Abstract
A chlorozincate ionic liquid-functionalized biochar sulfonic acid (BC-SO3H-IL-Zn) was designed and conveniently prepared via multistep processes, involving the syntheses of biochar sulfonic acid (BC-SO3H) and 1-trimethoxysilylpropyl-3-methylimidazolium chloride (IL)-ZnCl2 (IL-Zn), followed by grafting the IL-Zn onto BC-SO3H. The proposed catalyst was found to show a much higher turnover number (TON, 5.91 for cellulose and 1.78 for bamboo) for the microwave-assisted hydrolysis of cellulose and bamboo to reducing sugars (RSs) in water compared to the corresponding IL-functionalized BC-SO3H (BC-SO3H-IL, TON, 3.23 for cellulose and 0.46 for bamboo) and BC-SO3H (TON, 1.51 for cellulose and 0.15 for bamboo). Furthermore, it, like BC-SO3H-IL, possessed an excellent repeatability for cellulose hydrolysis. The excellent catalytic performance of BC-SO3H-IL-Zn is likely due to the following reasons: Firstly, the introduction of ZnCl2 bestows the catalyst with a delignification function. Secondly, in comparison with the OH groups of BC-SO3H, the IL and especially IL-Zn groups flexibly bound to BC-SO3H, like cellulose binding domain of cellulose, show an stronger affinity for cellulose molecules, on the other hand, they play a better synergistic role and improved acidity in the SO3H groups (as a catalysis domain of cellulase) catalyzing cleavage of the β-1,4-glycosidic bonds of cellulose, as supported by the adsorption experiments of catalysts to oligosaccharides, their thermogravimetric analysis and catalytic reaction results.
Graphical Abstract
Chlorozincate ionic liquid functionalized biochar sulfonic acid (BC-SO3H-IL-Zn) proposed by us was found to be an efficiently biomimetic catalyst for the heterogeneous hydrolysis of cellulose and lignocelluloses to RSs and 5-hydroxymethyl furfural (5-HMF) under microwave irradiation.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulosic biomass is an extremely abundant and renewable resource on earth, consisting of various agricultural residues, fruit and vegetable wastes, woods, municipal solid waste, waste from the pulp and paper industry, and herbaceous energy crops (Ragauskas et al. 2006). The development of some highly efficient transformation technologies for cellulosic biomass has gained increasing research attention due to their immense potential for the production of sugars, alternative fuels, and chemical feedstocks. Among these transformation technologies, the hydrolysis of cellulosic biomass into reducing sugars (RSs) is still the one of the key technologies for the transformation of cellulosic biomass, because its hydrolysis products RSs can be converted into a range of important industrial chemicals, including ethanol, hydrocarbons and starting materials for the production of polymers (Ragauskas et al. 2006; Zhang 2008; Zhang and Lynd 2004). Thus far, available technologies for cellulose conversion into the RSs include enzyme hydrolysis (Fan et al. 1987; Yang et al. 2006), dilute acid hydrolysis (Fan et al. 1987; Mok et al. 1992), concentrated acid hydrolysis (Kontturi and Vuorinen 2009), alkaline hydrolysis (Bobleter 1994) and supercritical water hydrolysis (Sasaki and Fang 2000; Deguchi et al. 2006), but none of these methods are cost-effective for large-scale applications. For example, acid hydrolysis has a long industrial history, but usually proceeds at high temperature and pressure, which leads to high operating costs, low selectivity for RSs and the production of various environmental pollutants (Mok et al. 1992; Calvini et al. 2008). Supercritical water hydrolysis has been proven to be very effective owing to its unique physical and chemical effects (Broll et al. 1999), but it is carried out in an extremely short residence time to avoid further degradation and dehydration of produced RSs (Sasaki and Fang 2000; Saka and Ueno 1999), which will cause difficulties in developing a commercial reaction system with an effective heat recovery. Enzymatic hydrolysis possesses mild reaction conditions, high hydrolysis activity and excellent selectivity for RSs, These merits of enzymatic hydrolysis originate from the inherent characters of cellulases with catalytic domain (CD) and cellulose binding domain (CBD), as well as full topology structure (Gao 2003). Thus, enzymatic hydrolysis is one of the most promising hydrolysis technologies (Hamelinck et al. 2005). The commercialization of enzyme hydrolysis is, however, hampered by the prohibitive high cost of the currently available enzyme preparations, the hard and fast conditions of enzyme reaction, and the inconvenience of enzyme separation (Mok et al. 1992). To solve these problems, Toda et al. (2005), Onda et al. (2008), Zhang and Zhao (2009), Suganuma et al. (2008) and our research groups (Wu et al. 2010) have developed a process in which the hydrolysis of cellulose is catalyzed by some solid acids; however, this heterogeneously catalytic process shows poor hydrolytic efficiency due to a mass transfer resistance between the solid acids and the insoluble cellulose in water. Recently, ionic liquids (ILs)-mediated cellulose hydrolysis has gained increasing research attention (Li and Zhao 2007; Wu et al. 2004), because some ILs possess a good solubility for cellulose (Swatloski et al. 2002; Lee et al. 2009; Fort et al. 2007; Fukaya et al. 2008). However, the high cost, high viscosity and troublesome separation of ILs still hamper their commercialized application. Consequently, there is an urgent demand for simpler and more efficient green technologies aimed at the hydrolysis of cellulose and especially lignocellulose, for the production of various biomass products.
More recently, we reported that an 1-(trimethoxy propyl silane)-3-methyl imidazolium chloride (IL) functionalized biochar sulfonic acid (BC-SO3H-IL) shows much higher hydrolysis efficiency and better repeatability for the hydrolysis of cellulose to RSs in water compared to the corresponding biochar sulfonic acid (BC-SO3H), being due to its unique functionality mimicking cellulose (Zhang et al. 2012). Here, a novel chlorozincate ionic liquid-functionalized BC-SO3H (BC-SO3H-IL-Zn) was prepared to strengthen its biomimetic functionality for the heterogeneous hydrolysis of cellulose and bamboo in water under microwave irradiation.
Experimental
Preparation of BC-SO3H-IL-Zn catalyst
Synthesis of ZnCl2 acidic 1-(trimethoxy propyl silane)-3-methyl imidazolium chloride (IL-Zn) is described as follows (see Scheme 1): 0.05 mol of 1-methyl-1H-imidazol and 0.05 mol of 3-chloropropyl trimethoxy silane were mixed in 100 mL of anhydrous toluene and the mixture stirred at the reflux temperature of toluene (120 °C) for 12 h to obtain an orange viscous ionic liquid 1-(trimethoxy propyl silane)-3-methyl imidazolium chloride (IL-Cl). Then, the IL-Cl (0.05 mol) was mixed with 0.25 mol of ZnCl2·2H2O in acetonitrile (MeCN, 100 mL) and the resulting mixture stirred at the temperature of 85 °C for 12 h to obtain a chlorozincate ionic liquid (IL-Zn).
A bamboo carbonization (BC) and its sulfonation (BC-SO3H) materials were prepared following a previously reported procedure (see Scheme 2) (Wu et al. 2010; Zhang et al. 2012; Cao et al. 1994).
Preparation of the BC-SO3H-IL-Zn (see Scheme 3): 5 g of BC-SO3H powder was treated with 20 mL of saturated aqueous solution of NaCl for 45 min under ultrasonic vibration (KQ-400LDB ultrasound bath), then washed repeatedly with hot distilled water and dried at 120 °C for 12 h to obtain BC-SO3Na powder. The BC-SO3Na was treated with the IL-Zn (4.5 g) in anhydrous MeCN (60 mL) at the refluxing temperature of MeCN for 12 h. After silanization, the precipitate was filtered and washed with anhydrous MeCN three times to obtain the solid BC-SO3Na-IL-Zn. Finally, the BC-SO3Na-IL-Zn was acidified by concentrated HCl, then washed by water three times and dried at 120 °C for 12 h to yield the catalyst BC-SO3H-IL-Zn. For the sake of comparison, an IL-Cl-grafted BC-SO3H material (BC-SO3H-IL) was also prepared based on our reported procedure (Zhang et al. 2012).
Measurement and characterization of catalysts
Referring to published work (Mbaraka et al. 2003), the measurement of SO3H groups on the catalysts is described as follows: a catalyst (0.050 g) was treated with 6.02 mol L−1 of NaCl solution (20 mL) for 45 min at 20–40 °C under ultrasonic vibration. After centrifugal separation, the supernatant solution was titrated by 0.01 mol L−1 of NaOH solution using phenolphthalein as an indicator. The loading amount of IL groups was measured by following our reported procedure (Zhang et al. 2012). In addition, the content of Zn ions on the catalyst was determined by ethylenediaminetetraacetic acid disodium salt (EDTA) complexometric titration. 0.2 g of catalyst was treated with a solution of EDTA (0.01 mol L−1, 25 mL) at 100 °C for 15 min, resulting in the catalyst’s Zn ions being completely complexed by EDTA. After centrifugal separation, the remaining EDTA in the filtrate was measured by a standard solution of Zn ions (0.01 mol L−1). The content of Zn ions on the catalyst was calculated from the total consumption of EDTA subtracting its remaining amount.
The thermogravimetric analysis (TGA) was carried out in flowing N2 (10 mL min−1) at a heating rate of 20 °C min−1 on a NETZSCH-STA 409PC.
Hydrolysis of cellulose and bamboo under microwave irradiation
In a typical experimental run, 0.2 g pure microcrystalline cellulose (particle size, 20–100 μm; degree of polymerization, 200–1,000) or bamboo powder (particle size, 50–100 μm, consisting of cellulose, 40.52 ± 0.5 %; lignin, 21.8 ± 0.4 %; hemicellulose, 15.6 ± 0.5 %; moisture, 19.1 ± 0.5 %; ash, 2.0 ± 0.5 %.) and 0.1 g catalyst were milled for 20–30 min in an agate bowl, and then the solid mixture was removed to a Pyrex reactor, followed by adding the distilled water (1.5 mL). The reactor, equipped with a microfeeder, was irradiated by an experimental microwave oven with a frequency of 2.45 GHz, power from 0 to 1,000 W and an IR remote sensing temperature controller (JQ NANJING Model NJL07-3). In the irradiation process, the distilled water was successively added to the reactor through a microfeeder to compensate for water consumed. After hydrolysis, the reaction mixture was diluted with cold water, filtered and neutralized with 0.5 mol L−1 NaOH solution. The resulting filtrate was subjected to the analysis of reducing sugars (RSs) and HMF. Referring to the published literature (Miller 1959; Li et al. 2008), the measurement of RSs is described as follows: the filtrate (0.2 mL) was treated with 3,5-dinitrosalicylic acid (0.15 mL) at 100 °C for 5 min, then cooled to room temperature, and diluted to 50 mL. The absorbance of the diluted solution was measured on an Agilent 8453 spectrophotometer at 520 nm, and the concentration of RSs was calculated based on a standard curve obtained with glucose. The measurement of HMF is described as follows: the filtrated sample solution (0.2 mL) was diluted to 50 mL with the distilled water, and then its absorbance was measured on an Agilent 8453 UV–Vis spectrophotometer at 283 nm, and the concentration of HMF was calculated based on a standard curve obtained with its standard sample. The yield of RSs or HMF was calculated based on the following formula: RSs or HMF yield = [RSs or HMF concentration (mg mL−1) × 50 × (V1/V2) × 0.9/(A × M)] × 100 %. Here, V1 and V2 are the volume of a hydrolysis solution and its sample solution, respectively, M is the mass of raw material, A is the content of cellulose and hemicellulose in raw material (A = 1.00 for microcrystalline cellulose, A = 0.56 for bamboo).
Adsorption experiment
0.1 g of catalyst was immerged in a 10 mL of an aqueous glucose solution (1.0 mg mL−1) or a hydrolysis solution of cellulose (1.1 mg L−1 glucose and 0.5 mg mL−1 oligosaccharides) and stirred for 12 h at room temperature. After filtration, the concentration for glucose or the glucose plus oligosaccharides in the filtrate could be directly estimated based on the DNS method above described and subjected to the calculation of adsorption amount. Here, the hydrolysis solution was obtained from the following hydrolysis conditions: 0.2 g catalyst (BC-SO3H), 0.4 g cellulose, water, 3.0 mL, microwave power, 350 W, temperature, 90 °C, time, 2 h. The resulting hydrolysis solution was diluted to 10 mL and its glucose and oligosaccharides concentrations were measured via on a Shimazu 20AT HPLC equipped with ODS C18 column (Spherigel) and refractive index detector (ERC-7571A).
Results and discussion
Characterization of catalysts
The main functional groups of three catalysts BC-SO3H, BC-SO3H-IL and BC-SO3H-IL-Zn were measured by chemical titration before use and after the 4th operating run. It can be seen from Table 1 that the densities of SO3H groups on the fresh BC-SO3H-IL-Zn and BC-SO3H-IL were nearly equal to each other (1.23 and 1.28 mmol g−1), but obviously lower than that on the fresh BC-SO3H (1.80 mmol g−1). We have proven that the IL groups are mainly grafted on the OH sites of BC-SO3H (Zhang et al. 2012). It is probable that the silanization and then acidification processes for the preparation of both the IL-containing catalysts easily lead to the leaching of SO3H groups from the parent BC-SO3H. Secondly, the loading of IL-Zn groups on BC-SO3H-IL-Zn was obviously higher than that of IL groups on BC-SO3H-IL, thus implying that the IL-Zn groups are grafted on the OH sites of BC-SO3H via a silanization more easily than the IL groups. Thirdly, the content of Zn ions on BC-SO3H-IL-Zn was slightly higher than that of the corresponding IL groups, illustrating that the chlorozincate bonded to the IL group perhaps exists in the mixed form of ZnCl3 − and Zn2Cl5 − ions. Notably, after the three catalysts were used repeatedly for 3 times in cellulose hydrolysis (the results of recycling experiments will be described later), the leaching of SO3H groups occurred easily on the BC-SO3H, while such a leaching was almost negligible upon the two catalysts BC-SO3H-IL and BC-SO3H-IL-Zn, illustrating that the introduction of IL or IL-Zn groups can improve significantly the stability of SO3H groups. In the experiments of using three fresh catalysts to absorb a glucose solution (1.00 mg L−1) and a hydrolytic solution of cellulose (consisting of soluble glucose (1.10 mg L−1) and oligosaccharides (0.50 mg mL−1), respectively), we found that these catalysts possessed a poor adsorption capacity for glucose (adsorption value, 3.00–3.25 mg g−1), but a very strong adsorption capacity for the hydrolysis solution (25.44–54.40 mg g−1), illustrating that they can selectively adsorb the oligosaccharides in the solution, as previously reported by Suganuma et al. (2008) and our groups (Wu et al. 2010). This should be due a hydrogen bond interaction of the catalysts with the β-1,4 glycosidic bonds of oligosaccharides. Furthermore, the adsorption capacity followed an increasing sequence of BC-SO3H-IL-Zn > BC-SO3H-IL > BC-SO3H. Our adsorption experiments clearly indicate that the introduction of IL and especially IL-Zn groups into BC-SO3H material can improve significantly such a hydrogen bond interaction, which is likely due to their Cl− ions being easily inserted into the hydrogen bonding network of cellulose molecules. Additionally, a high concentration of Cl− ions and a coordinated effect of Zn2+ ions with oligosaccharides (Cao et al. 1994) are likely responsible for the ultra-high adsorption capacity on BC-SO3H-IL-Zn.
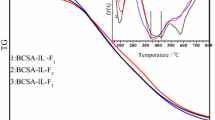
Thermal stability of the aforementioned materials was checked by thermogravimetric analysis (TGA) technique and their TGA and differential thermogravimetry (DTG) curves are shown in Fig. 1. Three main peaks for loss of weight in the range 25–800 °C were observed in the DTG curves of these materials. Among them, the first strong peak at 90–100 °C should arise from an evaporation of the adsorbed water molecules, the second weak peak in the range 240–310 °C can likely be assigned to a decomposition of the surface’s SO3H groups (Wu et al. 2010; Zhang et al. 2012; Xu et al. 2008) and the third broad peak appearing in the range 470–600 °C may be attributed to the further carbonization of these materials (Wu et al. 2009). When the IL or IL-Zn groups were anchored to the BC-SO3H material, a new peak near 350–370 °C was noticed upon the DTG curves of both the materials (see curves 2 and 3), which was likely due to the decomposition process of IL or IL-Zn groups anchored on the OH sites. We previously reported that the introduction of IL into the BC-SO3H could cause both the decomposition peaks for SO3H and IL groups to be shifted to the high temperature region and partly overlap each other (Zhang et al. 2012). Here, the decomposition peaks for SO3H and IL-Zn groups in the DTG curve of BC-SO3H-IL-Zn appeared at 310 and 370 °C, respectively, showing much bigger shifts toward the high temperature region than both corresponding peaks in the DTG curve of BC-SO3H-IL. We believe that an intramolecular electrostatic bonding interaction between SO3H and IL or IL-Zn groups should be responsible for the thermal stability of both the groups. In comparison with the IL group, the IL-Zn group can strengthen such an interaction due to its high concentration of Cl− ions, as supported by the foregoing chemical titration.
Hydrolysis of cellulose and lignocellulose
The hydrolysis of cellulose and lignocellulose (bamboo powder) catalyzed by BC-SO3H, BC-SO3H-IL or BC-SO3H-IL-Zn in water, [BMIM]Cl-water or DMA-LiCl-water was checked under microwave irradiation and the results are listed in Table 2. The heterogeneous hydrolysis process for cellulose in a water medium could efficiently proceed over these catalysts, giving RSs (yield, 22.0–58.7 %) as main hydrolysis products with turnover numbers (TONs) of 1.51–5.91. In addition, a trace amount of HMF (usually lower than 0.2 %) was also observed. In comparison with a pure water medium, the mixed media [BMIM]Cl-water promoted significantly the hydrolysis of cellulose, providing relatively higher TONs (1.69–6.30). This is likely due to a good solubility of [BMIM]Cl for cellulose (Swatloski et al. 2002; Lee et al. 2009; Fort et al. 2007; Fukaya et al. 2008; Ohno and Fukaya 2009; Amarasekara and Owereh 2009). Another mixed media DMA-LiCl-water were found to be less advantageous for this hydrolysis, giving relatively lower TONs (0.48–3.32).
In comparison with cellulose hydrolysis, the direct hydrolysis of bamboo in a pure water medium did not easily proceed, providing very low TONs (0.15–1.77) even if the high MW power (750 W) and temperature (110 °C) were used; this is likely due to the impediment of the lignin which was wrapped on the cellulose. The mixed media DMA-LiCl-water and especially [BMIM]Cl-water could promote obviously the hydrolysis of bamboo, providing relatively high TONs (0.45–2.84 in DMA-LiCl-water and 0.55–3.10 in [BMIM]Cl-water), being due to both the mixed media possessing a good solubility for lignocelluloses (Joseph and Ronald 2009; Vancov et al. 2012; Guo et al. 2012). Additionally, such two mixed media showed an obviously promoted effect on the further transformation of RSs into HMF in the hydrolysis of cellulose and lignocellulose. Notably, a two-steps process, which bamboo was pretreated with an 40 % aqueous ZnCl2 solution and then hydrolyzed over BC-SO3H or BC-SO3H-IL under microwave (MW) irradiation, could significantly accelerate the hydrolysis of bamboo, providing a high hydrolysis efficiency (TON, 1.52). This should be due to such accessional pretreatment efficiently removing the lignin in bamboo, as previously reported by us (Wu et al. 2012). When a catalytic amount of ZnCl2 was introduced into the hydrolysis system of bamboo, the BC-SO3H-IL’s TON (0.84) was improved obviously, but the BC-SO3H’s TON unchanged nearly. Interestingly, if ZnCl2 was anchored on the IL sites of BC-SO3H-IL to form a monolithic catalyst BC-SO3H-IL-Zn, the catalysis efficiency of such a catalyst bearing -SO3H and IL-Zn groups in all the solvents was found to be obviously superior to that of BC-SO3H-IL and especially BC-SO3H, whether cellulose or bamboo powder was used as a substrate and this predominance in activity was more strikingly apparent in a water medium. For example, the TONs of BC-SO3H-IL-Zn for cellulose and bamboo hydrolysis in water were ca. 5.91 and 1.77, being 0.83- and 2.85-fold higher than the corresponding TONs of BC-SO3H-IL, respectively. Furthermore, The TON of BC-SO3H-IL-Zn was also slightly higher than that the two-steps process described above, and by far better than that in the co-presence of BC-SO3H-IL and ZnCl2. This is likely due to a good synergistic effect between the IL-Zn and SO3H groups joined to the BC-SO3H, as supported by the aforementioned TGA characterization. Additionally, the BC-SO3H-IL-Zn was found to show much higher selectivity for HMF in a water medium and especially two mixed media than the other two catalysts, which was probably due to the catalytic interaction of its Lewis acids (Rasrendra et al. 2010).
The effects of microwave temperature and irradiation time on the heterogeneously catalytic hydrolysis of cellulose in a pure water medium were further investigated with 350 W of MW power and the results are summarized in Figs. 2 and 3, respectively. It can be seen in Fig. 2 that the yield of RSs increased gradually with the increase in temperature in the range of 70–90 °C, but decreased when the temperature was further increased to 100 °C, as a consequence of RSs to be further converted into HMF and other smaller molecules. Among the three catalysts examined, the catalyst BC-SO3H-IL-Zn showed the best activity for this hydrolysis, and this predominance in activity became more noticeable at a relatively low temperature (70 °C). For example, when the temperature was 90 °C, 58.7 % of RSs yield was obtained on BC-SO3H-IL-Zn, this yield being 0.76- and 2.67-fold higher than the corresponding yields on BC-SO3H-IL and BC-SO3H, respectively. When the temperature was 70 °C, the yield on BC-SO3H-IL-Zn was ca. 28.5 %, being 2.7- and −6.5 fold higher than yields on BC-SO3H-IL and BC-SO3H, respectively. On the other hand, at the high temperature (100 °C), the BC-SO3H-IL-Zn, unlike BC-SO3H-IL, showed a high activity for the further transformation of RSs to HMF (about 1 %), followed by the conversion of HMF into levulinic acid and formic acid (about 10 %), as well as other smaller molecules (no detection), this is probably due to the existence of its Lewis acid. Figure 3 illustrates that the curve of RSs yield upon BC-SO3H (curve 1) climbed sharply with time until it achieved a maximum at 60 min, after which it fell gradually due to serious degradation of RSs. On the contrary, an increase of RSs yield with time was monotonic upon the IL-or IL-Zn-containing catalysts although this increasing trend was weakened clearly after a relatively long time (curves 2 and 3), indicating that when relatively mild hydrolysis conditions were used, the degradation of RSs could be restrained efficiently in the presence of IL or IL-Zn groups.
Based on the relatively high selectivity for HMF in DMA-LiCl and water, we investigated the effect of microwave power on the BC-SO3H-IL-Zn-catalyzed hydrolysis of cellulose in this mixed media at 100 °C for 120 min. As shown in Fig. 4, when MW power was elevated from 350 to 750 W, the yield of RSs gradually decreased from 19.8 to 12.0 %, with a concomitant increase in HMF from 22.2 to 30.4 %. This is probably because a high MW power produces a stronger and more direct coupling of MW energy with the molecules in reaction system than a low MW power (Dallinger and Kappe 2007), easily leading to the further transformation of RSs into HMF.
The effect of irradiation temperature and time on bamboo hydrolysis was also examined in water using the best catalyst BC-SO3H-IL-Zn as an example and the results are shown in Fig. 5. Within the examined range of temperature, the accelerating effect of temperature on this reaction was evident, and the yield of RSs and HMF gradually increased with increasing temperature from 100 to 110 °C and time from 20 to 150 min. However, a further prolonging of time at the high temperature (110 °C) slightly lowered the yield of RSs, as a consequence of the RSs to be further converted into smaller molecules. The reaction solid mixture was easily spattered to the reactor’s internal wall at the temperature higher than 110 °C, which can seriously influence the hydrolysis efficiency. Therefore, the temperature higher than 110 °C was not checked here.
The results obtained from Table 2 and Figs. 2, 3, 4 and 5 fully indicate that the catalyst BC-SO3H-IL-Zn show much higher hydrolysis efficiency than BC-SO3H-IL and especially BC-SO3H whether cellulose or lignocellulose was used as a substrate. This is likely due to the following reasons: firstly, ZnCl2, as supported by our designed experiment in which about a half of the lignin was removed from the bamboo treated with 40 % aqueous ZnCl2 solution under MW irradiation (Wu et al. 2012), possesses a functionality of efficiently removing the lignin which is wrapped around cellulose, and its introduction can improve significantly the catalyst’s activity for bamboo hydrolysis. Secondly, the IL-Zn groups flexibly bound to BC-SO3H, like the CBD of cellulase, possess a good solubility and activated capacity for cellulose molecules via their Cl− ions efficiently breaking the hydrogen bonding network of cellulose molecules. Thirdly, their Cl− ions probably share the H+ ions of the SO3H groups (with a CD function of cellulase) through a strongly intramolecular electrostatic bonding interaction, which can lead to a good synergistic effect between the IL-Zn and SO3H groups, as well as the improved acidity. Furthermore, the IL-Zn groups with a high concentration of Cl− ions can bestow the catalyst with a more excellent functionality mimicking the CBD of cellulase, a better synergistic effect and improved acidity compared to the IL groups, as supported by the aforementioned characterization and reaction results.
Finally, the catalyst’s repeatability in MW-assisted hydrolysis of cellulose and lignocellulose medium was checked in water. After the first reaction was carried out under the optimum conditions, the solid residue containing the catalyst and substrate was separated and recovered from the hydrolytic solution via filtering and washing with water. After some cellulose or bamboo was supplemented, the ground residue was used directly for the next hydrolytic reaction under the same conditions. Figure 6 a shows the repeatability of three catalysts in cellulose hydrolysis (reaction conditions: 350 W and 60 min at 90 °C for BC-SO3H, 350 W and 120 min at 90 °C for BC-SO3H-IL and BC-SO3H-IL-Zn). The yield of RSs upon the catalyst BC-SO3H decreased gradually with recycling times, indicating that the gradual loss of activity occurs easily upon this catalyst. On the contrary, the yield of RSs upon BC-SO3H-IL or BC-SO3H-IL-Zn hardly decreased in each recycling run, indicating that both the catalysts have an excellent repeatability. We measured the content of SO3H, IL groups and Zn ions on the three catalysts before use and after the 3rd recycling run, and found that the leaching of surface SO3H groups on BC-SO3H was very serious after the 3rd operating run (see Table 1, 1.80 vs. 0.78 mmol g−1). However, this leaching could be severely restrained in the presence of IL or especially IL-Zn groups (1.28 vs. 1.16 mmol g−1 for the BC-SO3H-IL, 1.23 vs. 1.21 mmol g−1 for BC-SO3H-IL-Zn). The leaching of IL groups or Zn ions upon both the catalysts was almost negligible (IL groups for BC-SO3H-IL, 1.27 vs. 1.31 mmol g−1; Zn ions for BC-SO3H-IL-Zn, 2.38 vs. 2.03 mmol g−1). Evidently, the poor repeatability of BC-SO3H should be due to a serious leaching of its surface SO3H groups under microwave irradiation. A strongly intramolecular electrostatic bonding interaction between IL or IL-Zn and SO3H groups is responsible for the excellent repeatability of both the groups.
Figure 6b illustrates the repeatability of BC-SO3H-IL-Zn in bamboo hydrolysis (reaction conditions: 750 W and 120 min at 110 °C). As shown in Fig. 6b, the yields of RSs and HMF upon BC-SO3H-IL-Zn had a definite decrease in the 1st recycling run, but hardly decreased in the following 2nd to 3rd recycling run, illustrating that the catalyst still has a good repeatability for bamboo hydrolysis. The measured content of SO3H groups and Zn2+ ions on the catalyst after the 3rd recycling run was slightly lower than that on the fresh catalyst (1.02 vs. 1.23 mmol g−1 for SO3H groups and 2.03 vs. 2.38 mmol g−1 for Zn ions), indicating that the leaching of both the functional groups in bamboo hydrolysis occurs more easily than that in cellulose hydrolysis due to its harsh reaction conditions (high MW power and temperature).
Conclusion
In summary, for the first time we have designed and prepared chlorozincate ionic liquid functionalized biochar sulfonic acid (BC-SO3H-IL-Zn) as an efficient catalyst for the heterogeneous hydrolysis of cellulose and lignocelluloses to reducing sugars and HMF under microwave irradiation. The new catalyst has the following merits: (1) Using more available and cost-effective lignocellulosic bamboo as a raw material of the preparation of the catalyst. (2) The introduction of IL-Zn groups bestows the catalyst with more excellent mimetic CBD and CD functions of cellulose, as well as a delignification function compared to that of IL groups, which can improve significantly the activity and repeatability of the catalyst. (3) Facile operation and an environmentally benign process. It is anticipated that this kind of catalyst will have competitive potential for direct and highly efficient conversion of lignocellulosic materials into useful sugars and other chemicals if its delignification function is further improved.
References
Amarasekara AS, Owereh OS (2009) Hydrolysis and decomposition of cellulose in Brønsted acidic ionic liquids under mild conditions. Ind Eng Chem Res 48:10152–10155
Bobleter O (1994) Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19:797–841
Broll D, Kaul C, Kramer A, Krammer P, Richter T, Jung M, Vogel H, Zehner P (1999) Chemistry in supercritical water. Angew Chem Int Ed 38(20):2998–3014
Calvini P, Gorassini A, Merlani AL (2008) On the kinetics of cellulose degradation: looking beyond the pseudo zero order rate equation. Cellulose 15:193–203
Cao NJ, Xu Q, Chen CS, Gong CS, Chen LF (1994) Cellulose hydrolysis using zinc chloride as a solvent and catalyst. Appl Biochem Biotechnol Spring 45–46:521–530
Dallinger D, Kappe CO (2007) Microwave-assisted synthesis in water as solvent. Chem Rev 107:2563–2591
Deguchi S, Tsujii K, Horikoshi K (2006) Cooking cellulose in hot and compressed water. Chem Commun 31:3293–3295
Fan LT, Gharpuray MM, Lee YH (1987) Cellulose hydrolysis. Biotechnology Monograph. Springer, Berlin 57
Fort DA, Remsing RC, Swatloski MP, Moyna G, Rogers RD (2007) Can ionic liquids dissolve wood processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem 9(1):63–69
Fukaya Y, Hayashi K, Wada M, Ohno H (2008) Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions. Green Chem 10:44–46
Gao B (2003) China. Adv Nat Sci 13(1):21–28
Guo F, Fang Z, Xu CC, Smith JRL (2012) Solid acid mediated hydrolysis of biomass for producing biofuels. Prog Energy Combust Sci 38:672–690
Hamelinck CN, Hooijdonk GV, Faaij APC (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 28:384–410
Joseph BB, Ronald TR (2009) Biomass-derived platform chemicals: thermodynamic studies on the conversion of 5-hydroxymethylfurfural into bulk Intermediates. J Am Chem Soc 131(5):1979–1985
Kontturi E, Vuorinen T (2009) Indirect evidence of supramolecular changes within cellulose microfibrils of chemical pulp fibers upon drying. Cellulose 16:65–74
Lee SH, Doherty TV, Linhardt RJ, Dordick JS (2009) Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol Bioeng 102(5):1368–1376
Li CZ, Zhao ZBK (2007) Efficient acid-catalyzed hydrolysis of cellulose in ionic liquid. Adv Synth Catal 349:1847–1850
Li CZ, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem 10:177–182
Mbaraka IK, Lin VS-Y, Shanks BH (2003) Organosulfonic acid-functionalized mesoporous silicas for the esterification of fatty acid. J Catal 219:329–336
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mok WS, Antal MJ, Varhergyi G (1992) Productive and parasitic pathways in dilute acid-catalyzed hydrolysis of cellulose. Ind Eng Chem Res 31:94–100
Ohno H, Fukaya Y (2009) Task specific ionic liquids for cellulose technology. Chem Lett 38:2–7
Onda A, Ochi T, Yanagisawa K (2008) Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem 10:1033–1037
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Rasrendra CB, Makertihartha IGBN, Adisasmito S, Heeres HJ (2010) The catalytic conversion of d-glucose to 5-hydroxymethylfurfural in DMSO using metal salts. Top Catal 53:1241–1247
Saka S, Ueno T (1999) Chemical conversion of various celluloses to glucose and its derivatives in supercritical water. Cellulose 6:177–191
Sasaki M, Fang Zhen (2000) Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Ind Eng Chem Res 39(8):2883–2890
Suganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, Hayashi S, Hara M (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc 130:12787–12793
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124:4974–4975
Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2005) Biodiesel made with sugar catalyst. Nature 438:178
Vancov T, Alston A-S, Brown T, McIntosh S (2012) Use of ionic liquids in converting lignocellulosic material to biofuels. Renew Energy 45:1–6
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in new ionic liquid. Biomacromolecules 5:266–268
Wu R, Wang TH, Xiu ZL, Guo F, Pan YQ, Yin JZ (2009) Preparation of a biomass carbon-based solid acid catalyst. Chin J Catal 12:1203–1208
Wu YY, Fu ZH, Yin DL, Xu Q, Liu FL, Lu CL, Mao LQ (2010) Microwave assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem 12:696–700
Wu YY, Zhang C, Liu YC, Fu ZH, Dai BH, Yin DL (2012) Biomass char sulfonic acids (BC-SO3H)-catalyzed hydrolysis of bamboo under microwave irradiation. BioResources 7(4):5950–5959
Xu Q, Yang ZG, Yin DL, Zhang F (2008) Synthesis of chalcones catalyzed by a novel solid sulfonic acid from bamboo. Catal Commun 9:1579–1582
Yang B, Willies DM, Wyman CE (2006) Changes in the enzymatic hydrolysis rate of Avicel cellulose with conversion. Biotechnol Bioeng 94:1122–1128
Zhang YHPJ (2008) Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. Ind Microbiol Biotechnol 35:367–375
Zhang YHP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: non-complexed cellulase systems. Biotechnol Bioeng 88:797–824
Zhang ZH, Zhao ZK (2009) Solid acid and microwave-assisted hydrolysis of cellulose in ionic liquid. Carbohyd Res 344:2069–2072
Zhang C, Fu ZH, Liu YC, Dai BH, Zou YH, Gong XL, Wang YL, Deng XL, Wu HT, Xu Q, Steven KR, Yin DL (2012) Ionic liquid-functionalized biochar sulfonic acid as a biomimetic catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Green Chem 14:1928–1934
Acknowledgments
We acknowledge the financial support for this work by the Specialized Research Fund for the Doctoral Program of Higher Education (20124306110005), the Innovation Platform Open Fund of Hunan College (11K044), the National Natural Science Fund of China (20873040, 21003043), the Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, the 100 Talents Program of Hunan Province, the Natural Science Fund of Hunan Province (10JJ2007) and the Hunan Provincial Innovation Foundation for Postgraduate of China (CX2012B208).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, C., Fu, Z., Dai, B. et al. Biochar sulfonic acid immobilized chlorozincate ionic liquid: an efficiently biomimetic and reusable catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Cellulose 21, 1227–1237 (2014). https://doi.org/10.1007/s10570-014-0167-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0167-9