based on natural dyeing was developed for producing antibacterial and biodegradable cotton fabrics. Using the aqueous extraction procedure, a natural dye was extracted from boiled pomegranate rinds and prepared into a fine powder. The whey protein isolate was used as an ecofriendly mordant to increase the affinity of cotton fabrics to the pomegranate rind extract. The different dyeing parameters, including dye concentration, temperature, time, and dyeing bath pH, were optimized via reflectance spectrophotometry using the three different pre-mordanting, simultaneous mordanting, and post-mordanting dyeing methods. The pre-mordanting method performed at a temperature of 80 °C yielded the best overall efficiency in terms of dye color strength and depth of shade under the optimized dyeing conditions of a concentration of 70%, pH = 4.5, time = 80 min, a material to liquid ratio of 1:20, and a temperature of 90 °C. The bio-fabrics thus produced exhibited excellent wash and light fastness. The results of FTIR spectroscopy confirmed the chemical interaction between cotton fabrics and the combination of WPI/dye agents. Moreover, SEM micrographs revealed the homogeneous surface morphology of the bio-fabrics without any microscopic cracks or discontinuities in the sample surface. Results also revealed that, with 18 h of contact time, the proposed ecofriendly method was able to produce bio-fabrics that maintained desirable antibacterial properties against pathogenic and spoilage bacteria, even after 10 washing cycles and also exhibited excellent protection against ultraviolet radiation. The results confirmed that this procedure is promising for producing dyed fabrics for biomedical applications without adverse environmental effects and that the natural mordant used is a good replacement for the commonly used metallic ones.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Substantial quantities of dyes are used in dyeing processes by the textile industry resulting in large volumes of industrial effluents that contain non-biodegradable contaminants ultimately released into the environment. This requires complex and expensive wastewater treatment processes to remove the dyes before the effluents are discharged into the environment. Included among the economical and cost-effective methods used to make dye processes environmentally safer are those that use natural dyes extracted from natural sources like minerals, insects/animals, and plants. The advantages of natural dyes over their synthetic counterparts make them ideal alternatives; these advantages include biodegradability, environment-friendliness, durability, antimicrobial properties, non-toxicity to humans, and non-allergic reactions (Horincar et al. 2019; Souissi et al. 2018; Cui et al. 2020; Yu et al. 2020). These advantages underly the global trend in using them by the apparel and textile industries. However, natural dyes suffer from such disadvantages as high solubility in water and low durability. These problems can be remedied if appropriate mordants are employed (Ibrahim et al. 2020; Benli and Bahtiyari 2015; Rehman et al. 2018; Nambela et al. 2020; Adeel et al. 2009; Sinnur et al. 2017; Kulkarni et al. 2011; Satyanarayana and Chandra 2013; Naveed et al. 2020).
The majority of mordants are metallic salts that properly combine with dye substances to form co-ordinate bonds, promote good bonding of dyes with textiles, help improve fastness of the dye against washing and sunlight, and enhance dye take-up by the fabric. However, these metallic ions are the source of wastewater contaminants and might agglomerate in living organisms and in the food chain to lead to diseases and disorders in humans. As a result, contamination of industrial wastewater with these non-degradable and toxic ions might affect both public health and the environment (Monier et al. 2010; Petrovic et al. 2017; Ru et al. 2018). To avoid these serious environmental impacts, natural products might be selected as alternative, totally eco-friendly mordants for use in dyeing systems.
Whey protein isolate might serve as an alternative eco-friendly mordant that additionally improves the affinity of cotton fabrics to natural dyes. Thanks to its vast applications and excellent properties, cotton is one of the oldest, most versatile, and complex of all fibers used by man. Basically comprised of cellulose with the chemical structure poly(1,4-β-D-anhydroglucopyranose), cotton releases only slight amounts of negative charges in alkali and neutral aqueous solutions (Gotoh et al. 2004) mainly due to its hydroxylic sites that are capable of oxidizing and producing carboxylic acid groups. Its negative surface charges increase significantly at pH values higher than 8 due to the ionization of certain side chain hydroxylic sites. The charge repulsion between anionic dyes and cotton materials leads to declining dye uptake and fastness in these materials (Monier et al. 2010; Pisitak et al. 2016). To overcome these problems in an eco-friendly manner, whey protein isolate, principally consisting of the three major proteins of bovine serum albumin (BSA), α-lactalbumin (α-la), and β-lactoglobulin (β-lg) (Pelegrine and Gasparetto 2005; Wijayanti et al. 2014) is used in the present study as a bio-mordant. The isolate is produced by the dairy industry and used extensively not only in food and pharmaceutical products but in biological systems as well.
Whey protein isolate used in conjunction with cotton fabrics may bind onto the fabric surface through the hydrogen-bonding interactions occurring between the amino and carbonyl groups present in whey protein isolate and the hydroxyl groups present in cotton (Zhang et al. 2011). This results in weakened negative charges on cotton surface allowing for the fabric to be dyed with natural agents. The bio-fabrics formed through cotton-protein reactions then enjoy a number of biological functions that offer promising biomedical applications.
The present study is focused on using the whey protein isolate as a bio-mordant for the natural dyeing of cotton fabrics using the tannin extracted from pomegranate rind. The study strives to produce bio-fabrics that are more environmentally-friendly.
Constituting about 50% of the total fruit weight, pomegranate rind contains various types of ingredients including ellagitannins (hydrolysable tannins) mainly punicalagin, pedunculagin, and punicalin (Seeram et al. 2005; Al-Zoreky 2009); flavonoids such as kaempferol, luteolin, and quercetin (Van Elswijk et al. 2004; Fawole et al. 2012); tannins, anthocyanidins are principally delphinidin, pelargonidin, and cyanidin (Noda et al. 2002); hydroxybenzoic acids such as gallic acid, ellagic acid, and EA glycosides (Davulcu et al. 2014), and minerals such as calcium, magnesium, phosphorus, potassium and sodium (Al-Zoreky 2009). It has been reported that pomegranate rind extract contained total of flavonoids and phenolic compounds at 47.27 mg QE/g and 104.68 mg GAE/g, respectively (Yehia et al. 2011). Since the essential bioactive products used in the present work are commonly discarded as waste and, therefore, cost not much to procure, the procedure proposed in this study seems promising for large scale production of economical biomaterials. To the best of the present authors’ knowledge, no study has yet been reported on the application of these materials in producing bio-fabrics.
To protect the ecosystem as much as possible against the hazards of releasing pollutants into the environment and to achieve excellent results at minimal resource costs, it is necessary to optimize the dyeing conditions so that minimum waste is produced. Toward these goals, the present study is conducted to achieve the following tow objectives: (1) To prepare cotton bio-fabrics in which whey protein isolate and pomegranate rind extract are used as biomaterials under optimized process conditions; and (2) to evaluate the color strength, color fastness, reaction mechanism, surface morphology, ultraviolet protection, and antibacterial properties of the bio-fabrics thus produced.
Experimental
Materials
Bleached, 100%-cotton fabrics with a plane weave (136 g/m2 in weight procured from Mazandaran Textile Co., Iran) and sour–sweet pomegranate rind (collected from Semnan, Iran) were used. Whey protein isolate with a purity of 93% (A.O.A. C., 1980, Method 38,012) was derived from cow milk obtained from Pegah Dairy Co., Golpayegan, Isfahan, Iran. All other chemicals were analytically pure and were used without further purification. Deionized water was also used to prepare all the aqueous solutions.
Pre-treatment of cotton fabric
Cotton fabrics were scoured and cleaned by treating for 1 h at boiling temperature in a bath containing 7% nonionic detergent and 3% sodium carbonate at a liquid to material ratio of 20:1. The fabrics were subsequently rinsed in hot water and finally placed in cold distilled water to avoid the redeposition of the saponified contaminants on the samples before they were dried at room temperature.
Preparation of the dye powder
The aqueous extraction procedure was used to extract the dye. Briefly, dried pomegranate rind samples were ground using a food blender and soaked in deionized water for 24 h (M:L 1:3) before they were heated in a dye bath at 95 °C for 1 h at pH = 8 under constant stirring at 40 rpm. The mixture was then allowed to stand overnight at ambient temperature for its insoluble residuals to precipitate. The aqueous solution was subsequently filtered on a fine cotton cloth. Finally, the filtrate was dried in a hot oven before it was powdered in a grinding mill.
Dyeing procedure
Prior to the dyeing process, pretreated cotton fabrics were soaked in water for 25 min. This was followed by dyeing the samples using the pre-mordanting, simultaneous mordanting, and post-mordanting procedures under optimized conditions.
Pre-mordanting was carried out using WPI as a natural mordant under two different sets of optimum conditions. First, 10 g of the cotton fabric sample was loaded into a plastic tube with 200 mL of a normal pH whey protein isolate solution at a concentration of 40 g/L. The tube was then loaded into a thermostatic water bath at 80 °C for 30 min. To avoid further thermal denaturation, the tube was immediately cooled in an iced water bath. The second treatment involved exposing 10 g of the cotton fabric sample to 200 mL of WPI with a normal pH at a concentration of 40 g/L at ambient temperature for 2 h. All the samples were subsequently pressed using a padding machine to achieve 80% expressions. The pre-mordanted fabrics were then squeezed, air dried, and subjected to the dyeing process, which was performed by dipping the fabrics pre-mordanted with WPI in an AHIBA 1000 polymath apparatus containing various concentrations of pomegranate rind powder with a material to liquid ratio of 1:20. The soaking time was 10 min, which started at a temperature of 35 °C to rise at a heating rate of 1.5 °C/min. Various dyeing times, temperatures, and pH levels were applied to obtain the optimum dyeing conditions (Table 1).
In the simultaneous dyeing procedure, the cotton fabrics were mordanted at the same time that they were dyed in the same bath. The dyebath was prepared by dissolving 70% o.w.f. of pomegranate rind powder and 40 g/L of whey protein isolate at a material to liquid ratio of 1:20 to obtain a pH level of 4.5. Prior to loading the cotton fabric samples, bath temperature was raised to about 35 °C at which it was kept for 10 min before it was gradually raised at a heating rate of 1.5 °C/min to 90 °C at which the dyeing process was continued for 80 min.
Post-mordanted fabric samples were prepared by dyeing as described in the simultaneous dyeing procedure but in the absence of the whey protein isolate. After dyeing, 10 g of the cotton fabric sample was thoroughly squeezed before being introduced into the mordant bath containing 40 g/L of the whey protein isolate at ambient temperature where it was maintained for 2 h.
At the end of the dyeing process, all the samples were removed from the solution, rinsed with deionized water, and soaped in a bath containing 2 g/L of the non-ionic detergent at 80 °C for 15 min. Finally, the dyed fabrics were washed with deionized water while the solution was repeatedly replaced during each washing cycle before they were ultimately squeezed and dried at room temperature.
Color characteristics
The CIELAB color coordinates (L* a* b* c* h°) and color efficiency (K/S) over a range of 400–700 nm were recorded with an X-Rite Sp64 Portable Sphere Spectrophotometer (USA) (ASTM E308, ASTM E1331) using illuminant D 65 and a 10° standard observer. Color efficiency (K/S) defined as a function of color depth was determined using the Kubelka–Munk equation as follows:
where, K, S, and R represent adsorption coefficient, scattering coefficient, and reflectance of the dyed substrate, respectively (Sarkar and Seal 2003).
Relative color strength (RCS) defined as the ratio of WPI-dyed fabric color to that of the untreated plain cotton one may be calculated using the following equation:
IR Spectroscopic analysis
Fourier Transform Infrared (FTIR) spectroscopy was performed and absorbance spectra were obtained using a NICOLET Nexus 670 in order to investigate the structural changes of cotton fabrics after their modification. The spectra were averaged over 40 scans recorded at a resolution of 4 cm−1 over the range 400–4000 cm−1. The absorption peaks in the FTIR spectra were analyzed using Omnic software.
Color fastness
The bio-fabrics thus produced were subjected to a repeated washing test according to ISO 105-C01: 2006. The samples were studied with respect to their stability by antibacterial measurements after 0, 5, and 10 washing cycles in a nonionic detergent. The light fastness test of the coated samples was carried out according to ISO 105 B02: 2014.
UV protection measurement
AATCC 183-2010 standard was used to determine the ultraviolet protection properties of the untreated and dyed cotton fabric samples over a range of 290–400 nm at intervals of 10 nm. The values for ultraviolet protection factor for the dry, tensionless, and flat samples were determined using the relevant software as follows:
where, \(T_{{}} (\lambda )\), \(\Delta_{{}} (\lambda )\), \(\varepsilon (\lambda )\), and \(E_{{}} (\lambda )\) represent spectral transmittance at \(\lambda\) 290, wavelength interval of the measurements (nm), arithmetic action spectrum, and measured solar irradiance (Wm−2 nm−1), respectively. The percentage of UV-B and UV-A were also estimated and reported as the average value of four determinations using the average transmittance data in the ranges of 290–315 nm and 315–400 nm, respectively.
SEM analysis
A Philips scanning electron microscope (XL-30, The Netherlands) was used to investigate the surface morphologies of the untreated plain samples as well as the WPI-treated and dyed biofabrics.
Antibacterial activity
Using the AATCC 100-2004 test method, the bio-fabrics prepared under the optimized conditions were quantitatively investigated in terms of their antibacterial activities against the two microorganisms of Staphylococcus auras, a Gram-positive bacterium (American Type Culture Collection No. 6538), and Escherichia coli, a Gram-negative bacterium (ATCC 25,922) (Baseri 2016).
Results and discussions
Optimization of the dyeing parameters
The samples were subjected to different dyeing conditions to determine their effects on the color strength of the dyed cotton fabrics. Figure 1 presents the effects of different dyeing conditions (dye concentration, pH, temperature, and time) on color efficiency of the dyed cotton fabrics.
As can be seen, color strength exhibited a considerable enhancement with an increase in dye concentration. This can be explained by the increased number of dye molecules made available for reaction with the functional groups as a result of increasing dye concentration, a situation that is characterized by increased color efficiency as well.
Regarding dyebath temperature, a relatively slow increase in color strength was observed with increasing dyeing temperature up to 60 °C, beyond which the enhancement in color strength took a fast trend up to a temperature of 90 °C (Fig. 1). This could be due to the effect of temperature on the kinetic energy between the dye and the fabric molecules. Indeed, the kinetic energy of the dye molecules increased but their aggregation decreased with increasing temperature. Also, the increasing temperature increased the movement speed of the segment cotton chains leading to more open structures in the fabrics that created greater fractional free volumes (Baseri 2015) that made more active sites available for more dye molecules to attach to the fabrics. Thus, a temperature of 90 °C was identified as the best dyebath one under the conditions used in the experiments.
It is also clear from Fig. 1 that color strength declined in the samples as dyeing bath pH rose from 5 to 11 so that maximum color strength was obtained at a pH equal to 4.5. This might be attributed to the isoelectric point of whey protein isolate as whey protein exhibits its minimum solubility and surface charge at this pH due to the significantly decreased electrostatic forces of the molecules. This resulted in the facilitated transfer of dye molecules to the fabric such that more tannin molecules were able to form complexes with the polar groups of the whey protein isolate in the fabric. It is well known that the isoelectric point of whey protein isolate is about 4.5 (Pelegrine and Gasparetto 2005). At lower or higher pH values, the protein surface becomes either positively or negatively charged, which increases its solubility. It may, therefore, be concluded that, for the conditions used in the present experiments, maximum color strength is obtained at pH 4.5.
Another important point to note in Fig. 1 is the considerable increase in color efficiency with increasing dyeing duration to 80 min, beyond which dye uptake increased at a lower rate. This can be explained by the fact that, at longer dyeing durations, more time is available for the dye molecules to diffuse and react with the functional groups present in the pretreated cotton fabric until a state of equilibrium is reached. It, therefore, appears that a dyeing duration of 80 min best suits the conditions used in the present experiments.
Based on the results obtained from the present study, the optimal conditions for the most efficient dyeing of pre-mordanted cotton fabrics were determined to be those summarized in Table 1.
Evaluation of the dyeing procedure based on color strength and dyeing efficiency obtained for dyed cotton fabrics
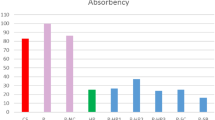
The effects of the different mordanting methods on cotton dyeing were investigated and the results obtained on dye depth, i.e. color efficiency (color strength), were presented in Fig. 2.
It is clear that, regardless of the dyeing procedure used, all the samples showed identical trends of decreasing color efficiency with increasing wavelength. In addition, both pre-mordanted samples exhibited higher values of color efficiency than did those solely subjected to dyeing. This may suggest a twofold increase in color efficiency in cotton fabrics dyed through the pre-mordanting procedure as compared to others. This may be explained along the following lines. First, the whey protein isolate might bind onto the fabric surface not only through the hydrogen-bonding interactions of the amine and carbonyl groups present in it with the hydroxyl groups in the cotton but also via van der Waals forces. These result in reduced negative charges on cotton surface and the fabric’s increased dyeability. Second, pomegranate rind has a tannin content. Hence, the polar groups in whey protein isolate might react with the phenolic hydrogen groups in tannins to form an insoluble complex through hydrogen bonding (Kadam et al. 1990). This claim will also be confirmed by the results obtained from FTIR analysis in the present study. On the other hand, it may be claimed that the whey protein isolate not only reduces the negative charges on cotton surface, but also improves dye uptake by pre-mordanted fabrics due to dye–whey protein isolate complexation.
It is seen in Fig. 2 that the color efficiency of the cotton fabrics exhibited a more pronounced effect as a result of pre-mordanting at 80 °C than at room temperature. This indicates adsorption of more of the whey protein isolate followed by a reduction of the negative charges on the cotton surface as a result of pre-mordanting at 80 °C, which eventually increases the dyeability of the fabric. This phenomenon could also be explained by the greater tannin-whey protein isolate complexes formed due to the higher amount of whey protein isolate absorbed on cotton fabrics as a result of pre-mordanting at 80 °C. These results are consistent with those observed in weight changes of the fabrics pre-mordanted at two different temperatures. For the conditions used in the present experiments, pre-mordanting of cotton fabrics with whey protein isolate at 80 °C and at room temperature resulted in 12.3% and 7.5% (w/w), respectively, of whey protein isolate contained in the final cotton fabrics treated with whey protein isolate.
Another important point to note in Fig. 2 is that the cotton fabrics dyed with pomegranate rind extract through the simultaneous and post-mordanting procedures exhibited lower color efficiencies than did those dyed only with the natural dye. On the other hand, using whey protein isolate as a bio-mordant in both procedures led to declines in color efficiency. The lower color efficiency of cotton fabrics dyed through the simultaneous mordanting method could be explained with recourse to the solubility of whey protein isolate in water. It is well known that the solubility of whey protein isolate is significantly affected by temperature such that, at high enough temperatures, some secondary and tertiary structures of the whey protein isolate transform or break down in time. This causes the hydrophobic groups of the whey protein isolate to come in contact with each other to form aggregate molecules; this coagulation causes protein solubility in water to decline (Langerdorff et al. 1999). Another explanation put forth is that the dye and the mordant in the simultaneous dyeing procedure come into contact with each other to form certain tannin-whey protein isolate complexes in the dyeing bath. Part of the dye then possibly escapes adsorption on the cotton fabric due to precipitation, thereby leading to reduced color efficiency in the samples.
In the post-mordanting procedure, the fabrics were initially dyed only with the pomegranate rind extract followed by treating in a separate bath with the whey protein isolate used as a bio-mordant. As a result, some of the dye molecules might have escaped the dyed fabric to form an insoluble tannin–protein complex in the mordanting bath. This situation is characterized by reduced color efficiency of the post-mordanted fabrics only relative to that of the fabrics merely dyed.
Table 2 reports the colorimetric characterization of the different samples tested and their relative color strengths.
In this Table, the chromatic coordinates of a* and b* represent green (a* < 0) to red (a* > 0) and blue (b* < 0) to yellow (b* > 0), respectively. The L* values show color brightness ranging from 0 (black or darkness) to 100 (white or lightness). C* denotes the distance between a color and the achromatic point; it may be termed ‘color clarity’ or ‘degree of color saturation’. Hue angle implying purity of color is designated by h°. Finally, while ∆E* signifies the total color differences, ∆E* > 1 indicates the total color difference between two dyed samples as distinguished by the naked eye (Gawish et al. 2017; Harane et al. 2014).
It is evident from Table 2 that all the dyed samples recorded ∆E* values larger than unity, implying a significant difference between the samples. Moreover, compared to the cotton fabric solely dyed, simultaneous mordanting and post-mordanting of the samples caused reductions not only in their relative color strengths but also in their b* and c* values. These observations confirm the reduced dye uptake in the simultaneous and post-mordanting procedures, indicating that the whey protein isolate is not a suitable bio-mordant for use in these dyeing procedures. Conversely, both pre-mordanted fabrics exhibited higher values of relative color strength than did the fabrics solely dyed, a situation which is characterized by a significant decrease in color brightness. Hue angle, yellowness, and color clarity increased as a result of pre-mordanting with the whey protein isolate. Moreover, compared to the fabrics pre-mordanted at room temperature, those subjected to pre-mordanting at 80 °C exhibited higher values of relative color strength, color clarity, color coordinate b*, hue angle, and total color difference but a lower value of brightness. This is not only an indication of the higher dye uptake by the fabrics pre-mordanted at 80 °C but also a confirmation of the strong reliance of whey protein isolate absorption on both the dyeing procedure used and the temperature applied.
Color tone is represented by the two color coordinates a* and b*. The relevant graphical representations for different dyed cotton fabrics are presented in Fig. 3.
It is evident that all the dyed cotton fabrics are located in the red-yellow zone of the CIE space and that they all exhibit yellowness but with different reddish shades. This is also confirmed by the hue angle values greater than 45° reported in Table 2. Another point worth noting in this Figure is the total color difference whose highest value was recorded with pre-mordanting at 80 °C. The results obtained from the present experiments reveal that the best results obtained with pre-mordanting at 80 °C. It is, hence, recommended as the best procedure for dyeing cotton bio-fabrics produced by using the whey protein isolate and the pomegranate rind extract. The total content of polyphenols in dyebaths before and after dyeing was analyzed by the procedure described in (Singleton and Rossi 1965) and the add-on% of the total polyphenols on the fabric surface may be calculated using the following equation:
where, Cb and Ca represent the total polyphenols concentrations in dye bath before and after dyeing, respectively (Table 3).
Color fastness of the dyed cotton bio-fabrics
Since textiles are subjected to frequent laundering, dye durability might have drastic effects on the final application and properties of cotton bio-fabrics. In order to investigate the durability of the samples under repeated washing cycles, they were washed in a Launder-O-meter to investigate their fastness properties before and after pre-mordanting with WPI and the results were reported in Table 4.
Clearly, the cotton fabrics dyed with the pomegranate rind extract exhibited excellent wash fastness properties as evidenced by their no shade change or staining to the adjacent multifiber strip after multiple washes. Additionally, pre-mordanting with WPI exhibited no effect on the wash fastness of the fabrics as they retained their excellent wash ability (Table 4). However, pre-mordanting with WPI was observed to improve the light fastness properties of cotton fabrics dyed with the pomegranate rind extract. It may be suggested that whey protein isolate as a bio-mordant had a negative catalytic influence on the photochemical degradation of the natural dye extracted from pomegranate rinds. Moreover, calorimetric analysis revealed that WPI pre-mordanting not only increased dye uptake by the cotton fabrics but also increased dye concentration in the samples, a situation characterized by elevated values of light fastness. It may be concluded that the conditions used in the experiments led to stable interactions between the WPI/pomegranate rind natural dye and the cotton fabrics.
Characterization of the surface of the bio-fabric (SEM)
Microstructural images were prepared of both the plain and the WPI/dyed samples using a scanning electron microscope at a magnification of 2.00 KX, the analytical results of which are presented in Fig. 4.
It is clear from these micrographs that a homogeneous layer of whey protein isolate lacking microscopic cracks or discontinuities formed on the fabric surface. The pre-mordanted cotton fabrics exhibited some particles on their surfaces, indicating that the whey protein isolate was bonded to the fabric. As expected, these structures persisted even after dyeing with pomegranate natural dye (Fig. 4d). This might have occurred due to the presence of a chemical bond between the whey protein isolate and the pomegranate rind extract with the cotton fabric as also confirmed by the FTIR analysis. It is not far from expectation that a continuous layer of both the bio-mordant and the dye could have been formed under the experimental conditions in this study.
Chemical interaction of cotton fabric, whey protein isolate, and pomegranate rind dye
FTIR spectra from 4000 to 400 cm−1 were used to determine whether the pomegranate rind extract and the whey protein isolate were chemically bonded to the cotton fabrics. The results obtained on the plain cotton fabrics and those dyed and pre-mordanted with WPI at 80 °C are presented in Fig. 5. The spectra are presented with no shift in the absorbance axis while they have also been normalized and baseline corrected.
The FTIR spectra for the plain cotton fabrics as well as those for the pre-mordanted dyed ones exhibit characteristic adsorption peaks that can be divided into three important regions: a broad region in the range of 3500–3200 cm−1 corresponding to the stretching of the hydrogen bonded (O–H), a region from 3000 to 2700 cm−1 corresponding to the stretching vibrations of CH2 and CH3, and finally, adsorption peaks in the range of 1800–700 cm−1 representing the (O–H) bending, (C=O) and (C–N) stretching, as well as (N–H) oop vibrations (Alizadeh-sani et al. 2018; Batool and Shah 2018; Cintron and Hinchliffe 2015; Kondo et al. 1994; Haque et al. 2017; Yang et al. 2000).
FTIR spectra of the (O–H) stretching region
The chemical structure of cotton is basically poly(1,4-β-D-anhydroglucopyranose) composed of glucose units. It contains three different hydroxyl groups in each repeating glucose unit, with each distinct hydroxyl group exhibiting a single stretching vibration at a given wavenumber, thereby giving a broader band. It is well known that vibration band in the range 3310–3230 cm−1 might be associated with the intermolecular hydrogen bonding for 6-OH…O-3′ while those in the ranges 3375–3340 cm−1, and 3455–3410 cm−1 might correspond to the intramolecular hydrogen bonds for 3-OH…O-5 and 2-OH…O-6, respectively (Kondo et al. 1994; Haque et al. 2017; Yang et al. 2000).
Figure 5 shows that a broad adsorption peak is observed at 3450 cm−1 for the plain cotton fabrics. Moreover, a broad adsorption peak appears at 3428 cm−1 with a shoulder on the low wavenumber side for the WPI-treated/dyed fabrics, suggesting new intermolecular interactions as well as widely distributed H-bond geometries and distances (Zhang et al. 2011). The observed shoulder may be equally attributed to the (NH2) stretching vibrations in the WPI-treated and dyed samples. Another important point to note in Fig. 5 is the shift from 3450 to 3428 cm−1 in the spectra for the pre-mordanted and dyed cotton fabrics. This might be attributed to the strengthening of the intramolecular hydrogen bonds for 2-OH…O-6, with the stronger bonds shifting to a lower energy. For this band, the hydrogen bonding distance (R) and the energy of the hydrogen bond (EH) can be estimated from the following equations (El-Zaher et al. 2019; Popescu et al. 2009):
where, v is the frequency of the bonded OH group observed in the FTIR of the sample, v0 is the standard frequency corresponding to free OH groups (at 3650 cm−1) and K is a constant (1/K = 2.625 × 102 kJ) (El-Zaher et al. 2019). Also, v0″ is the monomeric OH stretching frequency (v0″ = 3600 cm−1) (Popescu et al. 2009). The results are shown in Table 5.
Clearly, the WPI-treated/dyed cotton fabrics exhibited a higher value of hydrogen bonding energy than did the plain fabrics, a situation which is characterized by a decrease in the hydrogen bonding distance. These must be due to the specific hydrogen-bonding interactions between the hydroxyl groups in the cotton fabrics, the whey protein isolate, and the pomegranate rind extract.
FTIR spectra of (C–H) stretching region
The collection of bands between 3000 and 2700 cm−1 belong to the (C–H) stretching region. The baseline in this region exhibits an (O–H) absorbance (Haque et al. 2017; Yang et al. 2000; Forteir et al. 2017). It is evident from Fig. 5 that the (C–H) stretching vibrations decline in intensity once the cotton fabrics have been pre-mordanted and dyed. This may be explained by the fact that dyeing the cotton fabrics with the whey protein isolate and pomegranate rind dye might have led to more interactions between the hydroxyl groups in the cotton and the WPI/natural dye, which ultimately led to reduced free hydroxyl groups of the cotton bio-fabrics. This situation is characterized by a decrease in the intensity of the (C–-H) stretching bands in the bio-fabrics.
FTIR spectra of the (O–H) bending as well as the (C=O) and (C–N) stretching regions
The vibrational modes at 1800–700 cm−1 are attributed to the (O–H) bending region and to those of (C=O) and (C–N) stretching vibrations (CCintron and Hinchliffe 2015). The absorption peaks at 1850–1650 cm−1 were attributed to the overlapping of the bending vibration of (C–H) and to the presence of carbonyl/carboxyl functional groups (Allen et al. 2007). As is evident from Fig. 5, compared to plain fabrics, the WPI/dyed samples exhibited enhanced intensities in their (C =O) stretching vibrations in the relevant spectra, demonstrating that the carbonyl/carboxyl groups increased as a result of treatment with WPI and the natural dye. The presence of carboxyl groups in the WPI/dyed fabrics is also confirmed by the acidic (C–O) stretching peak at around 1260 cm−1. The plain control fabric did not display such a vibration mode, whereas the WPI-treated and dyed fabrics showed a small band at 1260 cm−1.
It is also interesting to note that a small peak appeared at about 800 cm−1 when the cotton fabrics were treated with WPI and pomegranate rind dye, which is indicative of the presence of (N–H) oop in amines (Pavia et al. 1996). The presence of amines in these samples was also verified in “FTIR spectra of the (O–H) stretching region” above. In the light of these arguments, it may be concluded that WPI and pomegranate rind dye were chemically absorbed to the cotton fabric. On the other hand, it is well known that the two spectral bands at around 1700 and 1580 cm−1 indicate water adsorption (Garside et al. 2003). This may be verified by Fig. 5 showing that the cotton fabrics treated with the WPI and the natural dye display slight increases in intensity at these modes, which might have been due to increased water absorption and hydrophilicity of the cotton bio-fabrics.
Based on the FTIR spectra and their analysis, the model in Fig. 6 was developed for the possible reaction route among the functional groups present in cotton, WPI, and pomegranate rind dye.
The adsorption of pomegranate rind dye into the cotton fabric is believed to take place in the following two steps:
-
(1)
The first step involves the initial adsorption of WPI onto the cotton fabric. Upon contact with the cotton fabric, the amine and carboxyl groups present in the WPI are oriented toward the cotton surface to allow for intra- and inter-molecular hydrogen bonds to form between the hydroxyl groups in the cotton and the amino/carboxyl groups in the WPI molecules. This decreases the negative charges on the cotton surface, resulting in its enhanced dyeability.
-
(2)
The second step involves the subsequent formation of hydrogen bonds between the various functional groups present in the WPI-premordanted cotton fabric and the phenolic compounds in the pomegranate rind dye. The phenolic compounds include Ellagic acid, N-methyl Granatonine, and Quercetin, all of which are prominent colored structures in pomegranate rind (Shaukat et al. 2016).
In addition to the predominant hydrogen bonds, hydrophobic interactions between the non-polar domains in tannins and the whey protein isolate might play an important role in the adsorption of pomegranate rind dye by the WPI-treated cotton fabrics (Deaville et al. 2007).
UV Protection of the cotton bio-fabrics
Table 4 reports the UV protection factors for the dyed samples before and after pre-mordanting with WPI at 80 °C. It is clear from this Table that the non-mordanted cotton fabrics dyed with the pomegranate rind natural dye recorded excellent UPF values (50+). This could be attributed not only to the chemical structures but also to the functional groups present in the pomegranate rind dye. The presence of tannins, ellagic and gallic acids, and other phenolic compounds in the pomegranate rind extract have been shown to exhibit photo protection properties due to their reflection or absorbance of UV radiations (Negi and Jayaprakasha 2003; Murugesh Babu and Ravindra 2014; Soobrattee et al. 2005). Also, it was well-known that flavonoids and phenolics are highly effective free radical scavengers and antioxidants (Yehia et al. 2011).As a result, they are well capable of preventing the destructive effects of UV radiation such as genomic and mitochondrial DNA fragmentation, mutagenesis, and DNA cellular death (Davulcu et al. 2014). The protective effects of pomegranate polyphenolics against UVA- and UVB-induced cell death of human skin fibroblasts may also be attributed to reduced generation of intracellular ROS and increased intracellular antioxidant capacity (Viuda-Martos et al. 2010). Additionally, pre-mordanting with WPI at 80 °C leads to reductions in both UV-A and UV-B transmittances that, in turn, increase the ultraviolet shielding efficiency of the fabric (Table 4).
It is well established that proteins give a two-fold increase in protection against ultraviolet radiation. Specifically, proteins remain as aggregates of molecules on the sample surface to scatter UV radiations. Moreover, the carbonyl groups of the protein molecules are well capable of absorbing ultraviolet radiation (Banga 2015).
Antibacterial efficiency of cotton bio-fabrics
Natural fibers such as cotton are not resistant to microorganisms. They usually provide suitable media for microorganisms to grow in. However, especially-prepared textile materials have been widely used as antibacterial materials in such varied applications as surgical clothing, bandages, medical dressings, apparel, protective clothing, veterinary materials, household tools, and healthcare (including disposable) appliances. The treatment of cotton fabrics for antibacterial effects has been studied widely (Lu et al. 2019; Gao et al. 2020; Liu et al. 2018; Wen et al. 2020). The conventional antibacterial substances used for treating cotton fabrics (i.e., metallic salts, quaternary ammonium, and triclosan) suffer from such disadvantages as escaping from the substrate to contaminate the wearers’ skin as well as high cost, toxicity, excessive tendering of the fabric, and inadequate reactivity (Mohamed et al. 2016).
Some of the natural dyes, especially those containing quinines, curcuminoids, and tannins, have been shown to offer antibacterial effects (Mohammed et al. 2016). Cotton bio-fabrics dyed with pomegranate rind dye seem to offer antibacterial effects and might, therefore, be an ideal substrate for this purpose. In an attempt to gain a better understanding of this aspect of the bio-fabrics manufactured in this study, the effects of whey protein isolate and pomegranate rind dye were investigated on their antibacterial properties against the two Gram-positive Staphylococcus aureus (ATCC 6538) and Gram-negative Escherichia coli (ATCC 25,922) bacteria in terms of percentage reductions in the number of bacteria after 18 h of incubation on the surface of plain cotton fabrics, those pre-mordanted with WPI at 80 °C, and those dyed with pomegranate rind dye using Eq. (7). The results are reported in Table 6 and Fig. 7.
where, A and B represent the numbers of bacterial colonies (CFU/ml) reduced on the plain and treated fabrics, respectively (Baseri 2018).
It is clear from Table 6 that the plain cotton fabrics and those pre-mordanted with whey protein isolate at 80 °C showed no antibacterial activity; the one dyed with pomegranate rind dye, however, exhibited an efficient antibacterial activity against both E. coli and S. aureus, which can be attributed to the antibacterial property of the dye used. The antibacterial activity of pomegranate rind has been attributed to its secondary metabolites such as phenolic compounds, tannins, ellagitannins especially punicalagin, ellagic and gallic acids, and anthocyanin as well as the flavonoids (Su et al. 2012; Gupta et al. 2005; Rosas-Burgos et al. 2016; Sorrenti et al. 2018).
In general, the antibacterial mechanisms of pomegranate rind extract can be explained with respect to its chemical active ingredients along the following lines:
-
1.
Phenolic compounds are capable of cross-linking, coagulating, and clumping bacterial cells (Davulcu et al. 2014). Moreover, they are able to react with sulfhydryl groups of proteins and interference with bacterial protein secretions, or the unavailability of substrates to microorganisms (Rosas-Burgos et al. 2016; Viuda-Martos et al. 2010; Al-Zoreky 2009).
-
2.
It seems that numerous mechanisms such as inhibition of nucleic acid synthesis, inhibition of cytoplasmic membrane function, inhibition of energy metabolism, inhibition of the attachment and biofilm formation, inhibition of the porin on the cell membrane, alteration of the membrane permeability, and attenuation of the pathogenicity are involved in the antibacterial activities of flavonoids which are used to explain their antibacterial properties (Xie et al. 2015).
-
3.
The antibacterial activity of ellagitannins, which include punicalagin isomers, has been considered as a possible result of their ability to precipitate protein and/or remove metal cofactors through their strong affinity for metal ions (Rosas-Burgos et al. 2016).
-
4.
The tannins present in pomegranate rind extract are well capable of binding to bacterial proteins, create stable complexes with them, and change their structural conformation, thereby preventing their growth (Gupta et al. 2005; Goel et al. 2005).
The WPI-treated/dyed fabrics exhibited a higher antibacterial activity than did the ones solely dyed, indicating that pre-mordanting with the whey protein isolate led to the adsorption of more pomegranate rind dye, that naturally increased the antibacterial efficiency of the fabric. This is confirmed by the findings regrading color efficiency.
It is also worth noting in Table 4 that antibacterial efficiency is more pronounced against E. coli than against S. aureus. This might be related to the cell walls of E. coli whose outer membrane is composed of phospholipids, lipoprotein, and lipopolysaccharide. Thus, the cell walls of E. coli, as a Gram-negative bacterium, act as potential barriers against foreign matter (Mohamed et al. 2016).
Finally, the samples were studied in terms of their antibacterial behavior against S. aureus and E. coli bacteria under different conditions including 5 and 10 wash cycles. It is evident from the results reported in Table 6 that the samples exhibited a consistent antibacterial performance against washing up to ten cycles, indicating the stability of the antibacterial properties of the cotton bio-fabrics.
Conclusion
Cotton fabrics were successfully dyed using whey protein isolate, as a bio-mordant, and pomegranate rind dye, as a natural dye. The effects of different dyeing conditions, including dye concentration, pH, duration, and dyeing bath temperature as well as dyeing procedure were investigated. The best results were obtained with pre-mordanting of cotton fabrics with WPI at 80 °C. The optimum dyeing conditions were determined to include a dyeing temperature of 90 °C, a duration of 80 min, a concentration of 70% o.w.f, and a pH level of 4.5. FTIR analysis revealed that the cotton bio-fabrics exhibited peaks that indicated chemical reactions between the WPI/ pomegranate rind dye and the cotton fabrics. These interactions were shown to favor hydrogen bonding and hydrophobic activities. These findings were further confirmed by visual observation of SEM images. These images also showed a homogeneous coating layer with no microscopic cracks or discontinuities formed on the fabric surface. The cotton bio-fabrics were found to offer an excellent fastness against washing cycles and light due to the chemical bonds formed between the pre-mordanted fabrics and the pomegranate rind dye. Moreover, the cotton fabrics subjected to both pre-mordanting and dyeing exhibited an excellent protection not only against the two microorganisms examined but also to the harmful ultraviolet radiation. Finally, the bio-fabrics exhibited a stable antibacterial efficiency in up to 10 washing cycles, confirming the chemical reactions between the cotton fabric and the WPI/dye. The procedure proposed in this study seems to be of potential use for the production of eco-friendly and economical textiles without adverse environmental impacts.
References
Adeel S, Ali S, Bhatti IA, Zsila F (2009) Dyeing of cotton fabric using pomegranate (Punicagranatum) aqueous extract. Asian J Chem 21(5):3493–3499
Alizadeh-sani M, Khezerlou A, Ehsani A (2018) Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind Crops Products 124:300–315
Al-Zoreky NS (2009) Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int J Food Microbiol 134:244–248
Allen A, Foulk J, Gamble G (2007) Textile technology preliminary fourier-transform infrared spectroscopy analysis of cotton trash. J Cotton Sci 11(1):68–74
Banga AK (2015) Therapeutic peptides and proteins: formulation, processing, and delivery systems. CRC Press, Boca Raton
Baseri S (2018) Improvement of dyeing, electro resistivity, and anti-microbial properties of acrylic fibers. Indian J Fiber Textile Res 43:143–152
Baseri S (2016) Preparation and characterization of conductive and antibacterial polyacrylonitrile terpolymer yarns produced by one-step organic coating. J Textile Institute 108(1):1–11
Baseri S (2015) Effect of drawing temperature on the structure and free volume of semicrystalline polyester yarns. Polymer Eng Sci 55(9):2030–2041
Batool I, Shah GB (2018) Chemical bonding of organic dye onto cotton fibers using silane as coupling agent (I). Fibers Polymers 19(4):790–796
Benli H, Bahtiyari Mİ (2015) Use of ultrasound in biopreparation and natural dyeing of cotton fabric in a single bath. Cellulose 22:867–877
Cintron MS, Hinchliffe DJ (2015) FTIR examination of the development of secondary cell wall in cotton fibers. Fibers 3:30–40
Cui L, Hu J, Wang W, Yan C, Guo Y, Tu C (2020) Smart pH response flexible sensor based on calcium alginate fibers incorporated with natural dye for wound healing monitoring. Cellulose 27:6367–6381
Davulcu A, Benli H, Sen Y, Bahtiyari MI (2014) Dyeing of cotton with thyme and pomegranate peel. Cellulose 21:4671–4680
Deaville ER, Green RJ, Mueller-Harvey I, Willoughby I, Frazier RA (2007) Hydrolyzable tannin structures influence relative globular and random coil protein binding strengths. J Agric Food Chem 55:4554–4561
El-Zaher NA, El-Bassyouni GT, Esawy MA, Guirguis OW (2019) Amendments of the structural and physical properties of cotton fabrics dyed with natural dye and treated with different mordants. J Natural Fibers. https://doi.org/10.1080/15440478.2019.1689884
Forteir C, Cintrón MS, Rodgers J, Fontenot K, Peralta D (2017) Fourier-transform imaging of cotton and botanical and field trash mixtures. Fibers 5(20):1–11
Fawole OA, Makunga NP, Opara UL (2012) Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement Altern Med 12:200. https://doi.org/10.1186/1472-6882-12-200
Gao D, Li Y, Lyu B, Jin D, Ma G (2020) Silicone quaternary ammonium salt based nanocomposite: a long-acting antibacterial cotton fabric finishing agent with good softness and air permeability. Cellulose 27:1055–1069
Garside P, Wyeth P (2003) Identification of cellulosic fibres by FTIR spectroscopy I: thread and single fiber analysis by attenuated total reflectance. Stud Conservation 48(4):269–275
Gawish SM, Mashaly HM, Helmy HM, Ramadan AM, Farouk R (2017) Effect of mordant on UV protection and antimicrobial activity of cotton, wool, silk, and nylon fabrics dyed with some natural dyes. J Nanomed Nanotechnol 8:1–9
Goel G, Puniya AK, Aguilar CN, Singh K (2005) Interaction of gut microflora with tannins in feeds. Naturwissenschaften 92:497–503
Gotoh T, Matsushima K, Kikuchi KI (2004) Adsorption of Cu and Mn on covalently cross-linked alginate gel beads. Chemosphere 55:57–64
Gupta D, Jain A, Panwar S (2005) Anti-UV and anti-microbial properties of some natural dyes on cotton. Indian J Fiber Textile Res 30:190–195
Haque MA, Akhtar M, Halilu A, Yun HD (2017) Validation and extended application of cellulose microfibril swelling enzyme assay method to alkali induced swelling of cellulose. J Chem Eng Bioanal Chem 2(1):62–69
Harane RS, Mehra NR, Tayade PB, Adivarekar RV (2014) A facile energy and water-conserving process for cotton dyeing. Int J Energy Environ Eng 5(96):1–10
Horincar G, Aprodu I, Barbu V, Rapeanu G, Bahrim GE, Stanciuc N (2019) Interactions of flavonoids from yellow onion skins with whey proteins: mechanisms of binding and microencapsulation with different combinations of polymers. Spectrochimica Acta A Molecular Biomolecular Spectroscopy 215:158–167
Ibrahim NA, Eid BM, Kafafy H (2020) Sustainable colorants for protective textiles (chapter 21). Advances in functional and protective textiles. Woodhead Publishing, Cambridge, pp 569–629
Kadam SS, Salunkhe DK, Chavan JK (1990) Dietary tannins: consequences and remedies. CRC Press, Florida
Kondo T, Sawateri C, Manley RSJ, Gray DG (1994) Characterization of hydrogen bonding in cellulose-synthetic polymer blend systems with regioselectively substituted methylcellulose. Macromolecules 27:210–215
Kulkarni SS, Gokhale AV, Bodake UM, Pathade GR (2011) Cotton Dyeing with Natural Dye Extracted from Pomegranate (Punica granatum) Peel. Universal J Environ Res Technol 1(2):135–139
Langerdorff V, Cuvelier G, Launay B, Michin C, Parker A, Kruif CG (1999) Casein micelle/iota carrageenan interactions in milk: Influence of temperature. Food Hydrocolloids 13(1):211–218
Liu G, Xiang J, Xia Q, Li K, Lan T, Yu L (2018) Superhydrophobic cotton gauze with durably antibacterial activity as skin wound dressing. Cellulose 26:1383–1397
Lu Z, Liu J, Dong C, Zhang Z, Wei D (2019) Durable multifunctional antibacterial and hydrophobic cotton fabrics modified with linear fluorinated pyridinium polysiloxane. Cellulose 26:7483–7494
Mohamed FA, Ibrahim HM, El-Kharadly EA, El-Alfy EA (2016) Improving dye ability and antimicrobial properties of cotton fabric. J Appl Pharmaceutical Sci 6(02):119–123
Mohammed GJ, Al-Jassani MJ, Hameed IH (2016) Anti-bacterial, antifungal activity and chemical analysis of Punica grantanum using GC-MS and FTIR spectroscopy. Int J Pharmacognosy Phytochem Res 8(3):480–494
Monier M, Ayad DM, Sarhan AA (2010) Adsorption of Cu (II), Hg (II), and Ni (II) ions by modified natural wool chelating fibers. J Hazardous Mater 176:348–355
Murugesh Babu K, Ravindra KB (2014) Bioactive antimicrobial agents for finishing of textiles for health care products. J Textile Institute 106(7):1–12
Nambela L, Venant Haule L, Mgani Q (2020) A review on source, chemistry, green synthesis and application of textile colorants. J Cleaner Production 246. Article 119036.
Naveed R, Bhatti I A, Adeel S, Ashar A, Sohail I, Ul Haq Khan M, Masood N, Iqbal M, Nazir A. (2020) Microwave-assisted extraction and dyeing of cotton fabric with mixed natural dye from pomegranate rind (Punica Granatum L.) and turmeric rhizome (Curcuma Longa L.). J Natural Fibers. https://doi.org/10.1080/15440478.2020.1738309
Negi PS, Jayaprakasha GK (2003) Antioxidant and antibacterial activities of Punica Granatum peel extracts. J Food Sci 68(4):1473–1477
Noda Y, Kaneyuka T, Mori A, Packer L (2002) Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyaniding, and pelargonidin. J Agri Food Chem 50(1):166–171
Pavia D, Lampman G M, Kriz G S (1996) Introduction to spectroscopy, 2nd edn, Washington.
Pelegrine DHG, Gasparetto CA (2005) Whey proteins solubility as function of temperature and pH. Lebensm-Wiss u Technol 38:77–80
Petrovic M et al (2017) Mechanism of adsorption of Cu+2 and Zn+2 on the corn silk (Zea mays L.). Ecol Eng 99:83–90
Pisitak P, Hutakamol J, Thongcharoen R, Phokeaw P, Kanjanawan K, Saksaeng N (2016) Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Industrial Crops Products 79:47–56
Popescu CM, Singurel G, Popescu MC, Vasile C, Argyropoulos DS, Willfor S (2009) Vibrational spectroscopy and X-ray diffraction methods to establish the differences between hardwood and softwood. Carbohyd Polym 77:851–857
Rehman F, Sanbhal N, Naveed T, Farooq A, Wang Y, Wei W (2018) Antibacterial performance of Tencel fabric dyed with pomegranate peel extracted via ultrasonic method. Cellulose 25:4251–4260
Rosas-Burgos EC, Burgos-Hernández A, Noguera-Artiaga L, Kaˇcániová M, Hernández-García F, Cárdenas-López JL, Carbonell-Barrachina AA (2016) Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J Sci Food Agri 97(3):802–810
Ru J, Qian X, Wang Y (2018) Low-salt or salt-free dyeing of cotton fibers with reactive dyes using liposomes as dyeing/level-dyeing promotors. Sci Reports 8(13045):1–9
Sarkar AK, Seal CM (2003) Color strength and colorfastness of flax fabrics dyed with natural colorants. Clothing Textiles Res J 21(4):162–166
Satyanarayana DNV, Chandra KR (2013) Dyeing of cotton cloth with natural dye extracted from pomegranate peel and its fastness. Int J Eng Sci Res Technol 2(10):2664–2669
Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D (2005) In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutritional Biochem 16(6):360–367
Shaukat A, Sobia J, Tanveer H, Sadia N, Umme H (2016) Optimization of extraction condition of natural dye from pomegranate peels using response surface methodology. Int J Eng Sci Res Technol 5(7):542–548
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticulture 16:144–158
Sinnur HD, Samanta AK, Verma DK, Kaware R (2017) Studies on coloration and UV protective action of Anar Peel (Pomegranate Rind) as an effective natural colorant for cotton khadi fabric. J Inst Eng India Ser E. https://doi.org/10.1007/s40034-017-0106-z
Sorrenti V, Randazzo CL, Caggia C, Ballistreri G, Romeo FV, Fabroni S, Timpanaro N, Raffaele M, Vanella L (2018) Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front Microbiol 10:660
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res 579:200–213
Souissi M, Guesmi A, Moussa A (2018) Valorization of natural dye extracted from date palm pits (Phoenix dactylifera) for dyeing of cotton fabric. Part 1: Optimization of extraction process using Taguchi design. J Cleaner Production 202:1045–1055
Su X, Howell AB, D'Souza DH (2012) Antibacterial effects of plant-derived extracts on methicillin-resistant Staphylococcus aureus. Foodborne Pathog Dis 9(6):573–578
Van Elswijk DA, Schobel UP, Lansky EP, Irth H, van der Greef J (2004) Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry 65(2):233–241
Viuda-Martos M, Fernandez-Lopez J, Perez-Alvarez JA (2010) Pomegranate and its many functional components as related to human health: a review. Comprehensive Rev Food Sci Food Safety 9:635–654
Wen W, Zhang Z, Jing L, Zhang T (2020) Synthesis of a Hein-Schiff base compound and its antibacterial activity on cotton fabrics. Cellulose 27:7243–7254
Wijayanti HB, Bansal N, Sharma R, Deeth HC (2014) Effect of sulphydryl reagents on the heat stability of whey protein isolate. Food Chem 163:129–135
Xie Y, Yang W, Tang F, Chen X, Ren L (2015) Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem 22(1):132–149
Yang G, Zhang L, Liu Y (2000) Structure and microporous formation of cellulose/silk fibroin blend membranes I. effect of coagulants. J Membrane Sci 177:153–161
Yehia HM, Elkhadragy MF, Abdel Moneim AE (2011) Antimicrobial activity of pomegranate rind peel extracts. Af J Microbiol Res 4(22):3664–3668
Yu Z, He H, Liu J, Li Y, Lin X, Zhang C, Li M (2020) Simultaneous dyeing and deposition of silver nanoparticles on cotton fabric through in situ green synthesis with black rice extract. Cellulose 27:1829–1843
Zhang S, Li F, Yu JY (2011) Novel cellulose/SPI blend bio-fibers prepared via direct dissolving approach. J Eng Fibers Fabrics 6:31–37
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baseri, S. Eco-friendly production of anti-UV and antibacterial cotton fabrics via waste products. Cellulose 27, 10407–10423 (2020). https://doi.org/10.1007/s10570-020-03471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03471-5