Abstract
Unsaturated Polyester (UP) based composites with 5, 15, 25, 35 and 45 wt% filler content have been fabricated by compression moulding technique. The NaOH treated discontinuous jute fibre has been used along with vinyl silane treated zirconia (ZrO2) particle as the dispersing phases. The structures were characterized by scanning electron microscopy and Fourier transform infrared spectroscopy. The thermal stability of the UP and UP based composites were observed using electronic thermal insulation tester, thermo-gravimetric analyser, differential scanning calorimetry and limiting oxygen index analyser. The swelling behaviour has been investigated in different solutions having a range of pH values. The optimum results were obtained with 35 wt% filler content for UP/Jute/ZrO2 composites.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a substantial demand for new polymer composites incorporating natural fibers in today’s research. Thermosetting polymers are generally brittle and their properties can be improved by incorporation of fibre and particulates, individually or by suitable combination of both. In either case, the physical and mechanical properties of the polymeric matrix were enhanced significantly (Van de and Kiekens 2002; Victor and Arun 2003). The untreated fibres (like jute, sisal, flax, hemp etc.) have very little adhesion with the thermosetting matrices, but the adhesion can be improved by suitable chemical treatment. This yields better bonding and considerable wetting between the fibres and matrices that improves significantly the properties and hence the performance of the fabricated composites (Narendra and Yiqi 2011; Liu et al. 2009; Mohanty et al. 2000; Satyanarayana et al. 1990). Moreover, the particles are also incompatible with the thermosetting polymer, but they can be made compatible with superior wettability by subjecting them to suitable silanization. The most essential merit of using the particulate as filler within polymeric matrix with respect to its fibre counterpart is that a small content of the former can fetch similar or better results to that of the latter (Mohammed 2011; Kim et al. 2012; Singh et al. 2002). Another approach is to fabricate the composites by incorporation of both particle and fibre that will yield a superior resultant property than that of the individual constituent phases. This approach has been adopted by various researchers, where a small quantity of particulate has been used along with significant amount of fibrous filler which reduces the processing cost and hence yield superior properties of the composites (Sing et al. 1995). UP based, jute-reinforced, and alumina- or zirconia-filed composites possess enhanced mechanical property and better thermal stability with 20 wt% of filler content. Such types of composites display a high resistance towards chemicals and moisture absorption (Adhikari et al. 2017). Ray et al. (Ray and Easteal 2007) investigated clay based nano polymer composites that demonstrate a high barrier performance and improved thermal stability, which make these composite suitable for several applications. In this present investigation the key objective is to fabricate an economical, thermally stable and chemical resistant polymer matrix composite by dispersion of jute fibre and ZrO2 particles within UP matrix.
Materials and methods
UP resin of GP 333 grades with 35 wt% styrene, methyl ethyl ketone peroxide (MEKP) as catalyst and cobalt naphthenate as accelerator were procured from M/s A K B Agencies, 1:3, Beleghata Road, Kolkata-700015, India. UP resin is synthesized through a reaction involving C4H2O3 (Methyl anhydride), C6H4 (CO)2O (Phthalic anhydride) and C3H8O2 (Propylene Glycol). The molecular weight, viscosity and density of the UP resin are estimated around 2800 g/mol, 600 cps and 1.12 g/cm3, respectively. The molecular weight and density of MEKP and Cobalt napthalate are around 210.22 g/mol and 1.15 g/cm3 for the former and 401.02 g/mol and 0.96 g/cm3 for the latter, respectively. The procured discontinuous jute fibre (~ 3 cm) with density ~ 1.5 g/cm3 and tensile strength ~ 360 MPa was treated with 5 wt% of NaOH solution for 2 h as per the following reaction (Ray et al. 2007).
The ZrO2 particles (size ~ 50–70 μm; 97% Extra Pure; Loba Chemie) with density ~ 5.85 g/cm3 and molecular weight ~ 123.22 g/mol were chemically modified with 1.0 wt% of silane (vinyl methoxyethylesilane) in a water and methanol solution for 5 min. During the silanization process the silane is placed on the surface of the ZrO2 particles followed by drying of the wet powder at 93 °C for 30 min that yields the condensation of silanol to siloxanes (Tanoglu et al. 1998). The schematic sequence (Fig. 1) of the silanization for the ZrO2 particles is depicts in the chemical reaction furnished below.
Processing of the composites
Compression moulding technique was used for the fabrication of UP based composites with a filler (jute fibre and ZrO2 particle) loading of 5, 15, 25, 35 and 45 wt%, respectively. The quantity of the ZrO2 particle was selected as 10 wt% of the total filler content incorporated within the UP matrix. Initially, waxpol was applied on the inner surface of the mould for ease released of the fabricated composites followed by pre-laying of the fibre in the mould. The silanized ZrO2 particle was mixed with the UP resin by mechanical stirring process. The mixture was degassed in vacuum followed by addition of accelerator and catalyst within the particle-resin mixture. The resultant mixture was then poured on the pre-layed fibre and subsequently a uniform pressure (1 kgf) was applied on the mould followed by room temperature curing for 24 h. Finally, the composite was released from the mould and subjected to post curing at 80 °C for 4 h.
Characterization of the composites
The morphological features and degraded surface of the composites were examined by scanning electron microscope (Hitachi S-3400N) with an accelerating voltage of 15 kV. Elemental mappings of the composite was analyzed using INCA software associated with energy dispersive X-ray spectroscope (Horiba EX-250) attached with the SEM facility. The transmission electron microscopy (TEM) of the cellulosic jute fibre was conducted using Jeol JEM-2100F HRTEM at an accelerating voltage of 200 kV to investigate its morphological features and to analyze the selected area electron diffraction (SAED) pattern of the lattice fringes. The specimen for the HRTEM investigation was prepared by drop casting technique.
Fourier transform infrared spectroscope (Bruker Tensor 27) with resolution of 1 cm−1, a DTGS detector, mid-IR source (4000 to 400 cm−1) and a KBr beam splitter was used. The thermal insulation property measurement was performed with electronic thermal insulation tester following the ASTM D1518-14 standard as per the Kawabata method (Hossen et al. 2010). The thermal stability and degradation of the composites were studied by using Thermogravimetric analyzer (NETZSCH instrument TG 209 f1; Germany) from 30 to 600 °C with a heating rate of 10 °C/min in N2 atmosphere. The differential thermogravimetric data was obtained from the TGA by using the software embedded within the instruments. Differential Scanning Calorimetric (DSC) analysis of the composites has been done by NETZSCH instrument. DSC analysis in N2 atmosphere was done from 30 to 300 °C with heating rate maintained as 10 °C/min. Flow rate of Nitrogen maintained as 60 ml/min. Limiting oxygen index (LOI) measurement was done according to ASTM standard D-2863.The minimum oxygen required for ignition of the composites is termed as LOI (%). The swelling behaviour of the fabricated composites was studied in various medium viz, neutral (pH ~ 7), alkaline (pH ~ 12), acidic (pH ~ 2) and simulated sea water (pH~7.15). A high precision balance with accuracy close to 10−5 g was used for measurements of the swelling (wt%) of the composites. The composites were regularly weighed after an interval of 24, 48, 72, 96, 120, 144, 168, 192, 216 and 240 h, respectively for all composites in different medium with varying pH values. In this context it is worthwhile to mentioned that for swelling in boiling water only, the samples were weighed at 60, 120, 180, 240, 300, 360, 420, 480, 540 and 600 min, respectively. The amount of swelling was calculated at above mentioned time intervals as per the relationship (Deo and Acharya 2008).
where, Wt is the weight of the sample after swelling as a time interval of t and Wo is the weight of the sample before swelling.
Results and discussions
Thermal insulation measurements
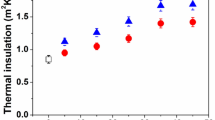
Figure 2 shows the variation in thermal insulation values of different UP based composites with various filler loading. The thermal insulation increases with addition of fillers for different composites. The peak value was observed with 35 wt% of filler contents for all types of composite. The increases in thermal insulation is monotonically from 5 wt% filler content to 35 wt% without any significant enhancement at 45 wt% of filler content. The increment in thermal insulation values up to 35 wt% of filler contents mainly due to the uniform dispersion of the insulating dispersing phase’s viz, jute fibre and ZrO2 within UP matrix. Another probable reason for the enhancement may be the increase in interfacial resistant with higher fillers addition (Han 2006; Lu et al. 2005). The hollow structure of natural jute fibre (lumen) and the presence of voids within UP matrix and interface between the matrix and fillers were also responsible for the augmentation of the thermal insulation values for composites as reported elsewhere (Biswas et al. 2016, 2018; Mitra et al. 1998; Dash et al. 2000; Padal et al. 2014; Mohanty et al. 2004; Ray et al. 2002). Beyond 35 wt% saturation was observed which may be due to non-uniform or irregular dispersion of fillers with 45 wt% filler content. It was clearly revealed from the thermal insulation measurements that the 35 wt% of filler content composites displays the peak value and hence the subsequent structural, thermal as well as the swelling behaviour were performed for the said composites.
Structural investigations
SEM images
The SEM images of jute fibre without and with 5% alkali treatment are shown in Fig. 3 a, b. It can be visualize from the images that the rough portion of the fibre, i.e. basically the lignocelluloses has been removed from its surface. This makes the fibre more water resistant, thereby significantly improving its adhesion with the UP matrix. The SEM images of 35 wt% UP/Jute and UP/Jute/ZrO2 has been presented in Fig. 3 c, d.The uniform and consistent dispersion of the fillers i.e., fibres and ZrO2 particles (marked with white circles) within the UP matrix is quite evident from the images.
HR-TEM analysis
Figure 4 shows the TEM micrographs, lattice fringes and SAED pattern of cellulosic jute fibre. Figure 4 a is the alkali treated single jute fibre at low magnification showing the arrangement of fibrils at different orientations. Figure 4 b presented enlarge view of the encircled portion (Fig. 4 a) of the jute fibre that revealed a clear and distinct arrangement of the fibrils within the same due to its treatment with alkali. The shiny and clear nature was mainly due to the alkali treatment that removed the amorphous lignin and hemicelluloses from the fibre surface. Figure 4 c shows the lattice fringes in the crystalline region of the cellulosic jute fibre and the corresponding SAED pattern as inset validate the same findings. Figure 5 shows the quantitative elemental analysis of the UP/Jute/ZrO2 composites with 35 wt% filler content. The elemental mapping of the composite corroborates the presence of carbon, oxygen, zirconium, cobalt, sodium and silicon, respectively that signifies the substantial existence of the fillers (jute and ZrO2) within the UP matrix.
FT-IR analysis
Figure 6 a–d shows the FTIR patterns for UP, alkali treated jute, vinyl silane treated ZrO2 and 35 wt% UP/Jute/ZrO2, respectively. The interaction between chemically treated fillers and UP matrix may be physical, mechanical and/or chemical (Sichina 2000). The detailed analyses of the FTIR spectra allow to draw the various interpretations. The peaks at around 3944.19 and 2364.58 cm−1 (Fig. 6 d) are of treated jute fibres which shifted from their original positions (3934.55 and 2362.65 cm−1 in Fig. 6 b) due to its in plane displacement. The peaks at around 3438.87 and 3859.33 cm−1 (Fig. 6 d) are due to intermolecular hydrogen bonding arises from the interaction between the carboxylic group (–COOH) of anhydride and the hydroxyl groups (–OH) of the UP. The 3770.61 cm−1 (Fig. 6d) peak is of UP matrix which shifted from 3776.39 cm−1 (Fig. 6 a) due to layer symmetric stretching vibration of aliphatic hydrocarbon C-H group. The two new peaks appeared at 3068 and 3026.13 cm−1 (Fig. 6 d) are mainly due to isometric stretching of –CH2 group at the matrix-filler interface between filler and matrix (Bar et al. 2015). The peak observed at 1730.04 cm−1 (Fig. 6 d) is due to the strong carbonyl stretching of UP matrix. The peaks at 1957.63 and 1593.10 cm−1 (Fig. 6 d) of UP matrix are shifted from 1959.56 to 1591.17 cm−1 (Fig. 6 a) due to the stretching vibration of phenyl group. The peaks at 746.40 and 543.89 cm−1 (Fig. 6 d) of UP matrix are shifted from 742.55 to 536. 17 cm−1 (Fig. 6 a) due to the matrix-filler interactions (Sichina 2000); (Han and Fina 2011)]. The peak at 1379.02 cm–1(Fig. 6 d) for UP matrix arises mainly due to layer symmetric stretching vibration of aliphatic hydrocarbon C-H group. The peaks at 1278.72, 1068.50 and 1132.14 cm−1 (Fig. 6 d) are due to ZrO2 particle which shifted from their original positions at 1277, 1064 and 1132 cm−1 (Fig. 6 c) as a results of chemical and mechanical interaction between matrix and fillers (Idicula et al. 2006).
Thermal properties measurements
Thermogravimetric analysis (TGA)
Figure 7 shows the TGA plots for UP matrix as well as UP based composites depicting the thermal stability and weight loss during thermal activation. The plots further corroborated the fact that the thermal stability of UP and UP based composites are in the range of 38–80 °C. The thermal stability temperature and corresponding residual weight (%) at 600 °C are illustrated in Table 1. It is clearly revealed that the thermal stability temperature (~ 80.27 °C) is highest for UP/Jute/ZrO2 as compared to UP matrix (~ 38.12 °C) and UP/Jute composites (~ 68.80 °C), respectively. This may be due to the better dispersion of the fillers (jute and ZrO2) within the UP matrix. In the case of UP/Jute composites, the uniform dispersion of jute within UP matrix is limited due to fibrous form of the former. As a result, for this type of composites, the stability of the polymeric matrix under thermal activation is lower than UP/Jute/ZrO2 composites. The UP/Jute/ZrO2 composites display a enhanced stability under thermal activation as compared to UP/Jute composites due to better dispersion and non-degradability of the ZrO2 particulate within UP matrix whereas in case of latter both matrix and jute are susceptible to degradation under the same condition. This also substantiates the fact that the residual weight at maximum temperature (~ 600 °C) is more for UP/Jute/ZrO2 (~ 3.725%) as compared to UP/Jute composites (~ 2.125%) and the UP matrix (~ 1.315%) (Adhikari et al. 2017; Victor and Arun 2003; Narendra and Yiqi 2011).
Differential thermogravimetric analysis (DTGA)
Figure 8 a–c shows the DTGA plots (obtain by differentiating the TGA plots) of UP and UP based composites which demonstrates the degradation temperature of the matrix as well as the composites. The DTGA plots revealed various associated events viz, pre-degradation and degradation during heating of the samples. The value of pre-degradation and degradation temperature are appended in Table 1. The DTGA plots show a maximum degradation temperature for UP/Jute/ZrO2 as compared to UP/Jute composites and UP matrix. This may be due to uniform dispersion of the ZrO2 particle and better bonding of the fillers (jute and ZrO2 particle) within the UP matrix. Moreover, higher energy is utilized in breaking the bonds between UP matrix and fillers (jute and ZrO2) in the case of UP/Jute/ZrO2 composites than UP/Jute composites (Narendra and Yiqi 2011; Idicula et al. 2006; Takagi et al. 2007). The degradation results in complete breakdown of both weak and strong bonds within matrix and fillers as well as between the same. In this context it is worthwhile to mention that the pre-degradation event may occur probably due to the breakage of C–C as well as C–H bonds in case of the UP matrix and for the cellulosic fibre (jute). It is quite interesting to note that a completely new peak (encircled) was evolved in the case of UP/Jute (~ 246.18 °C) and UP/Jute/ZrO2 (~ 247 °C) composites mainly due to breakage of weak Van der Waals and OH bonds in UP and jute fibre as well as the hydrogen bonds between UP matrix and fillers.
Differential thermal analysis (DTA)
The DTA plots of UP, UP/Jute and UP/Jute/ZrO2 composites are shown in Fig. 9. It is clearly observed from the plots that a mild endothermic reaction was occurred in the temperature range of 36–65 °C and high endothermic reaction take place at a temperature above 400 °C for UP, UP/Jute and UP/Jute/ZrO2 composites. It also observed that the reaction shifted towards right for UP/Jute and UP/Jute/ZrO2 composites than for the UP matrix. The initial peaks may reflect the glass transition temperature (Tg) and the higher value is for thermal degradation of the matrix and its composites, respectively. This confirmed the fact that with addition of fillers, an enhancement in thermal stability takes place.
DSC measurements
DSC measurement was performed on the UP and UP based composites (Fig. 10) in order to visualize the effect of fillers on Tg. The board endothermic peaks were observed due to the presence of absorbed water in the jute fibre. A very little broadening of endothermic peak was observed in this region below 100 °C. Hence it can be concluded that the fabricated composites possess a very negligible amount of moisture (Behzad and Sain 2007).
Several studies established that the enthalpy change in this region was much higher than that expected from the mere contribution of the moisture. Hence, the peak in this region was related to the Tg (Al-Nassar 2006) i.e., for UP (~ 40 °C), UP/Jute (~ 65 °C) and UP/Jute/ZrO2 (~ 85 °C), respectively. A significant increase in Tg was observed for the UP/Jute/ZrO2 composite than UP/Jute composites. This may be due to the proper distribution of the fillers within the UP matrix (Zou et al. 2003; Ning and Chou 1995; Verma et al. 1991; Mounika et al. 2012).
Limiting oxygen index (LOI) measurements
The Limiting oxygen index (LOI) measurements as per the ASTM D-2863 standard were used in the present investigation (Fig. 11) to indicate the relative flammability of UP and UP based composites. The value of the LOI signifies the minimum percentage of oxygen [O2] in the oxygen/nitrogen mixture (O2/N2) and expressed as:
The higher the LOI value, the better is the flame retardant property of the composites (Fenimore and Martin 1999; Camino et al. 1988; Laoutid et al. 2009).
It is clearly observed from Table 2, that the UP/Jute/ZrO2 composites have best LOI value of 25%. Thus, ZrO2 addition in the UP/Jute not only increases the thermal stability but also enhanced the fire retardant properties. In composites char formation occurred [Fig. 11b, d and f] which was the most vital cause for the improvement of flame retardancy as these char provide a physical mask of oxygen and heat around the unburnt portion of the composites that results in the enhancement of the LOI values (Knuutinen and Kyllonen 2006). Figure 11 shows the SEM micrographs of UP [Fig. 11 a, b], UP/Jute [Fig. 11 c, d] and UP/Jute/ZrO2 composite [Fig. 11 e, f] subjected to LOI measurements. The images revealed a clear formation of char in the case of UP and UP based composites. In this context, it is worthwhile to mentioned that severe air trapped zones were observed in the case of UP matrix (Fig. 11 b), whereas this tendency was minimal for the composites with better char formation in the case with UP/Jute/ZrO2 composite. [Figure 11 g–i] presented the photographical images of the UP, UP/Jute and UP/Jute/ZrO2 composites after LOI testing.
Swelling behaviour
Room temperature condition
Figure 12 a shows the swelling tendency of UP, UP/Jute and UP/Jute/ZrO2 composites carried out at the room temperature condition with pH~7. The swelling was measured at various time intervals viz. 24, 48, 72, 96, 120, 144, 168, 192, 216 and 240 h, respectively. It was clearly visualized that with passage of time, the swelling tendency was quite higher for composites as compare to the UP matrix. The swelling mainly take place through the macro and micro-voids, pores, cracks and the interfacial gap between matrix and fillers (Deo and Acharya 2008). In the case of UP/Jute composite, the tendency of swelling was severe as jute fibres tend to absorb higher amount of moisture whereas this propensity was lesser for UP/Jute/ZrO2 composite due to the hydrophobicity of ZrO2 particulate and its uniform dispersion within the UP matrix (Dhakal et al. 2007).
Figure 12 b shows the variation of microhardness subsequent to swelling at the room temperature condition. A significant microhardness reduction was observed for UP and.
UP based composites with the worst result observed for UP/Jute composites (~ 30% reduction) as jute fibres tend to absorb more moisture which drastically affects its surface property. On the contrary, a better result was obtained for UP/Jute/ZrO2 (~ 23% reduction only) due to uniform dispersion of the hydrophobic ZrO2 particulate within the UP matrix.
Figure 12 c, d presented the SEM micrographs of the swelled surfaces for 35 wt% UP/Jute/ZrO2 composite. It was observed that the swelling at the room temperature condition deteriorate the surface significantly. The matrix degrades drastically without any signature of fibre protruding from its surface, which is an indication of better interfacial bonding and hence superior stability of the fabricated composites in the room temperature swelling condition.
Boiling water condition
Figure 13 a shows the swelling behaviour of UP, UP/Jute and UP/Jute/ZrO2 composites carried out at boiling water condition. The swelling was estimated at various time intervals viz. 60, 120, 180, 240, 300, 360, 420, 480, 540 and 600 min, respectively. In this case, the swelling behaviour of UP and UP based composites was rapid as compare to the room temperature swelling. It was clearly visualized from the Fig. 13 a that with course of time, the swelling tendency was higher for UP based composites as compare to the UP matrix. In this condition the swelling mostly occurred through the voids, pores, cracks present on and below the surface of the composites as well as within the interfacial gap between matrix and fillers.
Figure 13 b revealed the variation of microhardness after swelling at the boiling water condition. A significant reduction in microhardness values was observed in this case when compared to the samples swelled at room temperature condition. The worst result noticed for UP/Jute composites (~ 43% reduction) as in boiling water the jute fibre absorbed more moisture thereby affecting its surface drastically. Moreover, the better result obtained for UP/Jute/ZrO2 (~ 28% reduction), as the hydrophobic ZrO2 particulate were well dispersed within the UP matrix.
The SEM micrographs as presented in Fig. 13 c, d shows the surface of 35 wt% UP/Jute/ZrO2 composite after swelling in boiling water. It was observed that the swelling at the boiling water leads to severe degradation of the surface when compared to the room temperature condition. The matrix degrades severely with a tendency of fibre protruding as well as cracks generation on its surface. This justify the facts that the degradation occurred in boiling water is more drastic than room temperature condition.
Alkaline water condition
The swelling tendency of UP, UP/Jute and UP/Jute/ZrO2 composites carried out at the alkaline water condition with pH~12 as shown in Fig. 14 a. The swelling was evaluated at various time intervals viz. 24, 48, 72, 96, 120, 144, 168, 192, 216, 240 h, respectively. Like previous two conditions, it was clearly visualized that with passage of time, the swelling tendency was quite higher for composites as compare to the UP matrix. The swelling mainly take place through various defects like voids, cracks, pores, as well as within the interfacial gap between matrix and fillers. For UP/Jute composite, the swelling tendency was extreme as jute fibres tend to attract higher amount of moisture while such tendency was limited for UP/Jute/ZrO2 composite due to the hydrophobicity of ZrO2 particulate and its uniform dispersion within the UP matrix.
Figure 14 b revealed the variation of microhardness after swelling at the alkaline water condition. A significant reduction in microhardness was observed which was much higher than the room temperature condition. The post swelling microhardness reduction for UP/Jute (~ 38%) and UP/Jute/ZrO2 (~ 26%) composites were more and rapid as compare to the room temperature condition as a results its affects the surface severely.
Figure 14 c, d presented the SEM micrographs showing the surfaces swelled in alkaline water for 35 wt% UP/Jute/ZrO2 composite. It was revealed that the surfaces swelled in the presence of alkaline water degraded drastically as compare to the room temperature condition.
Acidic water condition
Figure 15 a revealed the swelling behaviours of UP, UP/Jute and UP/Jute/ZrO2 composites which carried out at the acidic water condition with pH~2. The swelling was computed at different time intervals viz. 24, 48, 72, 96, 120, 144, 168, 192, 216, 240 h, respectively. It was clearly visualized from the Fig. 15 a that with passage of time, the swelling propensity was quite higher for composites as compare to the UP matrix that take place primarily through the voids, pores, cracks and the interfacial gap between matrix and fillers. Similar to the room temperature as well as alkaline conditions, the UP/Jute composite possesses maximum swelling tendency due to higher amount of moisture absorbance by jute fibre, whereas this affinity was less for UP/Jute/ZrO2 composite due to the hydrophobicity of ZrO2 particulate along with its uniform dispersion within the UP matrix.
Figure 15 b revealed the post swelling variation of microhardness values submerged within the acidic water. It was clearly noticed that a noteworthy microhardness reduction occurred for UP/Jute (~ 34% reduction) and UP/Jute/ZrO2 (~ 25% reduction) composites which is higher than the room temperature but lesser than the alkaline condition swelling.
Figure 15 c, d presented the SEM images of swelled surfaces for 35 wt% UP/Jute/ZrO2 composite in the acidic water condition. It was observed that the surface degradation due to swelling in the acidic water was severe than the room temperature condition. The matrix degrades extensively without any signature of fibre protruding although a significant presence of cracks on the surface was inevitable which is very similar to alkaline water condition.
Simulated sea water condition
The sea water condition was prepared following the ASTM D-1141 standards by maintaining a composition of NaCl (24.53 g/l), MgCl2 (5.20 g/l), Na2SO4 (4.09 g/l), CaCl2 (1.16 g/l), KCl (0.695 g/l), and NaHCO3 (0.201 g/l) (Saadatmanesh et al. 2010). Figure 16 a shows the swelling behaviours of UP, UP/Jute and UP/Jute/ZrO2 composites submerged in the simulated sea water with pH~7.15. The swelling calculated at various time intervals viz. 24, 48, 72, 96, 120, 144, 168, 192, 216, 240 h, respectively. Like all previous conditions it was clearly observed that with passage of time, the swelling that largely take place through the voids, pores, cracks and the interfacial gap was superior for composites as compare to the UP matrix. In the simulated sea water condition an identical swelling tendency was observed just like all other previous submerged cases with the maximum for UP/Jute as compared to UP/Jute/ZrO2 composite as the fibres be likely to absorb more amount of moisture whereas such tendency was minimum for UP/Jute/ZrO2 composite due to the hydrophobic nature of ZrO2 particulate as well as its uniform dispersion within the UP matrix.
Figure 16 b shows the variation of microhardness after swelling at the simulated sea water condition. A considerable microhardness reduction was observed i.e., ~ 33% for UP/Jute and ~ 24% for UP/Jute/ZrO2, the values much higher than the room temperature but lower than the alkaline and acidic swelling condition. This substantiate the fact that the composites absorbed moisture more quickly at simulated sea water condition than the room temperature water condition that significantly affects its surface.
Figure 16 c, d shows the SEM micrographs of swelled surfaces for 35 wt% UP/Jute/ZrO2 composite subjected to the simulated sea water condition. It was clearly revealed that the swelling at the simulated sea water condition slightly deteriorate the surface with formation of few cracks in comparison with the swelled surfaces submerged in alkaline, acidic and boiling water.
Conclusions
From the present investigation it was observed that along with fillers addition i.e., chemically treated discontinuous jute fibre and ZrO2, the thermal stability, degradation temperature and thermal insulation of the fabricated composites improved significantly. Moreover, the swelling behaviour shows a remarkable improvement with the addition of silane treated ZrO2 as filler. The composites viz. UP/Jute and UP/Jute/ZrO2 with 35 wt% filler content display the best results with respect to thermal stability, degradation temperature and insulation as well as swelling behaviours. Moreover, it was further established that the UP/Jute/ZrO2 composites show superior results with a significant improvement in thermal stability, thermal insulation and degradation temperature along with satisfactorily swelling behaviour after submerging in the solutions with a range of pH values.
References
Adhikari J, Biswas B, Chabri S, Bandyapadhyay NR, Sawai P, Mitra BC, Sinha A (2017) Effect of functionalized metal oxides addition on the mechanical, thermal and swelling behavior of polyester/jute composites. Eng Sci Technol 20:760–774
Al-Nassar YN (2006) Prediction of thermal conductivity of air voided-fiber-reinforced composite laminates part II: 3D simulation. Heat Mass Transf 43:117–122
Bar M, Alagirusamy R, Das A (2015) Flame retardant polymer composites. Fiber Polym 16:705–717
Behzad T, Sain M (2007) Measurement and prediction of thermal conductivity for hemp fiber reinforced composites. Polym Eng Sci 47:977–983
Biswas B, Chabri S, Sawai P, Mitra BC, Das K, Sinha A (2016) Effect of copper incorporation on the mechanical and thermal behavior of jute fiber reinforced unsaturated polyester composites. Polym Compos 39:E 1315–1321
Biswas B, Chabri S, Sawai P, Mitra BC, Das K, Sinha A (2018) Effect of aluminium addition on the mechanical and thermal behaviour of unsaturated polyester/jute composites. J Inst Eng 99:525–530
Camino G, Costa L, Casorati E, Bertelli G, Locatelli R (1988) J Appl Polym Sci 35:1863–1867
Dash BN, Rana AK, Mishra HK, Nayak SK, Tripathy SS (2000) Novel Low-cost jute-polyester composites. III. weatheringand thermal behavior. J Appl Polym Sci 78:1671–1679
Deo C, Acharya SK (2008) Preparation and properties of biodegradable poly (propylene carbonate)/thermoplastic dried starch composites. Carbohydr Polym 71:229–234
Dhakal HN, Zhang ZY, Richardson MOW (2007) Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos Sci Technol 67:1674–1683
Fenimore CP, Martin FJA (1999) Study of optimum fibre content in unit composite. Mod Plast 44:141–146
Han Z (2006) Two phase studies of unsaturated polyester. 3:11-19.
Han Z, Fina A (2011) Thermal conductivity of carbon nanotubes and their polymer nanocomposites: a review. Prog Polym Sci 36:914–944
Hossen MF, Hamdan S, Rahman MR, Rahman MM, Liew FK, Lai JC (2010) Effect of moisture absorption on mechanical properties of chopped natural fiber reinforced epoxy composite. J Reinf Plast Compos 29:2513–2521
Idicula M, Boudenne A, Umadevi L, Ibos L, Candau Y, Thomas S (2006) Thermo-physical. Properties of natural fibre reinforced polyester composites. Compos Sci Technol 66:2719–2725
Kim HJ, Jung DH, Jung IH, Cifuentes JI, Rhee KY, Hui D (2012) Enhancement of mechanical properties of aluminium/epoxy composites with silane functionalization of aluminium powder. Compos Part B 43:1743–1748
Knuutinen U, Kyllonen P (2006) E- preserv. Sci., 3:11-16.
Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta JMR (2009) New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng 63:100–106
Liu L, Iannyong YuJ, Cheng L, Qu W (2009) Mechanical properties of poly (butylene succinate) (PBS) biocomposites reinforced with surface modified jute fibre. Compos Part A 40:669–674
Lu J, Wu Z, Negulescu V (2005) Wood-fiber/high-density-polyethylene composites: coupling agent performance. J Appl Polym Sci 96:93–102
Mitra BC, Basak RK, Sarkar M (1998) Studies on jute-reinforced composites Its limitations, and some solutions through chemical modifications of fibers. J Appl Polym Sci 67:1093–1100
Mohammed MA (2011) Mechanical behavior for polymer matrix composite reinforced by copper powder. Coll Eng J (NUCEJ) 14:160–176
Mohanty AK, Misra M, Hinrichsen G (2000) Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mat Eng 276–277:1–24
Mohanty S, Nayak SK, Verma SK, Tripathy SS (2004) Effect of MAPP as a coupling agent on the performance of jute–pp composites. J Reinf Plast Compos 23:625–637
Mounika M, Ramaniah K, Prasad AVR, Rao KM, Reddy KHC (2012) Thermal conductivity characterization of bamboo fiber reinforced polyster composite. J Mater Environ Sci 3:1109–1116
Narendra R, Yiqi Y (2011) Completely biodegradable soyprotein–jute biocomposites developed using water without any chemicals as plasticizer. Ind Crops Prod 33:35–41
Ning QG, Chou TW (1995) Failure analysis of composite laminates with free edge. J Compos Mater 29:2280–2285
Padal KTB, Ramji K, Prasad VVS (2014) Thermal properties of jute nanofibre reinforced composites. Inter J Eng Res 3:333–335
Ray S, Easteal A (2007) Advances in polymer-filler composites: macro to nanomater. Manuf Process 22:741–749
Ray D, Sarkar BK, Basak RK, Rana AK (2002) Study of the thermal behavior of alkali-treated jute fibers. J Appl Polym Sci 85:2594–2599
Ray D, Sengupta S, Sengupta SP, Mohanty AK, Mishra M (2007) A study of the mechanical and fracture behavior of jute-fabric-reinforced clay-modified thermoplastic starch-matrix composites. Macromol Mater Eng 292:1075–1084
Saadatmanesh H, Tavakkolizadeh M, Mostofinejad D (2010) Environmental effects on mechanical properties of wet lay-up fibre-reinforced polymer. ACI Mater J 107:267–274
Satyanarayana KG, Sukumaran K, Mukherjee PS, Pavithran C, Pillai SGK (1990) Natural fibre-polymer composites. J Cem Concr Compos 12:117–136
Sichina WJ (2000) Characterization of epoxy resins using DSC, Perkin Elmer Instruments. https://shop.perkinelmer.com/Content/applicationnotes/app_thermalepoxyresinsusingdsc.pdf
Sing MB, Gupta M, Verma A (1995) Mechanical behaviour of particulate hybrid composite laminates as potential building materials. Constr Build Mater 9:39–44
Singh RP, Zhang M, Chan D (2002) Toughening of a brittle thermosetting polymer: effects of reinforcement particle size and volume fraction. J Mater Sci 37:781–788
Takagi H, Kako S, Kusano K, Ousaka A (2007) Effect of microstructure on multifunctional properties of natural fiber composites. Adv Compos Mater 16:377–384
Tanoglu M, McKnight SH, Palmese GR, Gillespie JW (1998) Use of silane coupling agents to enhance the performance of adhesively bonded alumina to resin hybrid composites. Int J Adhes 18:431–434
Van de V, Kiekens P (2002) Thermal degradation of flax: the determination of kinetic parameters with thermogravimetric analysis. J Appl Polym Sci 83:2634–2643
Verma LS, Shrotriya AK, Singh R, Chaudhary DR (1991) Characterization of polyester films used in capacitors. I. Transient and steady-state conductivity. J Phys D Appl Phys 24:1729–1734
Victor MFE, Arun S (2003) Fabrication, characterization, and dynamic behaviour of polyester/TiO2 nano composites. Mater Sic Eng A 361:358–366
Zou M, Boming Yu, Zhang D, Ma Y (2003) Transactions of the ASME, 125: 980-986
Acknowledgments
Author Bhabatosh Biswas is highly indebted to Indian Institute of Engineering Science and Technology (IIEST), Shibpur, India for providing the Institute fellowship to carry out his research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biswas, B., Sawai, P., Santra, A. et al. Thermal stability, swelling and degradation behaviour of natural fibre based hybrid polymer composites. Cellulose 26, 4445–4461 (2019). https://doi.org/10.1007/s10570-019-02383-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02383-3