Abstract
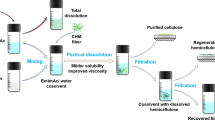
In this study, ionic liquid treatment for increasing the wet strength of cellulose paper was studied. The effects of treatment conditions with the ionic liquid 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) on the wet strength were investigated. First, the [BMIM]Cl (20 g) was melted at 80–100 °C and the paper was immersed in it for 5–60 s. Next, the paper was immersed in ethanol, and finally, it was washed with distilled water. The paper was dried in a hot press at 110 °C and 1.1 MPa for 5 min. The [BMIM]Cl treatment improved the wet strength of the paper. Disintegration of the papers was investigated by immersing them in distilled water, and the paper treated with [BMIM]Cl did not disintegrate. The minimum treatment time required for improvement of the wet strength of the paper was only 5 s. Excess [BMIM]Cl from the original treatment was recovered by vacuum distillation and dehydration was used to treat new paper. This recovered [BMIM]Cl enhanced the wet strength to a level similar to that obtained with the virgin [BMIM]Cl. Therefore, the [BMIM]Cl can be efficiently re-used in this method. The paper treated with [BMIM]Cl will be composed completely of cellulose. Therefore, this method is a viable alternative to treatment by adding chemical such as a polyamideamine epichlorohydrin (PAE).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paper with good wet strength is used in products such as tissue paper, paper towels, and packaging (Jocher et al. 2015; Lai et al. 2013; Obokata et al. 2005; Scott 2006; Siqueira et al. 2015; Su et al. 2012; Uematsu et al. 2011; Wang et al. 2015). The main component of paper is cellulose, which consists of β-1,4-glucosidic linked d-glucose units. The major determinant of paper strength is hydrogen bonds between hydroxyl groups in the cellulose fibers (Obokata et al. 2005; Scott 2006). However, these hydrogen bonds are broken on addition of water to paper, and this reduces the paper strength (Lai et al. 2013; Obokata et al. 2005; Scott 2006). To improve the wet strength of paper, treatment methods are required.
Polyamideamine-epichlorohydrin (PAE) resin has been widely used to treat paper and increase its wet strength through formation of chemical bonds between the cellulose fibers after thermal drying of the treated paper (Obokata et al. 2005; Scott 2006; Siqueira et al. 2015; Su et al. 2012). However, PAE is prepared from the organic halide epichlorohydrin, which is hazardous (Jocher et al. 2015). A method for increasing the wet strength that does not use hazardous materials is required. Some studies have successfully used carboxymethyl cellulose (CMC) in place of PAE to improve the wet strength of paper, and the prepared with CMC fibers had higher wet strength than that with no CMC content (Siqueira et al. 2015; Uematsu et al. 2011). This increase in wet strength was caused by inter-fiber bonds. This research shows that chemical bonds between cellulose fibers are important for the development of wet strength.
In recent years, ionic liquids, which are composed of organic cations and inorganic anions and have low melting points, have attracted attention for green chemistry applications (Goossens et al. 2016; Kerton et al. 2013). Ionic liquids are non-flammable, chemically stable, and highly polar, and have been used in biocatalysis for separation, polymer and chemical preparation, and as electrolytes (Ichiura et al. 2011; Isik et al. 2014; Li et al. 2010; Olivier-Bourbigou et al. 2010; Sivapragasam et al. 2016). Swatloski et al. (2002) reported dissolution of cellulose using 1-butyl-3-methyl-imidazollium chloride ([BMIM]Cl), and regeneration of cellulose using water or ethanol as a non-solvent. Ionic liquids have been actively studied as solvents for biomass dissolution (Feng and Chen 2008; Isik et al. 2014; Samayam and Schall 2010). It is well known that cellulose has poor solubility in both water and common organic solvents. Liquids that dissolve cellulose include viscose, sodium hydroxide/urea, N-methylmorpholine-N-oxide monohydrate, N,N-dimethylacetamide/LiCl, and dimethylsulfoxide/tetrabutylammonium fluoride trihydrate (McCormick et al. 1985; Williamson et al. 1998). However, these solvents are toxic, highly volatile, and difficult to recover. By contrast, ionic liquids are non-toxic, have low volatility, and are easy to recover. Therefore, ionic liquids could be effective solvents for dissolution of cellulose.
We previously found that regenerated cellulose film prepared using an ionic liquid had better wet strength than cellulose paper prepared without this treatment (Ichiura 2014). These results indicated that the chemical bonds formed in the cellulose film prepared using an ionic liquid were chemically stable, and that the cellulose film strength was not affected in wet conditions.
The aim of the present study was to investigate the wet strength of paper treated with [BMIM]Cl. We hypothesized that the [BMIM]Cl would cause partial dissolution of cellulose in the paper, and this dissolved cellulose would form a cellulose film on the surface of the paper and increase its wet strength. The effects of the treatment time on the wet strength of paper were studied. Paper treated with [BMIM]Cl was characterized using Fourier transform infrared spectroscopy (FT-IR), water contact angle testing, X-ray diffractometry (XRD), and scanning electron microscopy (SEM). To evaluate reuse of excess [BMIM]Cl, it was recovered by vacuum distillation, used to treat paper, and the wet strength was compared to that obtained with virgin [BMIM]Cl.
Materials and methods
Materials
Filter paper (No. 2, Toyo Roshi Kaisha, Ltd. Tokyo, Japan) was used as the cellulose paper. Dry tensile strength (DTS) and wet tensile strength of the filter paper were 2.02 and 0.090 kN/m, respectively.
[BMIM]Cl (≥95.0%) was purchased from Sigma Aldrich Co., Ltd. (St. Louis, MO). Ethanol (98.0% volume fraction) and silver nitrate (AgNO3) were obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Harmide PY (Harima chemicals, Inc., Osaka, Japan) was used as PAE wet strength resin.
Treatment of paper with [BMIM]Cl
First, the [BMIM]Cl (20 g) was melted at 80, 90, or 100 °C, and the filter paper (ø 55 mm) was immersed in the melted [BMIM]Cl for 5, 10, 20, 30, 40, 50, or 60 s. Next, the filter paper was immersed in ethanol (45 mL) for 1 min to induce precipitation of the cellulose film. Finally, the filter paper was washed by shaking with distilled water until no chloride ions were detected with 0.02 mol/L AgNO3 (45 mL), and then dried in a hot press at 110 °C and 1.1 MPa for 5 min.
Untreated paper was prepared as follows. The filter paper was immersed in ethanol (45 mL) for 1 min and then, in distilled water for 1 min. The filter paper was dried in a hot press at 110 °C and 1.1 MPa for 5 min.
The paper treated with PAE was prepared as follows. The filter paper was immersed in PAE for 1 min and then, was dried in a hot press at 110 °C and 1.1 MPa for 5 min. The paper treated with PAE was cured by additional heating at 110 °C for 10 min in an oven.
The mixture of [BMIM]Cl and ethanol obtained after immersion of the treated filter paper in ethanol was isolated by vacuum distillation at 40 °C and 74 hPa for 3 h. The recovered [BMIM]Cl was dried using a drying oven (DX-600, Yamato Scientific. Co, Ltd., Tokyo, Japan) for 1–9 h. This recovered [BMIM]Cl was used for treatment of the paper using the process described above.
The water content in the [BMIM]Cl was determined using an electronic moisture balance (MOC-120H, Shimadzu Corporation, Kyoto, Japan).
Characterization of paper treated with [BMIM]Cl
FT-IR spectra were acquired using a FT-IR-6100 (Jasco Inc., Tokyo, Japan) using attenuated total reflection at a resolution of 4 cm−1 throughout the spectral range (500–4000 cm−1), with an accumulation of scans.
The water contact angles between 100 and 400 ms after drop adhesion were measured by a sessile drop method at room temperature using a DM-500 instrument (Kyowa Interface Science Co., Ltd., Saitama, Japan).
XRD patterns were recorded with a Rigaku Type RINT 200 diffractometer fitted with a vertical goniometer and using Cu-Kα irradiation. The X-ray generation conditions were 30 kV and 40 mA. The scan speed was 4° min−1 for the scan range 5°–30° (2θ). The crystallinity was calculated from the ratio of crystalline and non-crystalline peak areas at 23° (2θ).
After applying an osmium coating (Neoc-ST; Meiwafosis Co., Ltd., Tokyo, Japan), the paper surfaces were analyzed using SEM (VE-9800 instrument, Keyence Corporation, Osaka, Japan), with an accelerating voltage of 3 kV.
Disintegration of the paper was investigated as follows. Distilled water (100 mL) was poured into a 110-mL glass bottle (height 120 mm, ø 40 mm), and then the filter paper was placed in the bottle. After 1 min, the glass bottle was inverted 100 times by hand and then allowed to stand for 1 min.
The water content in each paper was determined using the electronic moisture balance (MOC-120H, Shimadzu Corporation).
Dry and wet tensile strengths of the paper treated with [BMIM]Cl
The dry tensile strength (DTS) of the paper (machine direction) was determined using a tensile tester (STB-1225S, A&D Co., Ltd., Tokyo, Japan). The specimens tested measured 20 × 50 mm, and the test speed and span distance were 10 mm/min and 25 mm, respectively.
The wet tensile strength (WTS) of the paper was measured as follows. The paper was dipped in distilled water for 1 min, excess water was removed using a filter paper (ø 185 mm), and then the tensile strength was measured immediately using the method described above. The wet strength retention (WSR) of the paper was calculated using Eq. 1.
Results and discussion
Evaluation of treatment conditions
First, we determined the optimum treatment conditions for formation of the [BMIM]Cl-treated paper. At all [BMIM]Cl temperatures, when the treatment time was longer than 40 s, the filter paper did not form a sheet after treatment with [BMIM]Cl. Additionally, for [BMIM]Cl at 90 and 100 °C, the filter paper did not form a sheet after treatment. These results were attributed to excessive dissolution of cellulose by the [BMIM]Cl. To ensure only partial dissolution of the cellulose occurred, the [BMIM]Cl temperature was set at 80 °C and the treatment time was 5–30 s.
Characterization of the paper treated with [BMIM]Cl
In the FT-IR spectrum of the paper treated with [BMIM]Cl (Fig. 1), cellulose peaks (C–O and C–O–C stretching vibrations) were apparent at 1000–1200 cm−1. The presence of unreacted cellulose components was recorded in all conditions.
Hydrophilicity is an important property for paper used in wet conditions, such as tissue paper and paper towels. The hydrophilicity of the paper after [BMIM]Cl treatment was evaluated using photographs of the water contact angles (Fig. 2). For paper treated with [BMIM]Cl for 5–30 s, the water was adsorbed between 300 and 400 ms. By contrast, for the untreated filter paper, the water was adsorbed between 200 and 300 ms. The hydrophilicity of the paper treated with [BMIM]Cl was almost the same as that of the untreated paper, which showed that the paper retained its hydrophilicity after treatment.
After treatment with [BMIM]Cl for 5–30 s, the filter paper showed peaks for cellulose I at around 15°, 17° and 23° (2θ) (Fig. 3a). The peak areas at 23° (2θ) of the filter paper, and the paper treated with [BMIM]Cl for 5, 10, 20 and 30 s were 16,324, 15,755, 12,594, 10,707 and 6894, respectively. As the [BMIM]Cl treatment time increased, the areas for the peak decreased (Fig. 3b–e). The crystallinities of the filter paper, and the paper treated with [BMIM]Cl for 5, 10, 20 and 30 s were 88.0, 87.6, 84.9, 82.7 and 75.5%, respectively and then decreased increasing treatment time. These results indicate the cellulose in the paper gradually changed into amorphous cellulose (Yousefi et al. 2011). It is well known that the crystalline structure of cellulose I changes to amorphous cellulose on dissolution in an ionic liquid (Ichiura et al. 2011; Li et al. 2010). Therefore, these results suggest that some of cellulose in the paper dissolves in the [BMIM]Cl, and this cellulose then precipitates on the paper surface on immersion in ethanol.
SEM images of the paper treated with [BMIM]Cl (Fig. 4) showed a film formation over part of the paper surface. The pores of the paper treated with [BMIM]Cl were covered by this film, which was formed by the cellulose dissolved in the [BMIM]Cl. The formation of this partial cellulose film on the paper was particularly obvious after treatment with [BMIM]Cl for 30 s (Fig. 4e). The cross section of the paper treated with [BMIM]Cl showed cellulose fibers, and a structure similar to the surface film was not observed in the interior of the paper (Fig. 4f). These results indicate that the cellulose fibers on the paper surface dissolve in the [BMIM]Cl and then form a cellulose film on the paper surface by precipitation upon immersion in ethanol.
DTS and WTS of the paper treated with [BMIM]Cl
The WTSs of the papers treated with [BMIM]Cl were higher than that of the original filter paper, and increased with increasing treatment time (Fig. 5a). The WSRs of the papers treated with [BMIM]Cl were also higher than that of the original filter paper (Fig. 5b). These results suggest that the cellulose film formed on the surface of the paper treated with [BMIM]Cl (Fig. 4e) is important in determining its WTS. The improved WTS was attributed to hydrogen bonds in the cellulose film on the paper surface. An earlier study found that the WSR of a regenerated cellulose film prepared using viscose was around 20%, and was higher than that of untreated filter paper (around 5%). Other research (showed that CMC fiber prepared from regenerated cellulose had better water resistance than a cellulose sheet prepared without treatment (Siqueira et al. 2015; Uematsu et al. 2011). Hydrogen bonds formed in regenerated cellulose after [BMIM]Cl treatment are probably harder to break than those in the original cellulose. The increase in WTS with increasing area of the cellulose film on the paper surface suggests the hydrogen bonds formed in the paper with [BMIM]Cl treatment are important.
The DTS values of the paper treated with [BMIM]Cl were two times those of the original filter paper (Fig. 5c), which could be attributed to the increased hydrogen bonding in the paper. The DTS remained constant at around 3.0 kN/m regardless of the treatment time. Duchemin (Duchemin et al. 2009) and Nishino (Nishino and Arimoto 2007) reported that the DTS of the filter paper increased with increasing ionic liquid treatment time. The treatment times in their study ranged from 20 min to 12 h and were much longer than the treatment times used in our study (from 5 to 30 s). Therefore, increases in the DTS of the paper treated with [BMIM]Cl would not be observed with the treatment times used in this study, and this is why the DTS remained constant.
Disintegration experiments with the papers in distilled water showed that the untreated filter paper disintegrated (Fig. 6a, b, Video S1), whereas the paper treated with [BMIM]Cl for 5–30 s did not. With the shortest treatment time (5 s), the paper had sufficient wet strength and the WTS was around 0.20 kN/m. Therefore, the minimum treatment time required for improvement of the wet strength of the paper was 5 s. The paper treated with PAE was not disintegrated as shown in Fig. 7. The WTS of the PAE treated paper was 0.23 kN/m. The performance of the PAE treatment was similar to that of [BMIM]Cl treatment for 5 s.
These results suggest that [BMIM]Cl treatment of the paper is an effective method for improving the wet strength. In addition, paper treated in this way will be composed completely of cellulose rather than having added chemical content as with other treatment methods. Therefore, this method is a viable alternative to treatment with PAE.
DTS and WTS of the paper treated with recovered [BMIM]Cl
The mass fraction of water in the recovered [BMIM]Cl decreased with increasing drying time (Table 1), and there was no water present after 9 h. Disintegration experiments were performed with the paper treated with recovered [BMIM]Cl for 5 s to evaluate the effect of water in the [BMIM]Cl. With a high mass fraction of water (>10%) in the recovered [BMIM]Cl, the wet strength was not enhanced by [BMIM] treatment (Fig. 8a–c). When the mass fraction of water in the recovered [BMIM]Cl was <10%, the paper structure partly disintegrated (Fig. 8d, e). By contrast, paper treated with recovered [BMIM]Cl that contained no water did not disintegrate (Fig. 8f). Mazza et al. dlx (2008) reported that the solubility of cellulose in [BMIM]Cl was affected by the water content of the [BMIM]Cl. Their results and ours indicate that treatment with hydrated [BMIM]Cl will not improve the wet strength of paper.
The WTS and DTS of the paper treated with recovered [BMIM]Cl containing no water for 5 s were 0.190 and 3.06 kN/m, respectively. By comparison, the WTS and DTS of the paper treated with virgin [BMIM]Cl were 0.215 and 2.85 kN/m, respectively. These results suggest that the performance of the dried recovered [BMIM]Cl was the same as that of the virgin [BMIM]Cl. This is consistent with our previous research (Ichiura et al. 2011), which showed that recovered [BMIM]Cl could be used repeatedly.
Conclusion
We found the wet strength of paper impregnated with [BMIM]Cl at 80 °C increased with increasing treatment time. This could be attributed to formation of a cellulose film on the paper surface after partial dissolution of cellulose fibers in the paper with [BMIM]Cl treatment. Hydrogen bonds formed by the regenerated cellulose make the paper resistant to degradation by water. The WTS of the paper obtained after [BMIM]Cl treatment for only 5 s was sufficient to prevent disintegration of the paper in water. In addition, the DTS of the paper treated with [BMIM]Cl was larger than that of untreated filter paper.
The [BMIM]Cl used for the increasing the wet strength of the paper was recovered using vacuum distillation and then dried before recycling it back into the treatment process. If it contained any water, the function of the recovered [BMIM]Cl was lower than that of the virgin [BMIM]Cl. The performance of the recovered [BMIM]Cl was similar to that of virgin [BMIM]Cl if the water was completely removed. Therefore, after drying to remove water, recovered [BMIM]Cl is effective for improving the wet strength of paper.
References
Duchemin B-JC, Mathew A-P, Oksman K (2009) All-cellulose composites by partial dissolution in the ionic liquid 1-butyl-3-methylimidazolium chloride. Compos Part A 40:2031–2037
Feng L, Z-l Chen (2008) Research progress on dissolution and functional modification of cellulose in ionic liquids. J Mol Liq 142:1–5
Goossens K, Lava K, Bielawski CW, Binnemans K (2016) Ionic liquid crystals: versatile materials. Chem Rev 116:4643–4807
Ichiura H, Nakatani T, Ohtani Y (2011) Separation of pulp and inorganic materials from paper sludge using ionic liquid and centrifugation. Chem Eng J 173:129–134
Ichiura H, Ono A, Kamada M, Ohtani Y (2014) Novel recycling technique of paper sludge using ionic liquid—preparation of functional materials for water purification. In: Proceedings of 9th IWA international symposium on waste management problems in agro-industries, pp. 24–26
Isik M, Sardon H, Mecerreyes D (2014) Ionic liquids and cellulose: dissolution, chemical modification and preparation of new cellulosic materials. Int J Mol Sci 15:11922–11940
Jocher M, Gattermayer M, Kleebe HJ, Kleemann S, Biesalski M (2015) Enhancing the wet strength of lignocellulosic fibrous networks using photo-crosslinkable polymers. Cellulose 22:581–591
Kerton FM, Liu Y, Omari KW, Hawboldt K (2013) Green chemistry and the ocean-based biorefinery. Green Chem 15:860–871
Lai X, Song Y, Liu M (2013) Preparation and application of cationic blocked waterborne polyurethane as paper strength agent. J Polym Res 20:222–227
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101:4900–4906
Mazza M, Catana DA, Vaca-Garcia C, Cecutti C (2008) Influence of water on the dissolution of cellulose in selected ionic liquids. Cellulose 16:207–215
McCormick CL, Callais PA, Hutchinson BH Jr (1985) Solution studies of cellulose in lithium chloride and N, N-dimethylacetamide. Macromolecules 18:2394–2401
Nishino T, Arimoto N (2007) All-cellulose composite prepared by selective dissolving of fiber surface. Biomacromol 8:2712–2716
Obokata T, Yanagisawa M, Isogai A (2005) Characterization of polyamideamine-epichlorohydrin (PAE) resin: roles of azetidinium groups and molecular mass of PAE in wet strength development of paper prepared with PAE. J Appl Polym Sci 97:2249–2255
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: recent progress from knowledge to applications. Appl Catal A-Gen 373:1–56
Samayam IP, Schall CA (2010) Saccharification of ionic liquid pretreated biomass with commercial enzyme mixtures. Bioresour Technol 101:3561–3566
Scott WE (2006) Wet end Chemistry. Tappi Press, Atlanta
Siqueira EJ, Salon M-CB, Belgacem MN, Mauret E (2015) Carboxymethylcellulose (CMC) as a model compound of cellulose fibers and polyamideamine epichlorohydrin (PAE)-CMC interactions as a model of PAE-fibers interactions of PAE-based wet strength papers. J Appl Polym Sci 132:42144–42153
Sivapragasam M, Moniruzzaman M, Goto M (2016) Recent advances in exploiting ionic liquids for biomolecules: solubility, stability and applications. Biotechnol J 11:1000–1013
Su J, Mosse WKJ, Sharman S, Batchelor W, Garnier G (2012) Paper strength development and recyclability with polyamideamine-epichlorohydrin (PAE). BioResources 7:913–924
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Sci 124:4974–4975
Uematsu T, Matsui Y, Kakiuchi S, Isogai A (2011) Cellulose wet wiper sheets prepared with cationic polymer and carboxymethyl cellulose using a papermaking technique. Cellulose 18:1129–1138
Wang M, He W, Wang S, Song X (2015) Carboxymethylated glucomannan as paper strengthening agent. Carbohydr Polym 125:334–339
Williamson SL, Armentrout RS, Porter RS, McCormick CL (1998) Microstructural examination of semi-interpenetrating networks of poly(N,N-dimethylacrylamide) with cellulose or chitin synthesized in lithium chloride/N,N-dimethylacetamide. Macromolecules 31:8134–8141
Yousefi H, Nishino T, Faezipour M, Ebrahimi G, Shakeri A (2011) Direct fabrication of all-cellulose nanocomposite from cellulose microfibers using ionic liquid-based nanowelding. Biomacromolecules 12:4080–4085
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (Grant Number 16K07810) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ichiura, H., Hirose, Y., Masumoto, M. et al. Ionic liquid treatment for increasing the wet strength of cellulose paper. Cellulose 24, 3469–3477 (2017). https://doi.org/10.1007/s10570-017-1340-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1340-8