Abstract
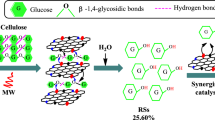
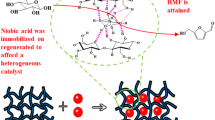
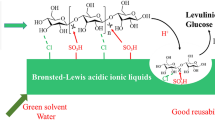
This article first discloses that the fluorine anion-containing ionic liquids-functionalized biochar sulfonic acids (BCSA-IL-F1–3s), which were simply synthesized by an ionic exchange of 1-trimethoxysilylpropyl-3-methylimidazolium chloride (IL-Cl) grafted on the BCSA with CF3SO3H (HF1), HBF4 (HF2), HPF6 (HF3), respectively, can efficiently catalyze cellulose hydrolysis into reducing sugars (RSs) and 5-hydroxymethyl furfural (HMF) in water under microwave irradiation. This process provides a very high catalysis efficiency (turnover numbers, 4.03–4.89) at mild temperature (80 °C) for 3 h, but also possesses an excellent repeatability. More outstandingly, they can achieve much higher HMF yields (12.70–27.94%) compared to the IL-Cl-functionalized BCSA catalyst (HMF yields are lower than 0.1%) under the same reaction conditions. This is likely because the introduction of IL-F1–3s groups can significantly improve the accessibility, acidity and thermal stability of BCSA’s SO3H sites, as supported by evidence from a solid 31P NMR spectrum and thermogravimetric analysis. It is proposed that the good selectivity for HMF perhaps originates from a co-catalysis action of the IL-F1–3s and SO3H groups on BCSA-IL-F1–3s in the further conversion of RSs to HMF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With growing concerns about diminishing fossil resources, global warming and environmental pollution, the research for renewable resources has attracted worldwide attention (Tilman et al. 2009). Cellulosic biomass, as an extremely abundant and renewable resource (Ragauskas et al. 2006; Zhou et al. 2011), can be converted to renewable chemicals and fuels by various catalysis processes (Tuck et al. 2012; Kobayashi and Fukuoka 2013). Among these processes, the hydrolysis of cellulosic biomass into reducing sugars (RSs) especially 5-hydroxymethylfurfural (HMF), is still the one of the key technologies for the transformation of cellulosic biomass, because its hydrolysis products RSs can be converted into a range of important industrial chemicals, including ethanol, hydrocarbons and starting materials for the production of polymers (Zhang and Lynd 2004; Zhang et al. 2011; Ragauskas et al. 2006; Zhang 2008). Another hydrolysis product HMF has a wide range of prospective applications as primary building blocks of the bio-refinery, polymers, bulk and fine chemicals (Alonso et al. 2012). Some efficient catalysis systems for cellulose hydrolysis to RSs have been developed (Fan et al. 1987; Mok et al. 1992; Bobleter 1994; Toda et al. 2005; Yang et al. 2006; Onda et al. 2008; Suganuma et al. 2008; Kontturi and Vuorinen 2009; Zhang and Zhao 2009; Wu et al. 2010). Among these systems, homogeneous catalysis usually shows high conversion efficiency, but it causes corrosion of equipment and involves troublesome separation of catalyst and products. Heterogeneous catalysis can efficiently overcome both the disadvantages of homogeneous catalysis, but generally presents low hydrolytic efficiency due to a mass transfer resistance between solid catalyst and insoluble cellulose in water. Consequently, there is an urgent demand for simpler and more efficient green technologies aimed at the hydrolysis of cellulose and especially lignocellulose, for the production of various biomass products.
Recently, organic solvents such as dimethyl sulfoxide, dichloromethane, methyl isobutyl ketone and especially ionic liquids, are widely used in the depolymerization and hydrolysis of cellulose to HMF (Swatloski et al. 2002; Chheda et al. 2007; Fort et al. 2007; Zhao et al. 2007; Rinaldi and Schuth 2009; Su et al. 2009; Zakrzewsk et al. 2011; Yang and Hu 2012; Shi et al. 2013), owing to their good solubility to cellulose (Swatloski et al. 2002; Chheda et al. 2007; Minnick et al. 2016). For examples, Qi et al. (2011) used acid resin to catalyze depolymerization of cellulose to glucose in ionic liquid [EMIM]Cl solvent by gradually adding trace water, then, removed acid resin and added CrCl3 to catalyze glucose isomerization, dehydration to get ca. 73% HMF. Su et al. (2009) reported that the one step conversion of cellulose to HMF in [EMIM]Cl solvent can be achieved upon catalysis with CuCl2–CrCl2 under mild conditions, providing ca. 55% HMF. Li et al. (2009) reported that the CrCl3-mediated conversion of glucose and cellulose in [C4min]Cl can be improved significantly under microwave assisted heating, affording HMF in ca. 90 and 60% isolated yields, respectively. Most of these reaction systems described-above, however, usually proceed at a relatively high reaction temperature (120–150 °C), which can lead to a high operating cost and a troublesome separation process (Rosatella et al. 2011). In addition, some corrosive or toxic reagents used easily result in the corrosion of equipment and serious environmental pollution and there is difficult to completely separate and recover the liquid acid catalyst and ionic liquid solvent from the reaction products. It is therefore desirable to develop an efficient green technology aimed at the conversion of cellulose to HMF in pure water under mild conditions.
More recently, Daorattanachai et al. (2012) reported that the earth phosphate α-Sr(PO3)2 can efficiently catalyze the conversion of cellulose to glucose and HMF under hot compressed water condition at 230 °C, yielding ca. 19% glucose and 15% HMF. Nandiwale et al. (2014) reported a more active Bimodal-HZ-5 zeolite catalyst for this reaction, which can provide ca. 67% cellulose conversion and 46% yield of HMF in distilled water at 170 °C. Fachri et al. (2015) reported the uncatalysed reaction of conversion inulin into HMF in water, the highest yield of HMF can reach 35%. These reaction systems have good activity for this reaction in water, but the harsh reaction conditions (high temperature and high pressure) hamper their commercial application. Also, we (Zhang et al. 2012, 2013, 2014a) reported that the ionic liquid functionalized biochar sulfonic acids, as biomimetic catalysts, show high hydrolysis activity and good repeatability for the microwave-assisted hydrolysis of cellulose to RSs in water under mild temperature (90 °C), but affording a very poor HMF yield (lower than 0.2%). Here, we would report that three fluorine anions-containing ionic liquid-functionalized BCSA (BCSA-IL-F1–3s) can significantly improve HMF yield for the microwave-assisted heterogeneous hydrolysis of cellulose in water under mild temperature.
Experimental
Preparation of fluorine anions-containing ionic liquid-functionalized BCSA catalysts
Firstly, a biochar sulfonate sodium-bearing chlorine anions-containing 1-(trimethoxy propyl silane)-3-methyl imidazolium (BCSANa-IL-Cl) was prepared referring to in our published work (Zhang et al. 2012). Firstly, synthesis of 1-(trimethoxy propyl silane)-3-methyl imidazolium chloride (Si(MeO)3PMIMCl) are described as follows: 4.12 g of 1-methyl-1H-imidazol and 10.84 g of 3-chloropropyl trimethoxy silane were mixed in 60 mL of anhydrous toluene and the mixture stirred at the reflux temperature of toluene (120 °C) for 12 h to obtain an orange viscous liquid Si(MeO)3PMIMCl (marked as IL-Cl). The resulting crude product was washed by ethyl acetate (15 mL × 3) to remove the unreacted reagents, then heated to remove the residual ethyl acetate under reduced pressure and yielded a pure liquid product (yield, 95%). Infrared spectrum of the product was recorded on a NICOLRT-Avatar370 FT-IR spectrometer. Additionally, its 1H and 13C NMR spectra were recorded on a Bruker Avance AC-500 MHz using CDCl3 as the deuterated solvent and TMS as the internal standard. Recorded results were as follows: IR (KBr): 3166 and 3121 [v(C–H) aromatic]; 2941and 2883 [v(C–H) aliphatic]; 1575 and 1472 [v(C=C)]; 1460 [v(C–N)]; 754 [v(C–Si)] m−1. The 1H NMR spectrum for Si(MeO)3PMIMCl (500 MHz, CDCl3) consisted of the following peaks: δ 0.56 (2H, t, –CH2–Si), 1.91 (2H, m, CH2), 3.46 (9H, s, 3(–OCH3)), 4.03 (3H, s, N–CH3), 4.23 (2H, t, –N–CH2), 7.36 (1H, d, pyr-H), 7.59 (1H, d, pyr-H), 10.37 (1H, broad, pyr-H). 13C NMR (125 MHz, CDCl3): results (ppm) include δ 5.8, 23.9, 36.3, 50.4, 77.0, 121.7, 123.5, 137.6.
Biochar sulfonic acid (marked as BCSA) was prepared using the carbonization of bamboo powder with 80% sulfuric acid and then sulfonation with oleum (50 wt% SO3), its specific preparation operations can be found in our published work (Zhang et al. 2012). The BCSA powder was treated with saturated aqueous solution of NaCl under ultrasonic vibration (KQ-400LDB ultrasound bath, power, 100 W), then washed repeatedly with hot distilled water and dried at 120 °C for 12 h to obtain BCSANa powder. Finally, the BCSANa was treated with IL-Cl in anhydrous acetonitrile for reflux reaction 12 h, after silanization, the precipitate was filtered, washed with anhydrous acetonitrile three times to obtain BCSANa-IL-Cl.
The resulting BCSANa-IL-Cl was respectively acidified by an excess of CF3SO3H (HF1), HBF4 (HF2), HPF6 (HF3) at room temperature for 24 h, then washed repeatedly with hot distilled water and dried at 120 °C for 12 h to yield the F1–3s anions-containing catalysts (BCSA-IL-F1–3s, see Scheme 1). For the sake of comparison, a biochar sulfonic acid (BCSA) and the IL-Cl-containing BCSA catalyst (BCSA-IL-Cl) were also prepared according to our published works (Zhang et al. 2012).
Measurement and characterization of catalysts
The loading amount of SO3H on the catalysts BCSA-IL-F1–3s was measured via a chemical titration method and the concrete procedure referred to in our previous publications (Wu et al. 2010; Zhang et al. 2012).
Thermal stability of the catalysts BCSA-IL-F1–3s was measured on a NETZSCH-STA 409PC in flowing N2 (10 mL/min) at a heating rate of 20 °C/min.
The solid 31P NMR spectra over these samples were performed on a Varian Infinitypuls-400 spectrometer with the use of a 4 mm double-resonance MAS probe at a spinning rate of 10 kHz. The Larmor resonance frequencies for 1H and 31P were 399.52 and 161.73 MHz, respectively. 31P MAS NMR spectra with high-power proton decoupling were recorded using a π/2 pulse length of 7.8 μs and a recycle delay of 30 s. The chemical shifts for the 31P resonance were externally referred to (NH4)2HPO4 (1.0 ppm). Prior to the solid 31P NMR spectra measurement, the sample was necessarily dehydrated at 180 °C for 10 h under vacuum (a pressure below 10−3 Pa) and then cooled. The following adsorption experiment was carried out at room temperature in a N2 glove box. In that, a known amount of TMPO adsorbate dissolved in anhydrous CH2Cl2 was added into a vessel containing the dehydrated sample, followed by evacuation of the solvent CH2Cl2 at room temperature. Then the sealed sample vessel was subjected to a thermal treatment at 160 °C for 2 h to ensure a uniform adsorption of TMPO probe molecules over the BCSA adsorbent. Finally, the sealed sample tube was opened and the sample was transferred into an NMR rotor with a Kel-F end cap under a dry nitrogen atmosphere in a glove box. Detailed procedures of the solid 31P NMR spectra measurement can be found elsewhere (Zheng et al. 2008, 2011; Feng et al. 2010).
Adsorption experiment
0.1 g of catalyst was immersed in 10 mL of aqueous glucose solution (1.0 mg/mL) or a hydrolysis solution of cellulose consisting of 1.1 mg/L glucose and 0.5 mg/mL oligosaccharides at room temperature for 12 h. After filtering to remove catalyst, the resulting filtrate was subjected to the analysis of glucose or oligosaccharides concentration according to our previous publication (Wu et al. 2010).
Hydrolysis of cellulose under microwave irradiation
Hydrolysis of microcrystalline cellulose (particle size, 20–100 mm; degree of polymerization, 200–1000) in water was carried out on a JQ NANJING Model NJL07-3 experimental microwave oven with a frequency of 2.45 GHz, power from 0 to 1000 W and an IR remote sensing temperature controller. The concrete experimental procedure and UV–Vis analysis method for the conversion products RSs and HMF are referred to in previous publications (Miller 1959; Hansen et al. 2010; Dutta et al. 2011; De et al. 2011; Zhang et al. 2013, 2014a). Here, in order to verify the UV–Vis analysis method’s accuracy, quantitative analysis of HMF was also performed with a High-Performance Liquid Chromatography (HPLC, Agilent 1100) method, using a Shimazu 20AT HPLC equipped with reversed-phase C18 column and an ultraviolet detector at 283 nm. A water and methanol volume ratio of 30:70 was used as the mobile phase at a rate of 1.0 mL/min, and column oven temperature was maintained at 30 °C. The concentration of HMF in samples was calculated based on the standard curve obtained with the standard substances. The results indicated that the yield of HMF measured from HPLC method agreed well with that measured by the present UV–Vis spectrophotometer (the error measured by both the methods was within ±5%.)
Catalyst recycling and re-use for bamboo hydrolysis
After the hydrolysis of cellulose (0.2 g) catalyzed by the catalysts BCSA-IL-F1–3s (0.1 g) in water under microwave irradiation was carried out under optimal conditions, the solid residue containing the catalyst and unreacted cellulose was recovered from the hydrolytic solution by filtering and washing with water. After drying and supplementing with a definite amount of fresh cellulose, the ground residue was directly used for the 2nd hydrolytic reaction under the same conditions. This recycling process proceeded repeatedly five times. The supplementing amount of free cellulose each recycling run was calculated based on the following formula:
Results and discussion
Characterization of catalysts
Content of the SO3H groups on various samples was measured by chemical titration before use and after the 4th operating run. As shown in Table 1, the densities of SO3H groups on the various F anions-containing BCSA-ILs (1.31–0.96 mmol g−1) were obviously lower than that on the parent BCSA (1.80 mmol g−1), which is probably due to the following reasons: (1) introduction of IL-F1–3s groups can increase catalyst’s mass; (2) the preparation process of catalyst leads to the leaching of SO3H groups from the parent BCSA (Zhang et al. 2012; Hu et al. 2014). Notably, after these catalysts were used repeatedly 3 times in cellulose hydrolysis (the results of recycling experiments will be described later), the leaching of SO3H groups was almost negligible upon the BCSA-IL-F3 (0.96 vs 1.05 mmol g−1), BCSA-IL-F1 (1.20 vs 1.31 mmol g−1) and especially BCSA-IL-F2 (0.95 vs 0.96 mmol g−1), illustrating that the introduction of these IL-F1–3s groups can significantly improve the stability of SO3H groups. In the absorption experiments of a glucose or hydrolysis solution over these catalysts, we found that all the catalysts possessed a poor adsorption capacity for glucose (adsorption value, 3.34–3.75 mg/g), but a very strong adsorption capacity for the hydrolysis solution (25.46–35.46 mg/g), illustrating that they can selectively adsorb the oligosaccharides in the solution, as previously reported by Suganuma et al. (2008) and our groups (Wu et al. 2010). This should be due to a good affinity of the catalysts with the β-1,4 glycosidic bonds of oligosaccharides. Furthermore, the adsorption capacity for β-1,4 glycosidic bonds followed an increasing sequence of BCSA-IL-F1 > BCSA-IL-F2 > BCSA-IL-F3.
Figure 1 shows thermogravimetric analysis (TGA) curves of the aforementioned materials. As shown in Inset of Fig. 1, three main weight loss peaks respectively appearing at 90–100, 330–360 and 370–425 °C were observed in the corresponding differential thermogravimetry (DTG) curves of these catalysts, which should be assigned to an evaporation of the adsorbed water molecules, a decomposition of the surface’s SO3H groups and a decomposition process of these IL-F1–3s groups grafted on the BCSA’s OH sites, respectively. We previously reported that the introduction of IL-Cl and especially IL-Zn groups into the BCSA could cause both the decomposition peaks for SO3H and IL groups to be shifted to the high temperature region (Zhang et al. 2012, 2014a). Here, the SO3H and IL-F1–3s groups on BCSA-IL-F1–3s showed very similar decomposition behaviors with our reported catalyst BCSA-IL-Zn (Zhang et al. 2014a), implying that the IL-F1–3s-containing catalysts, like BCSA-IL-Zn, should have an excellent repeatability. Table 1 lists the decomposition temperature (DT) of SO3H groups on various samples, in which the DT value for various samples followed an increasing sequence of BCSA-IL-F2 > BCSA-IL-F1 > BCSA-IL-F3 > BCSA-IL-Cl > BCSA.
The solid-state 31P MAS NMR of adsorbed TMPO over solid acids has been extensively used to investigate the acidity characterization of various solid acids (Zheng et al. 2008; Feng et al. 2010; Tagusagawa et al. 2010; Zheng et al. 2011). Such method was used to measure the Brønsted acid strength of various ILs-functionalized BCSA materials and the results are shown in Fig. 2. Curve a in Fig. 2 shows that the 31P MAS NMR spectrum of TMPO adsorbed on the parent BCSA exhibited highly overlapped 31P resonance peaks spanning from ca. 30 to 60 ppm. Further analysis by Gaussian simulation reveals the spectrum may be deconvoluted into two characteristic resonance peaks with 31P chemical shift of 38 and 46 ppm, each corresponding to a relative concentration of 39.5 and 60.5%, respectively. According to the range of the 31P chemical shift, the resonance with 31P chemical shift of 38 ppm originates from a physical adsorption of TMPO over the BCSA and another with 31P chemical shift of 46 ppm attributed to TMPO adsorbed on the weak H+ acid sites of BCSA’s OH groups. It is puzzling that the resonance with a low-field 31P chemical shift value (about 80 ppm) due to TMPO adsorbed on the strong SO3H acidic sites, which can be usually found in solid sulfate resins (Liu et al. 2013), was not observed upon the BCSA bearing SO3H groups. Also, a similar phenomenon was observed by Yang and co-workers in detecting the 1H MAS NMR spectrum of SO3H groups on PS-SO3H@ mesosilicas DSNs (Zhang et al. 2014b). This is likely because the compact stacking of BCSA’s aromatic carbon sheets caused by strong H-bonding interactions (Kitano et al. 2009) hampers the probing molecule TMPO to access the SO3H sites, leading to the absence of 31P MAS NMR signal attributable to the SO3H sites, When the IL-Cl was chemically grafted on the BCSA, its solid-state 31P spectra displayed very wide and overlapped 31P-NMR peaks in 40–82 ppm (Fig. 2b), which can be deconvoluted into four characteristic resonance peaks with 31P chemical shift of 45, 52, 65 and 80 ppm, each corresponding to a relative concentration of 34.9, 49.3, 11.3 and 4.5%, respectively. The observed 31P-NMR signals in 65–82 ppm region unambiguously confirm the existence of strong acidic sites on BCSA-IL-Cl. Furthermore, such strong acidic sites could be clearly found in the solid-state 31P spectra of three IL-F1–3s-containing BCSA materials (Fig. 2c–e) and their 31P-chemical shift and relative concentration are summarized in Table 1. Based on the 31P-NMR measurement results, the acidity of these ILs-containing BCSA catalysts followed an increasing sequence of BCSA-IL-F2 > BCSA-IL-F1 > BCSA-IL-F3 > BCSA-IL-Cl. The fact that the SO3H sites of these ILs-containing BCSAs can be detected by the solid-state 31P MAS NMR of adsorbed TMPO, supports that the intercalation of these bulk IL-Cl, IL-F3, IL-F2, and especially IL-F1 groups can make the compact stacking of BCSA’s aromatic carbon sheets to became loose and hence improve the accessibility of BCSA’s SO3H sites. In addition, the introduction of these ILs groups can significantly enhance the BCSA’s acidity via a strong intramolecular electrostatic bonding interaction between them and the SO3H groups.
Hydrolysis of cellulose
Table 2 lists the data for the various BCSA solid acids-catalyzed conversion of fructose, glucose or cellulose in pure water at 80 °C under microwave irradiation. On the whole, these BCSA solid acids were active for the heterogeneous conversion of these carbohydrates, providing HMF for fructose and glucose, as well as RSs and HMF for cellulose as main products, with a concomitant formation of a small amount of organic acids (levulinic acid and formic acid). Notably, they showed higher activity for cellulose hydrolysis (turnover number, TON, 0.77–4.89) than fructose (TONs, 0.59–4.43) and especially glucose conversion (TONs, 0.42–3.43), in contrast to the results that three liquid acids CF3SO3H, HBF4 and HPF6 catalyzed the conversion of these carbohydrates. Among the BCSA solid acids examined, the parent BCSA showed the lowest activity for these conversion reactions (TONs, 0.42–0.77) due to the inaccessibility of its SO3H sites. In contrast, the various ILs groups-grafted BCSA catalysts showed much higher TONs (0.90–4.89) for these reactions than the parent BCSA and their TON values basically accorded with their acid strengths above-measured. The high catalysis activity of these ILs groups-containing BCSA solid acids is likely due to the following reasons: (1) introduction of these ILs groups can make substrates be easily accessible to the SO3H sites of BCSA via breaking the strong H-bond network of BCSA, as supported by 31P-NMR spectra characterization; (2) the ILs groups flexibly anchored on the BCSA are able to efficiently improve the acidity and strengthen a synergistic effect with SO3H groups, as supported by the TGA and 31P-NMR characterizations above-described. Notably, the yield of HMF in the conversion of both the mono-sugars mainly replied on the acidic strength of these BCSA catalysts. But it was also dependant on the IL groups of these BCSA catalysts except catalyst’s acidity in cellulose hydrolysis. For example, the BCSA-IL-Cl bearing IL-Cl groups showed a good activity for cellulose hydrolysis to RSs (yield, 27.20%), but provided a very poor HMF yield (<0.1%). This should be due to its low activity for the further conversion of RSs to HMF, which can be confirmed by the results that it catalyzed the conversion of both mono-sugars to HMF (see Table 2, yield, 7.32–9.89%). The three IL-F1–3s groups-containing BCSA catalysts showed high activity in cellulose hydrolysis (TON, 4.34–4.89), but also could obtain a considerable amount of HMF (yield, 16.10–27.94%) except RSs (12.70–15.56%). This should originate from a promoted effect of their F ions on the further conversion of RSs into HMF, as observed in the conversion of both mono-sugars to HMF catalyzed by these catalysts (yield, 18.63–27.36%), and reported by Lansalot–Moreau (2003) and Binder and Raines (2009) in the use of [BMIM]+BF4 −, [BMIM]+PF6 − and [EMIM]+CF3SO3 −) as solvents to efficiently promote fructose or glucose conversion into HMF upon catalysis with various Brønsted or Lewis acids. Among the F anions-containing catalysts examined, the BCSA-IL-F1 with CF3SO3 − anions possessed an excellent activity (TON, 4.89) for cellulose hydrolysis, but also achieved the highest HMF yield (27.94%) with a selectivity of 62% at 80 °C for 3 h. To the best of our knowledge, this should be the first example that solid acids can efficiently catalyze the hydrolysis of cellulose to HMF in pure water at a temperature lower than 100 °C, which has significant progress in comparison with the currently reported solid acids to catalyze this reaction under hot compressed water condition (Daorattanachai et al. 2012; Nandiwale et al. 2014). And compared to some liquid B or L acids-efficiently catalyzed this reaction in ILs media (Zhao et al. 2011; Zakrzewsk et al. 2011; Hussein et al. 2013; Liu et al. 2013; Shi et al. 2013; Mondal et al. 2015; Zhang et al. 2015; Shen et al. 2016), the present catalytic system exhibited relatively low catalysis efficiency and HMF selectivity, but it possessed milder reaction conditions and especially simpler separation and recovery.
In the following experiments, the effects of reaction temperature and microwave irradiation power and time on the heterogeneously catalytic hydrolysis of cellulose in water were further investigated using the best BCSA-IL-F1 as catalyst and the results are shown in Figs. 3, 4 and 5, respectively. Figure 3 illustrates that the TON and yield of RSs and HMF obviously increased when temperature was enhanced from 70 to 80 °C, but slightly decreased with further increasing temperature, being due to a further conversion of RSs and HMF. The highest TON and HMF yield were obtained at 80 °C. Figure 4 illustrates that the hydrolysis of cellulose in water over BCSA-IL-F1 was accelerated with increasing microwave power from 350 to 550 W and the time to achieve the highest TON and HMF yield shortened from 3 to 2 h. Figure 5 is the effect of irradiation time on the yield of products for the BCSA-IL-F1-catalyzed cellulose hydrolysis in water at 80 °C. In that, the yield of HMF continuously and rapidly increased with time. After 180 min, the increasing trend of HMF yield with the time was nearly negligible. The yield curve of RSs rapidly climbed with the time and achieved a maximum value at 90 min. After that, it slightly decreased with further prolonged reaction time. The yield of levulinic acid and formic acid, which originates from further conversion of HMF, continuously and slowly increased with time.
Finally, the catalyst’s repeatability in the MW-assisted hydrolysis of cellulose in water was checked using BCSA-IL-F1 as a catalyst and the concrete operating procedure for the catalyst’s recycling experiment could be found elsewhere (Zhang et al. 2012). Figure 6 shows the repeatability of BCSA-IL-F1 in cellulose hydrolysis (reaction conditions: microwave power: 350 W, reaction temperature: 80 °C and time: 3 h). It is seen from Fig. 6 that the TON and HMF yield over the first recovered BCSA-IL-F1 slightly decreased compared to that over the fresh one, but were not further reduced in each following recycling run, indicating that the catalyst has a good repeatability. This should be due to a fact that a strongly intramolecular electrostatic bonding interaction between the IL-F1 and SO3H groups can significantly improve the thermal stability of both the groups, as supported by the results in Table 1 and Fig. 1.
Catalysis mechanism discussion
According to literature report (Zhao et al. 2011; Hussein et al. 2013; Cabiac et al. 2011; Seaman 1945), cellulose conversion to HMF generally includes two reactions: hydrolysis of cellulose to generate RSs and then further degradation of the RSs to produce HMF. Such two reactions are acid-catalyzed consecutive processes and the corresponding apparent activation energies measured by Rinaldi and co-workers (Rinaldi and Schuth 2009; Rinaldi et al. 2010) are approximately equal to each other (108–131 kJ/mol for cellulose hydrolysis to RSs, 114 ± 6 kJ/mol for RSs conversion to HMF). Generally, the degradation of RSs to HMF catalyzed by Brønsted or Lewis acid can efficiently proceed at a higher than 120 °C in DMSO, DMA-LiCl or ILs media (Su et al. 2009; Binder and Raines 2009; Musau and Munavu 1987; Wang et al. 2011), but it can not in pure water. This may be because a strong solvation of water to the substrates RSs leads to the enhanced apparent activation energy for this reaction. Based on the results presented here, a co-catalysis mechanism of the IL-F1–3s and SO3H groups on the catalysts BCSA-IL-F1–3s is proposed to realize the degradation of RSs into HMF in water at lower than 100 °C (using the BCSA-IL-F1-catalyzed glucose conversion to HMF as an example, see Scheme 2). In this mechanism, the catalyst’s H+ combines with the ether bond on glucose to form a cationic species 1. Then, the C2–OH and C3–OH on the 1 interacts with CF3SO3 − ion to form a cyclic hydrogen-bonded species 2. Species 2 undergoes a cleavage of its protonated ether bond and followed by a proton transfer from it to CF3SO3 − ion to form a species 3. Species 3 eliminates one molecule of CF3SO3H to yield a species 4. Species 4 can be converted to a stable fructose after undergoing coordination with CF3SO3 − ion to form an intermediate 5. Finally, fructose is easily converted to HMF through its intramolecular aldol condensation upon catalysis with Brønsted acid (Binder and Raines 2009; Roman-Leshkov et al. 2010; Zhao et al. 2011; Pag’an-Torres et al. 2012).
Conclusion
In summary, for the first time we have developed an efficient and environmentally benign method for the microwave-assisted heterogeneous hydrolysis of cellulose to reducing sugars and especially HMF. The present catalysis system possesses the following advantages: (1) using cheap and available BCSA-IL-F1–3s materials and water as catalysts and solvent, respectively; (2) the catalysts show a high activity and good selectivity for HMF, as well as an excellent repeatability; and (3) mild and facile operating conditions and a green process.
References
Alonso DM, Wettstein SG, Dumesic JA (2012) Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem Soc Rev 41:8075–8098
Binder JB, Raines RT (2009) Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc 131:1979–1985
Bobleter O (1994) Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19:797–841
Cabiac A, Guillon E, Chambon F, Pinel C, Rataboul F, Essayem N (2011) Cellulose reactivity and glycosidic bond cleavage in aqueous phase by catalytic and non catalytic transformations. Appl Catal A 402:1–10
Chheda JN, Román-Leshkov Y, Dumesic JA (2007) Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem 9:342–350
Daorattanachai P, Khemthong P, Viriya-empikul N, Laosiripojana N, Faungnawakij K (2012) Conversion of fructose, glucose, and cellulose to 5-hydroxymethylfurfural by alkaline earth phosphate catalysts in hot compressed water. Carbohydr Res 363:58–61
De S, Dutta S, Saha B (2011) Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride catalyst in water. Green Chem 13:2859–2868
Dutta S, De S, Patra A, Sasidharan M, Bhaumik A, Saha B (2011) Microwave assisted rapid conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by mesoporous TiO2 nanoparticles. Appl Catal A 409–410:133–139
Fachri BA, Abdilla RM, Rasrendra CB, Heeres HJ (2015) Experimental and modelling studies on the uncatalysed thermal conversion of inulin to 5-hydroxymethylfurfural and levulinic acid. Sustain Chem Process 3:8
Fan LT, Gharpuray MM, Lee YH (1987) Cellulose hydrolysis biotechnology monograph. Springer, Berlin
Feng N, Zheng A, Huang SJ, Zhang H, Yu NY, Yang CY, Liu SB, Deng F (2010) Combined solid-state NMR and theoretical calculation studies of Brønsted acid properties in anhydrous 12-molybdophosphoric acid. J Phys Chem C 114:15464–15472
Fort DA, Remsing RC, Swatloski RP (2007) Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem 9:63–69
Hansen TS, Woodley JM, Riisager A (2010) Production of 5-hydroxymethylfurfural in ionic liquids under high fructose concentration conditions. Carbohyd Res 345:1846–1850
Hu S, Smith TJ, Lou W, Zong M (2014) Efficient hydrolysis of cellulose over a novel sucralose-derived solid acid with cellulose-binding and catalytic sites. J Agric Food Chem 62:1905–1911
Hussein AY, El BH, Plilip S (2013) Rapid conversion of cellulose to 5-hydroxymethylfurfural using single and combined metal chloride catalysts in ionic liquid. J Fuel Chem Technol 41(2):214–222
Kitano M, Arai K, Kodama A, Kousaka T, Nakajima K, Hayashi S, Hara M (2009) Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal Lett 131:242–249
Kobayashi H, Fukuoka A (2013) Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem 15:1740–1763
Kontturi E, Vuorinen T (2009) Indirect evidence of supramolecular changes within cellulose microfibrils of chemical pulp fibers upon drying. Cellulose 16:65–74
Lansalot-Matras C, Moreau C (2003) Dehydration of fructose into 5-hydroxymethylfurfural in the presence of ionic liquids. Catal Commun 4(10):517–520
Li CZ, Zhang ZH, Zhao ZK (2009) Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett 50(38):5403–5405
Liu FJ, Zheng AM, Noshadi I, Xiao FS (2013) Design and synthesis of hydrophobic and stable mesoporous polymeric solid acid with ultra strong acid strength and excellent catalytic activities for biomass transformation. Appl Catal B 136:193–201
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Minnick DL, Flores RA, DeStefano MR, Scurto AM (2016) Cellulose solubility in ionic liquid mixtures: temperature, cosolvent, and antisolvent effects. J Phys Chem B 120(32):7906–7919
Mok WS, Antal MJ, Varhergyi G (1992) Productive and parasitic pathways in dilute acid-catalyzed hydrolysis of cellulose. Ind Eng Chem Res 31:94–100
Mondal S, Mondal J, Bhaumik A (2015) Sulfonated porous polymeric nanofibers as an efficient solid acid catalyst for the production of 5-hydroxymethylfurfural from biomass. ChemCatChem 7(21):3570–3578
Musau RM, Munavu RM (1987) The preparation of 5-hydroxymethyl-2-furaldehyde (HMF) from d-fructose in the presence of DMSO. Biomass 13:67–74
Nandiwale KY, Galande ND, Thakur P, Sawant SD, Zambre VP, Bokade VV (2014) One-pot synthesis of 5-hydroxymethylfurfural by cellulose hydrolysis over highly active bimodal micro/mesoporous H-ZSM-5 catalyst. ACS Sustain Chem Eng 2:1928–1932
Onda A, Ochi T, Yanagisawa K (2008) Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem 10:1033–1037
Pag’an-Torres YJ, Wang T, Gallo JMR, Shanks BH, Dumesic JA (2012) Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal 2:930–934
Qi XH, Watanabe M, Aida TM, Smith RLS Jr (2011) Catalytic conversion of cellulose into 5-hydroxymethylfurfural in high yields via a two-step process. Cellulose 18:1327–1333
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Rinaldi R, Schuth F (2009) Acid hydrolysis of cellulose as the entry point into biorefinery schemes. ChemSusChem 2:1096–1107
Rinaldi R, Engel P, Buchs J, Spiess AC, Schuth F (2010) An integrated catalytic approach to fermentable sugars from cellulose. ChemSusChem 3:1151–1153
Roman-Leshkov Y, Moliner M, Labinger JA, Davis ME (2010) Mechanism of glucose isomerization using a solid Lewis acid catalyst in water. Angew Chem Int Ed 49:8954–8957
Rosatella AA, Simeonov SP, Frade RFM, Afonso CAM (2011) 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem 13:754–793
Seaman JF (1945) Kinetic of wood saccharification. Hydrolysis of cellulose and decomposition of sugar in acid at high temperature. Ind Eng Chem Res 37(1):43–52
Shen Y, Zhang Y, Chen Y, Yan Y, Pan J, Liu M, Shi W (2016) Combination of Brønsted and Lewis polymeric catalysts for efficient conversion of cellulose into 5-hydroxymethylfurfural (HMF) in ionic liquids. Energy Technol 4:600–609
Shi N, Liu Q, Zhang Q, Wang T, Ma L (2013) High yield production of 5-hydroxymethylfurfural from cellulose by high concentration of sulfates in biphasic system. Green Chem 15:1967–1974
Su Y, Brown HM, Huang XW, Zhou XD, Amonette JE, Zhang ZC (2009) Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical. Appl Catal A 361:117–122
Suganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, Hayashi S, Hara M (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc 130:12787–12793
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124(18):4974–4975
Tagusagawa C, Takagaki A, Iguchi A, Takanabe K, Kondo JN, Ebitani K, Hayashi S, Tatsumi T, Domen K (2010) Highly active mesoporous Nb-W oxide solid-acid catalyst. Angew Chem Int Ed 49:1128–1132
Tilman D, Socolow R, Foley JA, Hill J, Larson E, Lynd L, Pacala S, Reilly J, Searchinger T, Somerville C, Williams R (2009) Beneficial biofuels—the food, energy and environment trilemma. Science 325:270–271
Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2005) Biodiesel made with sugar catalyst. Nature 438:178
Tuck CO, Pérez E, Horváth IT, Sheldon RA, Poliakoff M (2012) Valorization of biomass: deriving more value from waste. Science 337:695–699
Wang JJ, Xu WJ, Ren JW, Liu XH, Lu GZ, Wang YQ (2011) Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem 13:2678–2681
Wu YY, Fu ZH, Yin DL, Xu Q, Liu FL, Lu CL, Mao LQ (2010) Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem 12:696–700
Yang Y, Hu CW (2012) Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent System. Green Chem 14:509–513
Yang B, Willies DM, Wyman CE (2006) Changes in the enzymatic hydrolysis rate of avicel cellulose with conversion. Biotechnol Bioeng 94:1122–1128
Zakrzewsk ME, Bogel-Łukasik E, Bogel-Łukasik R (2011) Ionic liquid-mediated formation of 5-HydroxymethylfurfuralsA promising biomass-derived building block. Chem Rev 111:397–417
Zhang Y-HP (2008) Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J Ind Microbiol Biotechnol 35:367–375
Zhang Y-HP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulose systems. Biotechnol Bioeng 88:797–824
Zhang ZH, Zhao ZK (2009) Solid acid and microwave-assisted hydrolysis of cellulose in ionic liquid. Carbohyd Res 344:2069–2072
Zhang C, Fu ZH, Liu YC, Dai BH, Zou YH, Gong XL, Wang YL, Deng XL, Wu HT, Xu Q, Steven KR, Yin DL (2012) Ionic liquid-functionalized biochar sulfonic acid as a biomimetic catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Green Chem 14:1928–1934
Zhang C, Fu ZH, Dai BH, Zen SQ, Liu YC, Xu Q, Steven RK, Yin DL (2013) Chlorocuprate ionic liquid functionalized biochar sulfonic acid as an efficiently biomimetic catalyst for direct hydrolysis of bamboo under microwave irradiation. Ind Eng Chem Res 52:11537–11543
Zhang C, Fu ZH, Dai BH, Zen SQ, Liu YC, Xu Q, Steven RK, Yin DL (2014a) Biochar sulfonic acid immobilized chlorozincate ionic liquid: an efficiently biomimetic and reusable catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Cellulose 21:1227–1237
Zhang XM, Zhao YP, Xu ST, Yang Y, Liu J, Wei YX, Yang QH (2014b) Polystyrene sulphonic acid resins with enhanced acid strength via macromolecular self-assembly within confined nanospace. Nat Commun. doi:10.1038/ncomms4170
Zhang Y, Pan J, Shen Y, Shi W, Liu C, Yu L (2015) Brønsted acidic polymer nanotubes with tunable wettability toward efficient conversion of one-pot cellulose to 5-hydroxymethylfurfural. ACS Sustain Chem Eng 3(5):871–879
Zhao HB, Holladay JE, Brown H, Zhang ZC (2007) Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316:1597–1600
Zhao S, Cheng M, Li J, Tian J, Wang X (2011) One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted–Lewis–surfactant-combined heteropolyacid catalyst. Chem Commun 47:2176–2178
Zheng A, Zhang H, Lu X, Liu SB, Deng F (2008) Theoretical predictions of 31P NMR chemical shift threshold of trimethylphosphine oxide absorbed on solid acid catalysts. J Phys Chem B 112:4496–4505
Zheng A, Huang SJ, Liu SB, Deng F (2011) Probing the spatial proximities among acid sites in dealuminated H-Y zeolite by solid-state NMR spectroscopy. Phys Chem Chem Phys 13:14889–14901
Zhou CH, Xia X, Lin CX, Tong DS, Beltramini J (2011) Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem Soc Rev 40:5588–5617
Acknowledgments
We acknowledge the financial support for this work by the National Natural Science Fund of China (21676079, 21546010, 20873040), the Specialized Research Fund for the Doctoral Program of Higher Education (20124306110005), the Natural Science Fund of Hunan Province (10JJ2007, 14JJ2148), the Innovation Platform Open Fund of Hunan College (11K044), Collaborative Innovation Center of New Chemical Technologies for Environmental Benignity and Efficient Resource Utilization, Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province and the 100 Talents Program of Hunan Province.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, C., Cheng, Z., Fu, Z. et al. Effective transformation of cellulose to 5-hydroxymethylfurfural catalyzed by fluorine anion-containing ionic liquid modified biochar sulfonic acids in water. Cellulose 24, 95–106 (2017). https://doi.org/10.1007/s10570-016-1118-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1118-4