Abstract
This work investigates the flammability and antibacterial activity of cotton fabrics treated with DNA and chitosan, which have been deposited either by exploiting impregnation methods (building up two-layer structures or a single mix of the two components) or by the layer-by-layer approach (30 alternating chitosan-DNA bilayers). All the treated fabrics have been subjected to UV-curing and then washed. FTIR and SEM analyses have been employed for assessing the influence of the deposition method and the interactions of the biomacromolecules with the cellulosic substrate. Thermogravimetric analyses, flammability and cone calorimetry tests have been performed for evaluating the thermal stability and the fire behavior of the treated fabrics. The fire performances of the UV-cured and washed fabrics, and the antibacterial activity towards Staphylococcus aureus as well, have been found to be strictly correlated with the deposition method and hence with the presence of DNA or chitosan as the external layer. More specifically, the impregnation of the fabrics with a mixture of DNA and chitosan solution provided good stability in water and enhanced fire retardant properties, but very limited antibacterial activity. The layer-by-layer chitosan-DNA UV-cured architectures have shown the best overall behavior, i.e. enhanced water resistance, self-extinction in flammability tests and good antimicrobial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is today the most frequently used textile fiber in the world. Its current market share is 56 % of all fibers used for apparel and home furnishings. Nonwoven textiles and personal care items represent another contribution (USDA 2016).
Despite its appreciable characteristics, cotton is highly flammable and it burns readily and completely. As clearly described in the literature (Alongi and Malucelli 2015; Horrocks 2001; Horrocks et al. 1996; Low and Morterra 1985; Morterra and Low 1984, 1985a, b; Morterra et al. 1984; Price et al. 1997; Shafizadeh et al. 1985; Shafizadeh and Bradbury 1979), the thermal degradation of cotton (cellulose) occurs at 300–400 °C through two competitive processes, namely depolymerization and dehydration: this latter is initiated by the scission of acetal bonds between the chain glycosidic units, followed by splitting of volatile levoglucosan (the cyclic monomer of cellulose) from ensuing chain ends. Competing dehydration reactions promote the formation of thermally stable aliphatic structures (char I), which subsequently are converted into aromatic structures (char II), with water, methane, carbon monoxide and carbon dioxide formation (400–600 °C). Char II (ca. 18 %) is thermally stable at least up to 800 °C.
Nowadays, the most frequently used flame retardants for cotton fabrics are Proban® and Pyrovatex®, two phosphorous-based finishes (Horrocks et al. 2005; Horrocks 2011). The good water fastness of Proban® is due to the deposition of the chemical within the fibers by a construction of a polymer network during the heating process. Proban® is therefore not bound to the fabrics and is mechanically kept within the fiber interstices; one of the major drawbacks of the use of this flame retardant treatment refers to the possible release of formaldehyde during the fabric use (Muthu 2015).
Conversely, a conventional pad-dry-cure process can be exploited for applying N-methylol phosphonopropionamide derivatives (Pyrovatex®) to textiles: unlike Proban®, the required durability is ensured by the use of a methylolated crosslinking agent, which is responsible for the formation of covalent bonds with cellulose hydroxyl groups. However, about 50 % has been reported to be lost during the first laundry occasion, due to unreacted FRs, although it remains stably bound thereafter. The proportion of the FR applied in Pyrovatex treatment was found to remain at about 50 %, regardless of its concentration, in the treated material over a range of 8-48 % (Horrocks and Anand 2000).
The efficiency of phosphorus-based compounds can be further enhanced by the addition of some Nitrogen-containing additives, and this may give rise to a synergistic phosphorus-nitrogen action (Gaan and Sun 2009).
Nowadays, both scientific community and industry are seeking for renewable, bio-based or biodegradable chemicals and low temperature and energy saving processes.
In this context, preliminary results on the use of biomacromolecules as flame retardants have shown that proteins (e.g. caseins and whey proteins) and DNA (Alongi et al. 2013a, 2014) can be effectively applied to cotton fabrics. In particular, it has been found that LOI significantly increases, burning rates slow down and, in the case of DNA-treated fabrics, self-extinction of the flame can be achieved (Malucelli et al. 2014).
Besides, DNA has been successfully coupled with chitosan in layer by layer (LbL) structures with a good enhancement of the flame retardant properties and of the thermal stability of cotton (Carosio et al. 2013). Despite of these improvements, the durability (i.e. the resistance to washing treatments) of the assemblies was not assessed.
The flame retardant effect of these renewable products can be ascribed to the high phosphorus and nitrogen contents, which favor the formation of a stable protective char (Alongi et al. 2013a, b; Malucelli et al. 2014).
Pursuing this research, the effect of different DNA add-ons on the flammability of cotton, the resistance of treated cotton fabrics to an irradiating heat flux, and the correlation between some process parameters and morphology of coatings with the resulting flame retardant properties of the treated fabrics have been thoroughly investigated (Bosco et al. 2015). However, the washing fastness of the FR treatment still remains a challenge, as the biomacromolecule coating is dissolved in the washing medium, hence losing any FR effect (Malucelli et al. 2014).
Therefore, a chemical cross-linking strategy, which would bind DNA to the fabric or create an insoluble biomacromolecule complex could help to overcome this issue.
Chitosan (2-amino-2-deoxy-(1 → 4)-β-d-glucopyranan) is a biopolymer that could be a good candidate for this purpose. It derives from the deacetylation of the chitin component of the shells of insects and crustaceans. This biopolymer can easily be obtained from food industry wastes, at a relatively low cost (Crini 2005). It boasts various positive features, such as biodegradability, biocompatibility and low toxicity. It also shows high chemical reactivity and chelation and adsorption capacities that promote its use not only in wastewater treatments, but also in the textile, food, agricultural, cosmetic, pharmaceutical and medical fields (Kumar 2000).
The application of chitosan in the textile field, as a finishing agent, has been reported extensively in literature, mainly to confer antibacterial activity and to improve dyeability, anti-creasing and anti-shrinkage and durable press properties (Thakur and Thakur 2014; Mural et al. 2016; Thakur and Voicu 2016; Croisier and Jérôme 2013).
The antimicrobial activity of chitosan involves the damage of the microorganism structure, which causes the leakage of intracellular components and cell death. Although the specific mechanism of action is still unknown, the antibacterial activity is usually ascribed to the electrostatic interactions between the cationic structure of chitosan and the anionic cell surface (Helander et al. 2001). Chitosan has traditionally been applied to textiles by thermal wet processes involving glutaraldehyde as a crosslinking agent. A novel, eco-friendly approach was proposed by Ferrero and Periolatto (Ferrero et al. 2014; Periolatto et al. 2012; Periolatto and Ferrero 2013): in particular, chitosan was closely bound to cellulosic fibers by means of photografting. This approach exploits the impregnation of cotton with a chitosan solution, to which a radical photoinitiator has been added; this way, the treated fabric, dried and exposed to UV radiation, becomes water-resistant. The washing fastness of chitosan UV-grafted on cotton has already been confirmed by assessing the antimicrobial activity after several washing cycles: the strong antibacterial activity that was observed and maintained after 30 washing cycles, confirmed the efficiency of the UV grafting (Ferrero et al. 2015).
In a recent work, chitosan and ammonium polyphosphate (a common flame retardant system for cellulosic substrates) have been permanently fixed to cotton fabrics by adding a third UV-curable reactive component (i.e. an aliphatic acrylic polyurethane latex), in the presence of a suitable radical photoinitiator (Carosio and Alongi 2015). Conversely, the present work exploits UV-grafted chitosan as specific crosslinking agent, in order to fix DNA to cotton fabrics in a stable way: thus, by using this new approach, it may be possible to combine, in a durable coating, the flame retardant features provided by DNA with the antibacterial activity derived from chitosan.
The molecular structures of chitosan and DNA are reported in Fig. 1a, b. Chitosan contains several amino groups, which, at acidic pH, may undergo protonation. For this reason, it can establish electrostatic interactions with the negatively charged DNA to form complexes (polyplexes). The combination of chitosan and DNA has been studied above all in molecular biology and medical research for applications in gene delivery (Mao et al. 2001; Erbacher et al. 1998), or as films and membranes for biomedical applications (Rikimaru et al. 2003; Kawazoe et al. 2008). The electrostatic interactions can depend on the pH of the medium, the charge ratio (Liu et al. 2005; Strand et al. 2005), the chitosan molecular weight (Morán et al. 2009) and the degree of deacetylation (DA) of chitosan (Kiang et al. 2004; Huang et al. 2005). The best results, in terms of binding between chitosan and DNA, were obtained with pH values lower than chitosan pKa (6.5) (Liu et al. 2005), a high DA and using high molecular weight chitosan (Kiang et al. 2004). The effect of the charge ratio is modified by the above-mentioned properties, but a higher amount of positive charges to negative ones is always preferable for the complete binding of DNA.
In the present study, many approaches have been considered. First, release tests have been carried out on DNA and chitosan films, deposited as two separate layers or as a single mix on a glass slide and then photocured. On the basis of the obtained results, the same procedure has been applied on cotton fabrics. In this case, the release tests have been followed by flammability tests in horizontal configuration, thermogravimetric analyses and cone calorimetry tests. The influence of pH of the solutions, chitosan and DNA add-on and application parameters (e.g. mixed solutions, sequential application, layer by layer structuration) has been thoroughly investigated. Finally, antibacterial activity tests have been performed using Staphylococcus aureus ATCC 6538.
Materials and methods
Materials
Cotton fabrics (COT, 200 g/m2) were purchased from Fratelli Ballesio S.r.l. (Torino, Italy) and cut into 50 × 50 mm2 and 100 × 100 mm2 squares.
DNA powder from herring sperm (#D3159, size range 100–200 bp) was purchased from Sigma–Aldrich S.r.l. (Milano, Italy) and stored at 4 °C before use.
Chitosan was a pure low viscous product (Sigma Aldrich S.r.l., Milano, Italy), with 75–85 % deacetylation degree. Irgacure 1173 (BASF Corporation, Florham Park, NJ, USA) was used as radical photoinitiator (Fig. 1c) at 4 wt% with respect to chitosan.
Preparation of the solutions
Three different herring sperm DNA solutions (namely, 0.5, 2.5 and 8 wt%) were prepared by slowly dissolving the powder in distilled water under magnetic stirring (300 rpm) for 2 h at room temperature. The pH of the solutions at 2.5 wt% was corrected to 4 and 8; the pH of the solutions at 8 wt% was corrected to 4. Finally, only for layer by layer treatments, the pH of the DNA solutions at 0.5 wt% was corrected to 7, as reported elsewhere (Carosio et al. 2013). NaOH was used in all cases.
Chitosan was dissolved in aqueous solution containing 2 vol% acetic acid (Sigma Aldrich S.r.l., Milano, Italy) under magnetic stirring at room temperature for 24 h, at two concentrations (2 and 5 wt%) at pH 4; a further chitosan solution (0.5 wt%, pH 3.5) was prepared and used in the layer by layer treatments (Carosio et al. 2013).
The photoinitiator was added to the chitosan solution at 4 wt% with respect to chitosan.
The DNA solution (8 wt%, pH 4) and the solution of chitosan (2 wt%)-photoinitiator were mixed in a volume ratio of 1:1 and used immediately for the fabric treatment.
Samples preparation
Samples were prepared on glass slides and on cotton fabric squares used as substrates.
Glass slide samples
Samples were first prepared in the form of a film deposited on a glass slide.
Appropriate amounts of DNA (2.5 wt% at pH 4 or 8) and chitosan (5 wt%) solutions were homogeneously spread over the glass slide using a glass rod to obtain a micrometric layer.
A sample film with the same amount of DNA solution, which was just dried, and a second one made up of chitosan solution, which was both dried and UV-cured, were prepared as control samples.
The samples were prepared as follows:
-
1.
A first layer of chitosan solution (5 wt%) (in the presence of the photoinitiator) was spread over the glass substrate and dried, and it was then covered with the DNA solution at pH 4 or 8 and dried again. The obtained films were labeled as GLASS-CHI-DNApH4 and GLASS-CHI-DNApH8, respectively;
-
2.
The same procedure as (1) was used, but the chitosan and DNA layers were reversed. The obtained films were labeled as GLASS-DNApH4-CHI and GLASS-DNApH8-CHI;
-
3.
A chitosan solution (2 wt%) in the presence of Irgacure 1173 (4 wt%) was mixed with the DNA solution at pH 4 (1:1 volume ratio) and spread over the glass, and it was then dried. The obtained film was coded as Mix DNApH4-CHI.
Finally, all the obtained samples were dried in an oven at 100 °C for 15 min.
Cotton fabric samples
Chitosan and DNA were deposited on the fabrics by impregnation.
The DNA coating was prepared by dipping the cotton squares into the DNA solutions for 1 min at room temperature. During the impregnation step, a rocker–shaker was used to keep the solution moving. Multiple impregnations were adopted to achieve the desired final dry add-on value. After each step, the fabrics were dried in an oven at 100 °C for 15 min.
The chitosan coating was prepared by spreading, over the fabrics, an appropriate amount of the chitosan (2 wt%)-photoinitiator solution, and this was followed by drying at 100 °C for 15 min. The same procedure was used for the impregnation with the DNA and chitosan mixture.
Three types of samples were prepared: cotton fabrics impregnated with a chitosan solution, dried and then impregnated with a DNA solution (COT-CHI-DNApH4 samples); cotton fabrics impregnated first with a DNA solution, dried and then impregnated with a chitosan solution (COT-DNApH4-CHI); cotton fabrics impregnated with the mixture of chitosan and DNA solution and dried (Mix DNApH4-CHI). The add-on was adjusted to 2 % over the weight of fabric (owf) for chitosan (Ferrero and Periolatto 2012) and 8 % owf for DNA (Bosco et al. 2015).
The chitosan-DNA layer by layer (LbL) assembly was structured on cotton fabrics by alternate immersion into the positively charged chitosan solution (0.5 wt%, pH 3.5) and the negatively charged DNA solution (0.5 wt%, pH 7). More specifically, the first chitosan layer was obtained after an immersion time of 5 min; the subsequent layers were obtained after 1 min of immersion. The process was repeated until 30 bi-layers (BL) were built, being DNA the last layer in one case (LbL-DNA) and chitosan in the other (LbL-CHI).
Table 1 summarizes the main characteristics of the prepared samples.
The total dry add-on of the cotton samples (AO %) is the amount, in percentage, of the finishing agent added to the fabric with respect to its initial weight. This was determined by weighing each sample before (Wi) and after impregnation and the subsequent drying (Wf), using an analytical balance (Scaltec) (accuracy: ±10−4 g). The DNA and chitosan dry uptake was calculated according to the following equation:
UV-curing treatments
After impregnation and drying, all the samples (deposited on both the glass slides and cotton fabrics) were exposed to UV radiation to induce the grafting reactions.
The UV treatment was carried out using a medium pressure mercury lamp, with a light intensity of about 60 mW/cm2, in a small box equipped with a quartz window, under nitrogen atmosphere (oxygen content under 20 ppm). The required radiation dose was obtained by adjusting the distance between the sample and the lamp as well as the exposure time. The right setup was found choosing a sample distance from the lamp of about 20 cm and an irradiation time of 1 min. The cotton samples were irradiated on both sides to ensure the fabrics were completely cured.
Samples characterization
Release tests
Glass slides and treated cotton fabric samples (50 × 50 mm2) were immersed in water under low stirring at 30 and at 55 °C, respectively, for 1 h. The higher temperature was chosen as it is an average temperature in textile washing procedures. Samples of the washing water were collected and analysed at defined intervals to determine the DNA content. This was obtained by measuring of the absorbance of the samples at 260 nm (after proper dilution) with a HP 8452A diode array spectrophotometer.
The corresponding concentrations were evaluated according to the following equation (Carlos et al. 2007):
where OD260 is the absorbance value at 260 nm, DF is the dilution factor and 50 is a constant related to the fact that 50 µg/ml of DNA have an absorbance value of 1 with an optical path of 1 cm.
The fabric samples were also conditioned and weighed before and after the washing step to evaluate any variations in AO %.
All the tests were duplicated, except the ones with the DNA and chitosan mixtures, which were triplicated.
Infrared spectroscopy
The interactions between chitosan and DNA were investigated by means of Fourier Transformed Infrared (FTIR) spectroscopy carried out on biomacromolecule films embedded in KBr pellets. Not UV-cured chitosan and DNA films, as well as chitosan–DNA mixed films, both dried and UV cured, were analyzed. A Nicolet FTIR 5700 spectrophotometer was used for the measurements: each spectrum was collected by cumulating 128 scans, at a 4 cm−1 resolution and gain 8, in the 4000–600 cm−1 wavenumber range.
Surface morphology
The surface morphology of the treated fabric samples before and after the washing cycle and of the residues after flammability tests was studied using a LEO-1450VP Scanning Electron Microscope (beam voltage: 5 kV). Fabric pieces of 5 × 5 mm2 were cut and pinned up to conductive adhesive tapes and gold-metallized for the analysis.
Thermogravimetric analyses
Thermogravimetric analyses (TGA) were carried out both in nitrogen and air, from 50 to 800 °C with a heating rate of 10 °C/min, using a TA Q500 thermo balance (TA Instruments) (experimental error: ±0.5 wt%, ±1 °C). The samples (ca. 10 mg) were placed in open alumina pans and fluxed with nitrogen or air (gas flow: 60 mL min−1). T10 % (temperature, at which 10 % weight loss occurs), Tmax (temperature, at which maximum weight loss rate is achieved) and the mass of the final residues at 600 °C were evaluated.
Flammability tests
The fire behavior of all the treated and untreated cotton fabrics was assessed by flammability tests performed in a horizontal configuration. These tests were carried out by applying a methane flame (25 mm long) for 3 s to the short side of the samples (50 × 100 mm2), which were clamped to a U-shaped metallic frame tilted 45° with respect to the plane.
A total of three tests were performed on all the samples that had not exhibited self-extinction, while six tests were carried out on all the other samples. The total burning time, char length, total burning rate after flame application, calculated as the ratio between the char length and total burning time, and the final residue were evaluated. The flammability tests were conducted with the aim of mimicking the procedure described in the ASTM D4804 standard, which is usually employed for thin films, although the specimen size is different (50 × 200 mm2 in the ASTM D4804 standard).
Prior to flammability tests, all the specimens were conditioned at 23 ± 1 °C for 48 h at 50 % R.H. in a climatic chamber.
Cone calorimetry tests
The combustion behavior of square fabric samples (50 × 50 × 0.5 mm3) was investigated by cone calorimetry (Fire Testing Technology, FTT). The measurements were carried out under a 35 kW/m2 irradiative heat flow in horizontal configuration, following the procedure described elsewhere (Tata et al. 2011).
Time To Ignition (TTI, s), Total Heat Release (THR, MJ/m2g) and peak of Heat Release Rate (PHRR, kW/m2 g) were measured. The last two parameters were normalized with respect to the initial mass due to the significant difference between untreated and treated fabrics. The experiments were repeated three times for each material investigated to ensure reproducible and significant data; the experimental error was within 3 %. Prior to combustion tests, all the specimens were conditioned at 23 ± 1 °C for 48 h at 50 % R.H. in a climatic chamber.
Antibacterial tests
The antibacterial activity of all the cotton treated samples was determined on samples of about 1 g, according to ASTM E 2149-01 “Standard test method for determining the antimicrobial activity of immobilized antimicrobial agents under dynamic contact conditions” using Staphylococcus aureus ATCC 6538 as the microorganism, following the procedure detailed in a previous article (Periolatto et al. 2012).
Results and discussion
Radically photo-cured chitosan has here been proposed as a crosslinking agent to improve the water fastness of a fire-resistant DNA finishing for cotton fabrics.
In previously reported studies, the authors conducted a detailed investigation on the application of both DNA (Bosco et al. 2015) and chitosan (Ferrero and Periolatto 2012) to cotton fabrics as finishing agents.
Promising results were obtained, in terms of fire resistance, with low molecular weight DNA from herring sperm applied to cotton by means of wet impregnation, an add on of 8 % was reached. Unfortunately, the treatment was not waterproof and all the DNA was washed off after rinsing at room temperature for 10 min.
Chitosan was applied to cotton fabrics, by means of UV-grafting, to confer antimicrobial properties and resistance to washing, as proved by the remaining antimicrobial activity that was found after 30 washing cycles (Ferrero et al. 2015).
Release tests
Glass slide samples
In a preliminary series of experiments, the finishing agents were applied to glass slides in the form of films, in order to specifically focus the attention on the interactions between chitosan and DNA molecules and on the effect of the UV treatment.
A close interaction between chitosan and DNA was already observed during samples preparation. When the DNA solution was spread over the dried chitosan layer, the latter swelled and all the water was absorbed. The swelling process was much slower for the DNA at pH 8 than DNA at pH 4 and the absorption of water was incomplete. When the DNA was the first layer on the glass slide, the swelling again occurred, but in a more unstable way: in fact, when chitosan was added, the DNA layer was almost detached from the glass.
The results of the release test are reported in Fig. 2 and Table 2: it can be observed that the pure DNA film released the greatest amount of DNA (almost 100 %) after only 5 min.
The behavior of the layered films is reported in the same figure and the release curves are located somewhere in between those of the control samples. In all the cases, a release peak was registered during the first 5–10 min of the test. This is probably due to the release of all the DNA molecules that were not in direct contact with the chitosan film, i.e. not interacting with the chitosan layer.
A stable condition (i.e. a plateau) was reached after 1 h, so the corresponding release values can be considered for the discussion. In the presence of chitosan, all the samples released a lower amount of DNA than the pure DNA film, thus confirming that an interaction had occurred between the two biomacromolecules.
As far as the chitosan DNA films are concerned, the deposition of DNA solution at pH 8 increased the released amount of DNA compared to the pH 4 solution. There was an average DNA release of about 83 % for the former, while the release for the latter was about 60 % (Table 2).
The release was further lowered when chitosan was deposited under the DNA at pH 4 (GLASS-CHI-DNApH4 sample), probably because of a better mobility of the DNA molecules in the solution, which can be entrapped more easily by the chitosan molecules (54 % of DNA release instead of 66 %).
The best results were obtained when mixed chitosan and DNA solutions were used (Fig. 2): in fact, the release was limited to only 12 %. In this case, DNA was intimately mixed with chitosan in the liquid phase: as a consequence, a complex interlaced polymeric structure could have been formed in the solution, before the drying and curing steps. The final UV treatment blocked the macromolecules in a tangled morphology, so that the DNA molecules were trapped and their release was reduced significantly.
Cotton fabric samples
The results obtained from the preliminary set of experiments with the glass slides suggested that pH 4 was preferable to pH 8 for the DNA solution, and that the mixing of chitosan and DNA solutions before film spreading was a necessary step to significantly limit the DNA release after UV-curing.
For this reason, only the acidic solution of DNA was applied during the subsequent tests on cotton fabrics, while the three deposition method was maintained, as reported in the “sample preparation” section. The morphology and hydrophilicity of cotton fibers make this substrate very different from glass, indeed. The influence of the deposition method needs to be specifically investigated for this kind of substrate. In this case, chitosan and DNA molecules can partially penetrate the external layer of the fibers. The active surface, considering each single fiber of the fabric, is much higher than the smooth glass surface, and cotton fabrics are characterized by micro- and macro-porosity that can be occupied selectively by the modifiers.
As a consequence, the formation of two distinct covering layers may not be so distinct (i.e. the formation of an interphase occurs), even in the case of sequential impregnation. In addition, a grafting reaction takes place between the cotton and the chitosan molecules, which affects the mobility of the grafted polymeric chains.
The results of the release tests performed on the treated cotton fabrics are shown in Fig. 3 and Table 2: they confirm the positive effect exerted by chitosan on the DNA release, due to water contact. In this case, unlike the glass slide samples, the DNA release showed a continuous growing trend, with a final steady state after 20–30 min.
Even in this case, the mixture of the two solutions, before their application to cotton fabrics, gave the best results in terms of DNA release, namely 27 % compared to the 100 % of the control sample without chitosan.
As regards the spreading of the solution, the samples treated with the mixture were less uniform than the other fabric samples. Thus, the interactions between chitosan and DNA were more irregular, as shown by the higher standard deviations (almost doubled) in the corresponding release tests (Fig. 3).
An opposite trend, with respect to the glass slide samples, was found when the layered samples were considered. In this case, the chitosan was more effective when it was deposited on cotton after DNA. As previously mentioned, this finding could be due to the interaction between the chitosan molecules and the cotton fibers. The cotton fabrics were coated on both sides, with the formation of a “sandwich-like” structure: the DNA was inside and, therefore, the chitosan coating prevented its release. On the other hand, the chitosan molecules were hindered from coming into contact with the cellulosic fibers because of the presence of the DNA, consequently the grafting UV-induced reactions were less performing, and the anchoring was limited.
Finally, a release test was carried out on the best performing samples, i.e. cotton fabrics treated with the mix of chitosan and DNA solutions at pH 4, which were only dried and not exposed to UV radiation, in order to assess the importance of the grafting UV-induced reactions that can confer washing fastness to the finishing treatment (Fig. 3).
As shown in Table 2, the dried-only samples showed an average DNA release of 44 %, which was higher than that of the UV-cured samples (27 %) and similar to the COT-DNApH4-CHI samples (51 %).
In addition to these deposition procedures, cotton fabric samples were also treated with the LbL technique, adopting a negatively charged alkaline DNA solution, and a positively charged chitosan acid solution. In this case, after washing with distilled water, the fabric weight was unchanged, which means that there was a negligible loss of the coating elements (Table 2). The formation of an organized positive and negative monolayer structure, with the successive UV-grafting of chitosan, was found to provide stability to the coating in the presence of water. The electrostatic attraction between the opposite charged layers was strengthened by the thinness of the monolayers, which also showed the greatest effectiveness. Whether DNA or chitosan was the last monolayer, the release was not affected.
In short, the envisaged crosslinking was clearly obtained by the addition of the chitosan to the DNA solution, using a cost-effective and low environmental impact UV-grafting process.
FTIR characterization
FTIR analyses were carried out on the chitosan, the DNA and on the chitosan–DNA mixed films. The former two samples were not exposed to UV radiation, while the latter was also UV-cured, with the aim of investigating any possible molecular interactions between the two biomacromolecules.
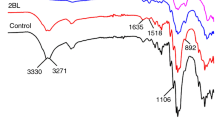
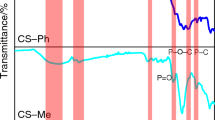
The typical bands of chitosan are located at 3360 cm−1 (OH groups), at 3290 cm−1 (NH stretching), at 1648 cm−1 (C=O) and at 1560 cm−1 and 1075 cm−1 (NH bending in amide group) (Sionkowska et al. 2006).
As far as DNA is concerned, the most informative FTIR vibrations are the guanine/thymine carbonyl stretch vibrations at 1715 cm−1, the thymine aromatic amine stretch ones at 1328 cm−1, the symmetric and asymmetric phosphate stretch ones at 1090 and 1223 cm−1, respectively and a strong coupled sugar-phosphodiester one at 970 cm−1. All these bands are traditionally taken as markers of the DNA B form and, upon complexation, their positions remain consistent with the presence of this form (Banyay et al. 2003).
As far as the obtained results reported in Fig. 4 are considered, the peaks at 965, 1095 and 1232 cm−1 are clearly visible on the spectrum of DNA alone and can be attributed to phosphate groups. These bands are not recognizable when chitosan is added to DNA, even when the samples were not exposed to UV radiation.
This finding confirms the involvement of the NH3 + groups of chitosan in the crosslinking process. As a consequence, this protonated groups were not available to interact with the microorganisms, and it can therefore be supposed that the antimicrobial activity, usually conferred by chitosan alone, was lost due to coupling with DNA.
Morphology of the treated fabrics
Figures 5, 6, and 7 show some typical structures of cotton, before and after the application of the different biomacromolecule-based architectures. As is well known, pure cotton fibers are characterized by a level of inhomogeneity due to their natural growth, as shown in Fig. 5a. When the fibers are treated with the biomacromolecules, a homogeneous and continuous coating appears on the surface. Furthermore, it also appears that, in the case of the fabric treated with Mix DNApH4-CHI, the biomacromolecule coating is present within the interstices (Fig. 6b). A similar surface morphology can be observed for LbL samples, irrespective of the sequence of the layers (Fig. 7).
As mentioned in the Materials and methods section, all the treated fabrics, after having been exposed to UV-radiation, were subjected to a washing cycle at 55 °C for 1 h. It has been noted that the morphologies of the washed fabrics are correlated to the washing resistance: in fact, the LbL architectures (i.e. LbL-DNA and LbL-CHI samples), for which DNA is not released after washing, show the same morphology as the unwashed counterparts (compare Figs. 7, 10). Conversely, the DNA release promotes a significant change in the surface morphology. For example, if the COT-DNApH4-CHI sample is compared before and after the washing treatment (Figs. 6a, 8b, respectively), the surface roughness of the latter can be seen to increase significantly, due to the release of DNA. Similar results can be observed for the Mix DNApH4-CHI samples (Figs. 6b, 9, respectively before and after washing).
Thermal and thermo-oxidative stability
The thermal and thermo-oxidative stability of the pure and treated cotton fabrics has been assessed by thermogravimetric analyses performed in nitrogen and air, respectively. Table 3 shows the obtained data.
As clearly reported in the scientific literature (Price et al. 1997; Alongi and Malucelli 2015), the pyrolysis of cotton in nitrogen takes place through a single step process, during which the cellulose degradation follows two competitive routes. The former involves the depolymerization of the main chain to produce volatile species (such as levoglucosan, furan and furan derivatives); the latter is a dehydration combined with further auto-crosslinking to form a thermally stable carbonaceous structure, known as char.
As a consequence of these processes, upon heating cotton starts to degrade at about 315 °C (Tonset10 %, Table 4), losing 10 wt%; then the fabric further goes on with a maximum weight loss rate at about 369 °C (Tmax1, Table 3) that corresponds to 58 % weight loss. The presence of the coating anticipates the cellulose decomposition: more specifically, irrespective of the structure of the deposited coating, Tonset10 % and Tmax1 values decrease, while, at the same time, the residues found at Tmax1 are within 50 and 58 %. This finding can be attributed to the phosphate groups of DNA that behaves similarly to ammonium polyphosphate (APP). During its decomposition, APP gives rise to the formation of phosphoric acid and favors cellulose decomposition towards the formation of char. Analogously, DNA starts to decompose at about 200 °C, releasing the same acid species, hence promoting the formation of a residue thermally stable up to 600 °C. This finding justifies the significant formation of the residue at Tmax1 and at 600 °C as depicted by comparing the values listed in Table 3.
In air, the thermo-oxidation of cotton takes place in a similar way, which differs from the degradation in nitrogen only for the presence of a second degradation step at high temperatures (Tmax2: 502 °C). This phenomenon can be attributed to the oxidation of the char formed during the first step and of all the hydrocarbon species still present. Once again, the biomacromolecule coatings are responsible for the decrease of both Tonset10 % and Tmax1 as well as for the increase of the residues at Tmax1, Tmax2 and 600 °C that confirm the protective effect exerted by the formed stable char.
Fire behavior
All the UV-cured and washed fabric samples tested for DNA release were subjected to flammability tests performed in horizontal configuration.
As already mentioned, the cotton fabrics treated with herring sperm DNA, with an add-on of 8 %, have been found to be self-extinguishing, to give rise to high residues (more than 70 %) and to show low burning rates (about 1.2 mm/s). These treatments also promoted a reduction in the total heat release and in the heat release rate peak, of about 40 % and 35 % respectively, in cone calorimetry tests (Bosco et al. 2015).
A similar AO % was here considered: about 8 % for the impregnated specimens for DNA and 2 % for chitosan. A total add-on of 14 % was reached with 30 bilayers for the LbL samples.
Table 4 and Fig. 11 summarize the flammability results.
The control fabric samples, treated with chitosan alone (AO = 2 %), burnt completely with a final glowing combustion (GC), and only negligible residues (Fig. 11a), but with a lower burning rate than the untreated cotton (Fig. 11g).
Self-extinguishment of the flame was achieved for COT-CHI-DNApH4 (Fig. 11b) and for both the LbL samples (Fig. 11e, f, for LbL-DNA and LbL-CHI, respectively). About half the length of the COT-CHI-DNApH4 samples burnt with a burning rate 30 % lower than cotton (Table 4). When the fabric was LbL-treated, there were residues of more than 85 % and the best results were obtained with DNA as the final layer (Fig. 11e). A very small char length and a low burning rate (10 mm and 0.44 mm/s, respectively) were observed. This is probably due to the presence of the DNA on the surface, which forms phosphoric acid that then dehydrates the fabric, releasing water and ammonia, which results in an improved fire retardant effectiveness.
With the mixture of DNA and chitosan (Mix DNApH4-CHI, Fig. 11d), the flame reached the end of the sample, which did not burn completely. In fact, more than 50 % of residue remained after the test. In this case, the char was very brittle and fragmented, probably because of the inhomogeneity of the DNA and chitosan distribution over the fabric. Most of the samples treated with the mixture showed a final glowing combustion, probably due to the presence of chitosan.
Lower levels of flame retardancy were obtained for the COT-DNApH4-CHI samples, where the DNA was placed on the cotton substrate, which was then coated by chitosan (Fig. 11c; Table 4). Although it was not possible to achieve flame out and the fabric burnt completely, the final residue (42 %) was thick and compact and the burning rate was reduced compared to untreated cotton.
As far as the morphology of the treated fabrics after the flammability tests is considered, all the coatings containing DNA showed the intumescent-like behavior of this macromolecule (Alongi et al. 2013b, 2015), which upon exposure to a flame, swelled and formed bubbles on the burnt surfaces. It is worth noting that, when DNA was the outermost layer, very small bubbles that homogeneously covered the burnt fibers could be observed (Fig. 12a). On the other hand, when the chitosan layer entrapped the DNA, the size of the bubbles increased and they were partially covered by the formed char (Fig. 12b). The same behavior was observed when chitosan and DNA were mixed (Fig. 13). These findings can be attributed to the coexistence of the two biomacromolecules. In fact, when the chitosan covered the DNA or was mixed with it, the DNA was able to better exploit the presence of chitosan as a carbon source, thus favoring the intumescent process and the formation of larger bubbles.
Finally, with reference to the LbL systems, irrespective of final layer, the morphology of the burnt surfaces was different from the previously described morphologies: the bubbles were larger than those of all the other samples and were homogeneously dispersed on the fibers (Figs. 14a, b, for LbL-CHI and LbL-DNA, respectively).
Cone calorimetry tests
Cone calorimetry tests have been performed in order to assess the reaction and efficiency of the biomacromolecules treatments upon exposure to a heat flux (35 kW/m2). Table 5 collects the obtained data, in terms of Time To Ignition, Total Heat Release (normalized with respect to the initial sample mass), peak of Heat Release Rate (normalized with respect to the initial sample mass) and final residue.
From the data shown in Table 5, it is worthy to note that the presence of the biomacromolecule coating, irrespective of its structure and composition, significantly reduces TTI: this behavior can be ascribed to the DNA that, similarly to ammonium polyphosphate (Carosio et al. 2012), starts to degrade in air, releasing phosphoric acid and promoting the dehydration and char formation reactions of cellulose. This latter is further confirmed by the increase of the final residues.
Besides, in the presence of the different coatings, both THR and PHRR values show a remarkable decrease, which indicates that the formation of a stable char during the exposure to the heat flux is able to protect the underlying substrate, slowing down the heat and mass transfer phenomena occurring in between the fabric specimen and its surroundings.
Antibacterial tests
The ability of UV-grafted chitosan to confer antibacterial activity to treated cotton against Staphylococcus aureus ATCC 6538, a gram-positive microorganism, has been reported extensively in literature. A total reduction in microorganism number (>99.9 %) has been reached with low chitosan add-ons (0.3 % ofw) and the antibacterial activity has been maintained after 30 washing cycles (Ferrero et al. 2015).
This suggests that even a few available protonated amino groups can be sufficient to act against bacteria. For this reason, antibacterial tests were carried out on chitosan–DNA treated fabrics to evaluate any possible residual antibacterial activity due to chitosan. The obtained results are shown in Table 6.
The chitosan antimicrobial activity on cotton fabrics treated by double impregnation (with chitosan and DNA solutions separately) was totally lost, regardless of the order of impregnation. A reduction of 0 % in microorganism number was measured on these samples, similarly to untreated cotton. This means that the protonated groups of chitosan were all involved in the interaction with the DNA and for this reason were not available for the microorganisms.
In the case of the cotton fabrics treated with the mixed solutions, a low antibacterial activity against S. aureus was observed, with an m.o. reduction of 6 %, which is too low to be considered responsible for antibacterial activity.
This behavior was further enhanced on the LbL-treated samples, where the mass ratio between chitosan and DNA was increased. The bacterial reduction of this system increased to 66 %, irrespective of the final covering layer. This finding is probably due to the higher amount of chitosan on the surface and, therefore, of the free protonated amino groups that are available.
Conclusions
In the present work, chitosan and DNA have been deposited on cotton fabrics using an impregnation method or a layer by layer approach, with the aim of obtaining a self-extinguishing substrate, with water resistance and antimicrobial properties. UV-curing was exploited to permanently fix the biomacromolecules onto the cotton substrate, thanks to the presence of chitosan that directly undergoes a photografting reaction.
Despite an anticipation of the degradation of cotton, the biomacromolecule coatings promoted the formation of a stable protective char, as assessed by thermogravimetric analyses. Cone calorimetry tests showed a remarkable decrease of both THR and PHRR values in the presence of the different coatings; furthermore, due to the activation of DNA, TTI was significantly reduced.
The best results were obtained for a layer by layer chitosan-DNA UV-cured architecture, which resulted to provide water resistance, self-extinction in flammability tests and a good antimicrobial activity to cotton fabrics.
As far as the standard deposition methods are concerned, all the samples showed no antimicrobial effect, whereas different properties were observed in relation to water resistance and fire retardancy.
The cotton fabrics treated with the DNA and chitosan solution mixture showed good stability in water and good fire retardant properties: in fact, although self-extinction was not reached, a significant reduction in the total burning rate and a remarkable increase in the final residue were observed.
Finally, the layered samples showed different behaviors: the COT-CHI-DNApH4 samples were self-extinguishing, but almost all the DNA was lost during washing, while the COT-DNApH4-CHI samples showed intermediate water resistance and provided slightly lower fire retardant features to cotton fabrics than the mixture of the two biomacromolecules.
References
Alongi J, Malucelli G (2015) Thermal degradation of cellulose and cellulosic substrates. In: Tiwari A, Raj B (eds) Reactions and mechanisms. Thermal analysis of advanced materials. Beverly, Wiley, pp 301–332. ISBN 978-1-119-11757-5
Alongi J, Carletto RA, Di Blasio A, Carosio F, Bosco F, Malucelli G (2013a) DNA: a novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A 1(15):4779–4785
Alongi J, Carletto RA, Di Blasio A, Cuttica F, Carosio F, Bosco F, Malucelli G (2013b) Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr Polym 96:296–304
Alongi J, Cuttica F, Di Blasio A, Carosio F, Malucelli G (2014) Intumescent features of nucleic acids and protein. Thermochim Acta 591:31–39
Alongi J, Di Blasio A, Milnes J, Malucelli G, Bourbigot S, Camino G (2015) Thermal degradation of DNA, an all-in-one natural intumescent flame retardant. Polym Degrad Stab 113:110–118
Banyay M, Sarkar M, Gräslund A (2003) A library of IR bands of nucleic acids in solution. Biophys Chem 104:477–488
Bosco F, Casale A, Mollea C, Terlizzi ME, Gribaudo G, Alongi J, Malucelli G (2015) DNA coatings on cotton fabrics: effect of molecular size and pH on flame retardancy. Surf Coat Technol 272:86–95
Barbas CF III, Burton DR, Scott JK, Silverman GJ (eds) (2001) Phage display: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Carosio F, Alongi J (2015) Few durable layers suppress cotton combustion due to the joint combination of layer by layer assembly and UV-curing. RSC Adv 5:71482–71490
Carosio F, Alongi J, Malucelli G (2012) Layer by layer ammonium polyphosphate based coatings for flame retardancy of polyester-cotton blends. Carbohydr Polym 88:1460–1469
Carosio F, Di Blasio A, Alongi J, Malucelli G (2013) Green DNA-based flame retardant coatings assembled through layer by layer. Polymer 54(19):5148–5153
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49(4):780–792
Erbacher P, Zou SM, Bettinger T, Steffan AM, Remy JS (1998) Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm Res 15(9):1332–1339
Ferrero F, Periolatto M (2012) Antimicrobial finish of textiles by chitosan UV curing. J Nanosci Nanotechnol 12(6):4803–4810. doi:10.1166/jnn.2012.4902
Ferrero F, Periolatto M, Vineis C, Varesano A (2014) Chitosan coated cotton gauze for antibacterial water filtration. Carbohydr Polym 103:207–212
Ferrero F, Periolatto M, Ferrario S (2015) Sustainable antimicrobial finishing of cotton fabrics by chitosan UV-grafting: from laboratory experiments to semi industrial scale-up. J Clean Prod 96:244–252
Gaan S, Sun G (2009) Effect of nitrogen additives on thermal decomposition of cotton. J Anal Appl Pyrol 84(1):108–115
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol 71(2–3):235–244
Horrocks AR (2001) Textiles. In: Horrocks AR, Price D (eds) Fire retardant materials. Woodhead Publishing Ltd., Cambridge, pp 128–181
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stab 96:377–392
Horrocks AR, Anand SC (2000) Handbook of technical textiles. Woodhead Publishing Limited, Cambridge
Horrocks AR, Price D, Akalin M (1996) FTIR analysis of gases from cotton and flame retarded cotton fabrics pyrolysed in air. Polym Degrad Stab 52:205–213
Horrocks AR, Kandola BK, Davies PJ, Zhang S, Padbury SA (2005) Developments in flame retardant textiles—a review. Polym Degrad Stab 88:3–12
Huang M, Fong CW, Khor E, Lim LY (2005) Transfection efficiency of chitosan vectors: effect of polymer molecular weight and degree of deacetylation. J Control Release 106(3):391–406
Kawazoe N, Narita Y, Chen G, Satomi T, Tateishi T (2008) Chitosan/DNA polyelectrolyte complex membranes for controlling cell spreading and aggregation. Open Biotechnol J 2:148–151
Kiang T, Wen J, Lim HW, Leong KW (2004) The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials 25:5293–5301
Kumar MNVR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW, Luk KDK (2005) An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials 26(15):2705–2711
Low MJD, Morterra C (1985) IR studies of carbons. V. Effects of NaCl on cellulose pyrolysis and char oxidation. Carbon 23:311–316
Malucelli G, Bosco F, Alongi J, Carosio F, Di Blasio A, Mollea C, Cuttica F, Casale A (2014) Biomacromolecules as novel green flame retardant systems for textiles: an overview. RSC Adv 4(86):46024–46039
Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW (2001) Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release 70(3):399–421
Morán MC, Laranjeira T, Ribeiro A, Miguel MG, Lindman B (2009) Chitosan-DNA particles for DNA delivery: effect of chitosan molecular weight on formation and release characteristics. J Disper Sci Technol 30(10):1494–1499
Morterra C, Low MJD (1984) IR studies of carbons. II. The vacuum pyrolysis of cellulose. Carbon 21:283–288
Morterra C, Low MJD (1985a) IR studies of carbons. IV. The vacuum pyrolysis of oxidized cellulose and the characterization of the chars. Carbon 23:301–310
Morterra C, Low MJD (1985b) IR studies of carbons. VI. The effect of KHCO3 on cellulose pyrolysis and char oxidation. Carbon 23:335–341
Morterra C, Low MJD, Severdia AG (1984) IR studies of carbons. III. The oxidation of cellulose. Carbon 22:5–12
Mural PKS, Kumar B, Madras G, Bose S (2016) Chitosan immobilized porous polyolefin as sustainable and efficient antibacterial membranes. ACS Sustain Chem Eng 4(3):862–870
Muthu SS (2015) Handbook of sustainable apparel production. CRC Press, Boca Raton
Periolatto M, Ferrero F (2013) Cotton filter fabrics functionalization by chitosan UV-grafting for removal of dyes. Chem Eng Trans 32:85–90
Periolatto M, Ferrero F, Vineis C (2012) Antimicrobial chitosan finish of cotton and silk fabrics by UV curing with 2-hydroxy-2-methylphenylpropane-1-one. Carbohydr Polym 88:201–205. doi:10.1016/j.carbpol.2011.11.093
Price D, Horrocks AR, Akalin M, Faroq AA (1997) Influence of flame retardants on the mechanism of pyrolysis of cotton (cellulose) fabrics in air. J Anal Appl Pyrolysis 40–41:511–524
Rikimaru S, Wakabayashi Y, Nomizu M, Nishi N (2003) DNA-chitosan bilayer membrane as a bi-functional biomedical adhesive. Polym J 35:255–260
Shafizadeh F, Bradbury AGW (1979) Thermal degradation of cellulose in air and nitrogen at low temperatures. J Appl Polym Sci 23:1431–1442
Shafizadeh F, Newell TP, Zeronian SH (1985) Cellulose chemistry and its applications. Ellis Horwood, Chichester
Sionkowska A, Kaczmarek H, Wisniewski M, Skopinska J, Lazare S, Tokarev V (2006) The influence of UV irradiation on the surface of chitosan films. Surf Sci 600:3775–3779
Strand SP, Danielsen S, Christensen BE, Vårum KM (2005) Influence of chitosan structure on the formation and stability of DNA-chitosan polyelectrolyte complexes. Biomacromolecules 6:3357–3366
Tata J, Alongi J, Carosio F, Frache A (2011) Optimization of the procedure to burn textile fabrics by cone calorimeter: part I. Combustion behavior of polyester. Fire Mater 35(6):397–409
Thakur VK, Thakur MK (2014) Recent advances in graft copolymerization and applications of chitosan: a review. ACS Sustain Chem Eng 2:2637–2652
Thakur VK, Voicu SI (2016) Recent advances in cellulose and chitosan based membranes for water purification: a concise review. Carbohydr Polym 146:148–165
USDA United States Department of Agriculture, Foreign Agricultural Service (2016) Cotton: world markets and trade. USDA United States Department of Agriculture, Foreign Agricultural Service, Washington
Acknowledgments
Authors acknowledge Dr. Claudia Vineis (CNR-ISMAC, Biella) for the technical support in antibacterial activity test. Authors are grateful to Prof. Franco Ferrero for the fruitful discussion on the obtained results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10570-016-1106-8.
Rights and permissions
About this article
Cite this article
Annalisa, C., Francesca, B., Giulio, M. et al. DNA-chitosan cross-linking and photografting to cotton fabrics to improve washing fastness of the fire-resistant finishing. Cellulose 23, 3963–3984 (2016). https://doi.org/10.1007/s10570-016-1067-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1067-y