Abstract
Nylon fabric possesses high strength, good resilience, abrasion resistance and moisture absorption which is widely used as the textile materials. However, there are some potential safety risks due to its high flammability, poor heat and light stability, and melt-dripping phenomenon during combustion. In this paper, nylon fabric was multifunctional modified by bio-based tannic acid (TA) and phytic acid (PA) to obtain flame retardancy, ultraviolet protection and antibacterial properties simultaneously. The multifunctional coating was constructed on nylon fabric using the simple finishing method. The limiting oxygen index (LOI) of treated nylon fabric significantly increased to 39%, and its dripping was completely eliminated, showing excellent flame retardancy and anti-dripping property. In order to improve the washing durability of the multifunctional coating, polyethyleneimine (PEI) was introduced to form covalent and ionic bonds with TA, PA and nylon fabric to enhance the interfacial binding strength. After four times washing, the LOI value of treated fabric was still higher than 29% showing good washing durability. The antibacterial rates of treated nylon fabric against E. coli and S. aureus were higher than 99%. And the ultraviolet protection factor (UPF) value of treated nylon fabric increased from 12.105 to 264.82, showing excellent antibacterial and ultraviolet protection properties. This research provides the experimental basis for multifunctional modification of textile fabrics by using bio-based compounds.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nylon fabric is used extensively in aerospace, automotive, electronic appliance, military, home decoration and industrial textile industries because of its excellent physical and mechanical properties, including exceptional wear resistance, high strength, and chemical resistance [1]. However, it has non-negligible shortcomings, such as the poor light stability and easy oxidation in prolonged high temperature environment, resulting in the discoloration and brittleness of nylon fabric [2]. And its high flammability and melt-dripping during combustion cause the potential safety hazards [3, 4]. Therefore, improving the flame-retardant properties and light stability of nylon fabrics has practical significance.
At present, the main flame-retardant methods for synthetic fiber and fabric are blending method, copolymerization method, and flame-retardant finishing method [5, 6]. Among the above methods, finishing method is widely used for the advantages of easy application, simplicity and low cost [7, 8]. The commonly used flame-retardant finishing methods include pad-dry-cure, coating, layer-by-layer (LBL) assembly, sol–gel and graft modification [9,10,11,12]. And the pad-dry-cure method is the widely used finishing method. In recent years, with the increasing awareness of environmental protection, green flame retardants have become an important development direction in flame-retardant field [13, 14]. Bio-based flame retardants have received more and more attention, such as DNA, protein, chitosan (CS), phytic acid (PA) and tannic acid (TA) [15].
PA is a natural organophosphorus compound rich in phosphorus content (28 wt%), which is known as a “green” compound and an excellent bio-based flame retardant. Our previous research has studied to construct a PEI/PA intumescent flame-retardant (IFR) coating with a gradient structure on cotton fabric using LBL method [16]. The PEI/PA-coated cotton fabric obtained excellent flame-retardant properties with the LOI value higher than 40%. Our research group has also studied to flame-retardant treatment of PET fabrics by PEI/PA coating. The LOI value of coated PET fabric could increase to higher than 40% from 21.3% of uncoated fabric. And the coated PET fabric had no melt-dripping [17]. The bio-based flame retardants have also been studied to flame retardant of nylon fabric. Kundu et al. [18] reported to construct the fully bio-based IFR coating on nylon fabric using CS, PA and oxidized sodium alginate (OSA). The dripping of treated nylon fabric was eliminated, while its LOI value increased slightly to 21.8% indicating that the flame retardancy of treated nylon fabric was not improved obviously. Kundu et al. [19] studied to synthesize the phosphorylated CS which was grafted onto the surface of nylon fabrics and modified by (3-aminopropyl) triethoxysilane (APTES) to form a cross-linking coating through sol–gel process. Though the LOI value of treated nylon fabric reached 24.3%, the flame retardancy of treated nylon fabric was not satisfactory.
TA as the bio-based polyphenol could be an excellent carbon source in intumescent flame-retardant system. Previous researches have confirmed that TA can play a good synergistic effect with phosphorus containing acid sources [20, 21]. Kulkarni et al. [22] reported that a novel surface functionalization method to improve the flame retardancy of nylon/cotton fabric using a synergistic combination of TA and PA. Moreover, TA has excellent antibacterial and ultraviolet (UV) protection properties [23,24,25,26]. Luo et al. [27] reported to prepare multifunctional protective cotton fabrics by LBL assembly TA and PA. The modified cotton fabrics exhibited excellent flame retardancy, antibacterial and UV protection properties. Zhou et al. [28] reported to prepare environmentally friendly cotton fabric with UV resistance and flame-retardant properties by using TA and PA. However, the multifunctional modification of nylon fabric using bio-based TA and PA has not been reported.
In this study, bio-based PA and TA were used for multifunctional modification of nylon fabric through finishing method to endow it with flame retardancy, antibacterial and UV protection properties. However, the durability of multifunctional fabric was poor due to the water solubility of both PA and TA. In order to improve the washing durability of multifunctional fabric, PEI was introduced into the PA/TA system in this research which could form insoluble polymer with PA [16]. The flame retardant, antibacterial and UV protection properties of multifunctional finished nylon fabric were studied, and the washing durability was also investigated.
2 Experimental
2.1 Materials and Chemicals
Phytic acid solution (PA, 50%) was purchased from Huangshan Shexian Xingcheng Phytic Acid Co., Ltd. Polyethyleneimine (PEI, M.W. 10,000, 99%), sodium dodecyl sulfate and glacial acetic acid were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Tannic acid (TA, AR) and sodium hydroxide (NaOH, 96%) were purchased from Shanghai Macklin Biochemical Reagent Co., Ltd. Nylon oxford fabric (130 g/m2) was purchased from Zhejiang Qianxun Textile Co., Ltd.
2.2 Multifunctional Finished Nylon Fabric
2.2.1 Treatment of Nylon Fabric with TA
The nylon fabric was put into sodium hydroxide solution (4 g/L), and then it was heated to 100 °C and kept for 30 min. After treatment, the nylon fabric was washed with deionized water and dried at 80 °C for 30 min.
A certain amount of TA were added into deionized water which was stirred until the TA was completely dissolved to prepare 10% TA solution. The sodium dodecyl sulfate (0.04 g) and glacial acetic acid (20 g) were added into this solution to adjust the pH to 3.5. Then, the TA solution was heated to 40 °C, and the nylon fabric was immersed in TA solution for 30 min, and then the solution was heated to 100 °C with a heating rate of 1 °C/min and kept at this temperature for 60 min. After that, it was washed in deionized water at 60 °C for 2 min and dried at 80 °C for 30 min.
2.2.2 Flame-Retardant Treatment of Nylon Fabric
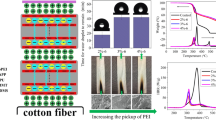
50% PA solution was diluted into the deionized water to prepare 20% PA solution. A certain amount of pure PEI was added into the deionized water to prepare 3% PEI solution. The TA-treated nylon fabric was immersed into 20% PA solution for 3 h to make nylon fabric combining with PA adequately, and then it was dried at 80 °C for 30 min. The treated nylon fabric was immersed in 3% PEI solution for 3 min, which was padded with the wet-pick up of 60 ~ 70%, and dried at 120 °C for 10 min. And then, the treated nylon fabric was immersed in 20% PA solution for 3 min, which was padded with the wet-pick up of 60 ~ 70%, and dried at 120 °C for 45 min. Repeating the above dip-pad-dry process for 2 and 4 times, the treated samples were named TA/PA/PEI-2 and TA/PA/PEI-4, respectively. And the samples without TA were named PA/PEI-2 and PA/PEI-4, respectively. Flame-retardant treatment process and the combination of nylon fabric, TA, PA and PEI are shown in Fig. 1.
2.3 Characterization
Fourier transform infrared spectroscopy test (FTIR) of nylon fabric was carried out on FTIR (IRPrestige-21, Shimadzu, Japan) in the range of 4000–500 cm−1. Flame-retardant properties were determined by LOI value on a JF-3 oxygen index instrument (Jiangning Analysis Instrument, China) according to GB/T 5454-1997, and the vertical burning test on a YG (B)815D-I fabric combustion tester (Wenzhou Darong, China) according to GB/T 5455-1997. The durability of flame-retardant nylon fabric was evaluated by determining the LOI values after four times washing. The washing process was performed at 49 °C for 45 min using 2 g/L synthetic detergent according to AATCC 61-2006 standard. The morphology of nylon fabrics and char residues were observed by field emission scanning electron microscope (FE-SEM, Hitachi S-4800, Japan). The thermal mass loss rates both under air and nitrogen atmosphere were measured on a microcomputer differential thermal balance (DTG-60H, Shimadzu, Japan) in the temperature range from 30 to 700 °C. The gas flow rate and the heating rate were 20 ml/min and 10 °C/min, respectively. The antibacterial properties of treated nylon fabrics were tested according to GB/T 20944.3–2008 standard, the antibacterial rates of the samples were calculated. The UV protection properties of nylon fabrics were determined on a YG902C UV transmittance tester according to GB/T 18830-2009 standard, and the values of ultraviolet protection factor (UPF), T(UVA) and T(UVB) were recorded, respectively.
3 Results and Discussion
3.1 Characterization of Treated Nylon Fabric
Figure 2 shows the FTIR spectra of nylon fabrics before and after treatment.
As shown in Fig. 2, the absorption peak at about 3400 cm−1 in the spectral curve of untreated nylon fabric corresponds to the amine group. And the peaks at 2938 and 2867 cm−1 correspond to the methylene group. The peak at 1638 cm−1 is assigned to amide I causing by the C = O bond. And the peak at 1542 cm−1 is attributed to the amide II stretching vibration absorption peak belonging to the N–H bond. As for the treated nylon fabric (PA/PEI-4 and TA/PA/PEI-4), the new absorption at 1650 cm−1 is attributed to absorption peak of the N–H bond from the PEI. The new absorption peaks at 1160, 1058 and 983 cm−1 belong to the characteristic peaks of the P = O, O–P–C and P–O bonds of PA. These results show that PA and PEI have been successfully attached to nylon fabrics. And the new peaks at 1307 cm−1 and 1177 cm−1 appear in the TA/PA/PEI-4 curve belong to the vibration peaks of the –C–NH–C– forming by TA and –NH2, indicating that TA has been covalently combined with nylon fabric and PEI of TA/PA/PEI-4. These results show that the multifunctional coating of PA, TA and PEI has been constructed on nylon fabrics, and the covalent bonds among them have been formed.
As shown in Fig. 3, the surface morphology of untreated nylon fiber is smooth and the fibers are clearly distinguishable. The treated nylon surface is covered with a layer of coating, and the fibers are bonded to each other. These results also show that the flame-retardant coating has been formed on nylon fabric.
3.2 Flame Retardancy of Nylon Fabric
The LOI values and vertical burning properties of nylon fabric are listed in Table 1. And the photographs of nylon fabrics after the vertical burning are shown in Fig. 4.
As shown in Table 1 and Fig. 4, the LOI value of untreated nylon is only 24.0%. And the damage length of untreated nylon fabric is 14 cm, and its dripping phenomenon is severe of the vertical burning. The LOI values of treated nylon fabrics increase with the treatment times of PA/PEI, which reach to 38.5% and 39% of PA/PEI-4 and TA/PA/PEI-4, respectively. Their char lengths reduce to 11 cm and 10 cm, and their dripping phenomenon is completely eliminated. As shown in Fig. 4, the damage length of nylon fabric reduces with the increasing of PA/PEI treatment times. Though the damage length of PA/PEI-2 and TA/PA/PEI-2 are larger than that of untreated nylon fabric, their dripping phenomenon is attenuated. After 4 times washing, the LOI values of PA/PEI-4 and TA/PA/PEI-4 are higher than 26%, especially that of TA/PA/PEI-4 is higher than 29%, indicating that the flame-retardant nylon fabric has good washing durability. The reason about this may be due to the strong covalent bonds and electrostatic attractions are formed through PEI with TA, PA and nylon fabric as shown in Fig. 1.
Cone calorimetry testing (CCT) was used to investigate the combustion characteristics of nylon fabrics as shown in Fig. 5 and Table 2.
As shown in Fig. 5 and Table 2, the TTI and TTF of TA/PA/PEI-4 are 18 s and 70 s which are much less than that of untreated nylon fabric. The results show that the combustion of nylon fabric is advanced and the combustion time is shortened after treatment. The pHRR of TA/PA/PEI-4 is 227.84 kW/m2 which is lower than that of untreated nylon fabric (289.25 kW/m2). The THR of TA/PA/PEI-4 significantly reduce from 10.05 MJ/m2 of untreated one to 5.38 MJ/m2. The pHRR and THR of TA/PA/PEI-4 reduce about 21.23% and 46.47% comparing with that of untreated nylon, respectively. The results demonstrate that the fire hazard of nylon fabrics was significantly reduced after multifunctional treatment.
The average effective heat of combustion (av-EHC) of TA/PA/PEI-4 is 13.99 MJ/kg, which is lower than that of untreated nylon (15.70 MJ/kg). This indicates that the degree of combustion of combustible volatiles is reduced. The CO2/CO ratio of TA/PA/PEI-4 is greatly reduced from 71.43 of untreated nylon to 35.40. The reason about this may be due to the incomplete combustion inhibit the conversion of CO to CO2, which confirms the gas phase flame-retardant action of the multifunctional coating on nylon fabric. The residual mass of TA/PA/PEI-4 is 22.60% comparing with no residue of untreated nylon. The result demonstrates that multifunctional coating can promote nylon fabric to form stable char showing obvious condensed phase flame-retardant action.
3.3 Thermal Stability of Nylon Fabrics
As shown in Fig. 6 and Table 3, nylon fabrics have two mass loss stages under air atmosphere. The first stage is caused by the breakdown of nylon to form small molecules and char residues. And the further oxidative degradation of unstable char residues results in the second mass loss stage. The T−10%, T1max and R1max of flame-retardant nylon fabrics significantly reduced comparing with that of untreated one. The T2max of flame retardant treated nylon is slightly higher than that of untreated nylon fabric. The char residues at 700 °C of PA/PEI-4 and TA/PA/PEI-4 are 26.37% and 24.35% comparing with 2.77% of untreated one. The results show that the PA/PEI and TA/PA/PEI coatings can promote nylon fabric to form char.
Both untreated and treated nylon fabrics have one mass loss stage under N2 atmosphere. The T−10%, Tmax and Rmax of nylon fabric after treatment are significantly lower than those of untreated nylon fabric. The amount of char residues increase from 3.472% to 27.09% and 28.78%. The results also indicate that the promoting char formation effect of PA/PEI and TA/PA/PEI flame-retardant coatings. And the PA/PEI-4 and TA/PA/PEI-4 show almost the same thermal decomposition process. Therefore, both PA/PEI coating and TA/PA/PEI coating on the nylon fabric can act through condensed phase action by promoting the formation of char to act as the barrier layer for inhibiting the diffusion of combustible gas and heat transfer.
3.4 SEM Images of Char Residues
SEM is used to observe the morphology of char residues, and the results are shown in Fig. 7.
As shown in Fig. 7, the char residue surface of untreated nylon fabric exists cracks and holes. However, the char residue surface morphology of treated nylon fabrics changes obviously comparing with that of untreated one. The char residue of treated nylon is more continuous and intact than that of untreated one. And many bubbles exist on char residues surface of PA/PEI-4 and TA/PA/PEI-4, which are formed by the blowing effect of nonflammable gas originating from the PA/PEI and TA/PA/PEI flame-retardant coating. Therefore, TA, PEI and PA can form IFR system to act through IFR action.
3.5 Antibacterial and UV Protection Properties
Table 4 lists the data of the antibacterial and UV protection properties of nylon fabrics. As shown in Table 4, the antibacterial rates of original nylon fabric against E. coli and S. aureus are only 19% and 40%, respectively. The antibacterial rates of PA/PEI-2 and PA/PEI-4 against E. coli increase to 98.62% and 97.93%, and the antibacterial rates of them against and S. aureus increased to 81.25% and 90.23%, respectively. PA has strong acidity, which is not conducive to the growth of bacteria showing certain antibacterial properties. The antibacterial rates of TA/PA/PEI-2 against S. aureus and E. coli could increase to 99.87% and 99.93%. TA as a kind of plant polyphenols, which can destroy bacterial cell membranes and inhibit bacterial activity is often used as a natural antibacterial agent. Therefore, TA can further enhance the antibacterial properties of nylon fabrics.
The UV protection properties of nylon fabrics are also shown in Table 4. The UPF value of original nylon fabric is 12.105, its T(UVA) and T(UVB) are 18.52% and 6.22%, respectively. The UPF values of PA/PEI-2 and PA/PEI-4 are almost the same with that of untreated one. And their T(UVA) values slightly decrease to 12 ~ 13%, while their T(UVB) values slightly increase. These results indicate that the UV protection properties of nylon fabric after flame retardant by PA/PEI coating have not been improved. While the UPF values of TA/PA/PEI-2 and TA/PA/PEI-4 significantly increase to 154.63 and 264.82. And their T(UVA) and T(UVB) values greatly decrease, especially those of TA/PA/PEI-4 reduce to 2.98% and 0.16%. According to the GB/T 18830-2009 standard, the samples with UPF value higher than 40 and the T (UVA) less than 5% could be considered to have good UV protection property. Thus, the nylon fabric treated with TA/PA/PEI could obtain excellent UV protection properties. The reason about this may be due to the strong UV absorption properties of TA originating from the benzene rings and carboxyl groups in its molecule.
Therefore, multifunctional modification of nylon fabric using bio-based PA and TA could endow it with excellent flame retardancy, antibacterial and UV protection properties.
4 Conclusion
In this paper, the multifunctional nylon fabric were prepared by simple finishing method using bio-based tannic acid and phytic acid. And PEI was introduced in this system to enhance the washing durability of multifunctional coating. The LOI value of treated nylon fabrics reach 39%, and its dripping was completely eliminated, showing the excellent flame retardancy and anti-dripping property. And the LOI value of treated nylon fabric after four times washing was 29%, indicating the treated nylon fabric with TA/PA/PEI could obtain good washing durability. The thermogravimetric analysis results showed that the TA/PE/PEI flame retardant coating could enhance the high temperature stability of nylon fabric and promote it to form stable char. The SEM of char residues confirmed that TA/PA/PEI could construct the IFR system to act through IFR action. The antibacterial rates of TA/PA/PEI treated nylon fabric against S. aureus and E. coli could reach to higher than 99%, showing excellent antibacterial properties. And UPF value of TA/PA/PEI treated nylon fabric increases from 12.105 of untreated one to 264.82, indicating the UV protection properties of nylon fabric are greatly improved after treatment with TA/PA/PEI coating. This research provides an experimental basis for multifunctional modification of textile fabrics using bio-based compounds to obtain excellent flame retardancy, antibacterial and UV protection properties.
Data Availability
The data related to this research can be obtained from the corresponding author by email request.
References
B.L. Deopura, N.V. Padaki, Synthetic textile fibres: polyamide, polyester and aramid fibres, in Sinclair, R, Textiles and Fashion. (Woodhead Publishing, Cambridge, 2015), pp.97–114. https://doi.org/10.1016/B978-1-84569-931-4.00005-2
M. Tian, Z. Wang, L. Qu, K. Wang, S. Zhu, X. Zhang, R. Liu, Enhanced UV photo-stabilization of Nylon 6 filament with reduced graphene oxide/polyurethane nanocomposite inks. In. J. Cloth. Sci. Tech. 30, 817–827 (2018). https://doi.org/10.1108/IJCST-07-2017-0107
W.J. Jin, X.W. Cheng, W.L. He, W. Chen, J.P. Guan, Q.F. Qian, J.L. Xu, Sustainable modification of polyamide 6 fabric by Maillard reaction product for mitigating fire hazards and molten-dripping. Polym. Test.. Test. 111, 107595 (2022). https://doi.org/10.1016/j.polymertesting.2022.107595
Y.C. Chen, B. Sun, H.X. Zhang, X.D. Zhou, Synthesis and application of a sulfur-containing phosphoric amide flame retardant for nylon fabric. Fire Mater. 40, 959–972 (2016). https://doi.org/10.1002/fam.2354
C.K. Kundu, Z. Li, L. Song, Y. Hu, An overview of fire retardant treatments for synthetic textiles: from traditional approaches to recent applications. Eur. Polym. J.Polym. J. 137, 109911 (2020). https://doi.org/10.1016/j.eurpolymj.2020.109911
X. Guo, L. Liu, H. Feng, D. Li, Z. Xia, R. Yang, Flame retardancy of nylon 6 fibers: a review. Polymers 15, 2161 (2023). https://doi.org/10.3390/polym15092161
M.S. Subbulakshmi, N. Kasturiya, P. Hansraj, A.K. Bajaj, Agarwal., Production of flame-retardant nylon 6 and 6.6. J. Macromol. Sci. C 40, 85–104 (2000). https://doi.org/10.1081/MC-100100580
J. Alongi, F. Carosio, G. Malucelli, Current emerging techniques to impart flame retardancy to fabrics: an overview. Polym. Degrad. Stabil. 106, 138–149 (2014). https://doi.org/10.1016/j.polymdegradstab.2013.07.012
O.Y. Wen, M.Z.M. Tohir, T.C.S. Yeaw, M.A. Razak, H.S. Zainuddin, M.R.A. Hamid, Fire-resistant and flame-retardant surface finishing of polymers and textiles: a state-of-the-art review. Prog. Org. Coat.. Org. Coat. 175, 107330 (2023). https://doi.org/10.1016/j.porgcoat.2022.107330
S.T. Lazar, T.J. Kolibaba, J.C. Grunlan, M.A. Razak, H.S. Zainuddin, M.R.A. Hamid, Flame-retardant surface treatments. Nat. Rev. Mater. 5, 259–275 (2020). https://doi.org/10.1038/s41578-019-0164-6
W. Zhang, R.C. Tang, Adsorption and flame retardant properties of potassium diphenyl sulfonate on nylon 6 fabric. React. Funct. Polym.Funct. Polym. 126, 36–43 (2017). https://doi.org/10.1016/j.react-functpolym.2018.03.005
X.Y. Li, X.Y. Gu, S. Zhang, H.F. Li, Q.L. Feng, J. Sun, Q. Zhao, Improving the fire performance of nylon 6,6 fabric by chemical grafting with acrylamide. Ind. Eng. Chem. Res. 52, 2290–2296 (2013). https://doi.org/10.1021/ie302452e
M. Wang, G.Z. Yin, Y. Yang, W. Fu, J.L.D. Palencia, J. Zhao, N. Wang, Y. Jiang, D. Wang, Bio-based flame retardants to polymers: a review. Adv. Ind. Eng. Polym. Res. 6, 132–155 (2023). https://doi.org/10.1016/j.aiepr.2022.07.003
S. Basak, A.S.M. Raja, S. Saxena, P.G. Patil, Tannin based polyphenolic bio-macromolecules: creating a new era towards sustainable flame retardancy of polymers. Polym. Degrad. Stabil. 189, 109603 (2021). https://doi.org/10.1016/j.polymdegradstab.2021.109603
L. Costes, F. Laoutid, S. Brohez, P. Dubois, Bio-based flame retardants: when nature meets fire protection. Mat. Sci. Eng. R 117, 1–25 (2017). https://doi.org/10.1016/j.mser.2017.04.001
X.H. Liu, H.L. Liu, Y.C. Fang, High-efficiency and durable flame retardant cotton fabric with good physical and mechanical properties by Layer-by-Layer assembly polyethylenimine/phytic acid coating. J. Nat. Fibers. 19, 11790–11801 (2022). https://doi.org/10.1080/15440478.2022.2044428
Y.C. Fang, W.H. Sun, H.L. Liu, X.H. Liu, Construction of eco-friendly flame retardant and dripping-resistant coating on polyester fabrics. Surf. Eng. 37, 1067–1073 (2021). https://doi.org/10.1080/02670844.2021.1911458
C.K. Kundu, W. Wang, S. Zhou, X. Wang, H. Sheng, Y. Pan, L. Song, Y. Hu, A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohyd. Polym.. Polym. 166, 131–138 (2017). https://doi.org/10.1016/j.carbpol.2017.02.084
C.K. Kundu, X. Wang, Y. Hou, Y. Hu, Construction of flame retardant coating on polyamide 6.6 via UV grafting of phosphorylated chitosan and sol-gel process of organo-silane. Carbohyd. Polym.. Polym. 181, 833–840 (2018). https://doi.org/10.1016/j.carbpol.2017.11.069
Z.Y. Xia, W. Kiratitanavit, P. Facendola, S.R. Yu, J. Kumar, R. Mosurkal, R. Nagarajan, A bio-derived char forming flame retardant additive for nylon 6 based on crosslinked tannic acid. Thermochim. Acta. Acta 693, 178750 (2020). https://doi.org/10.1016/j.tca.2020.178750
X. Wang, G. Yang, H. Guo, Tannic acid as biobased flame retardants: a review. J. Anal. Appl. Pyrol.Pyrol. 174, 106111 (2023). https://doi.org/10.1016/j.jaap.2023.106111
S. Kulkarni, Z.Y. Xia, S. Yu, W. Kiratitanavit, A.B. Morgan, J. Kumar, R. Mosurkal, R. Nagarajan, Bio-based flame-retardant coatings based on the synergistic combination of tannic acid and phytic acid for nylon-cotton blends. ACS Appl. Mater. Inter. 51, 61620–61628 (2021). https://doi.org/10.1021/acsami.1c16474
S. Qiu, J. Sun, H. Li, X. Sun, B. Fei, S. Zhang, A green way to simultaneously enhance the mechanical, flame retardant and anti-ultraviolet aging properties of polylactide composites by the incorporation of tannic acid derivatives. Polym. Degrad. Stabil. 196, 109831 (2022). https://doi.org/10.1016/j.polymdegradstab.2022.109831
Y. Yin, Z. Huang, P. Wang, Z. Zhu, J. Xu, S. Chen, H. Wang, The use of copper ions and tannic acid to enhance the UV protection of cotton fabrics. J. Nat. Fibers 19, 3492–3501 (2022). https://doi.org/10.1080/15440478.2020.1848708
W. Zhang, Z.Y. Yang, R.C. Tang, J.P. Guan, Y.F. Qiao, Application of tannic acid and ferrous ion complex as eco-friendly flame retardant and antibacterial agents for silk. J. Cleaner Prod. 250, 119545 (2020). https://doi.org/10.1016/j.jclepro.2019.119545
W. Zhang, Z.Y. Yang, X.W. Cheng, R.C. Tang, Y.F. Qiao, Adsorption, antibacterial and antioxidant properties of tannic acid on silk fiber. Polymers 11, 970 (2019). https://doi.org/10.3390/polym11060970
X.L. Luo, Y.N. Ma, H. Chen, L. Liu, Z.Q. Hu, Z.Y. Li, J.M. Yao, Highly fireproof, antimicrobial and UV-resistant cotton fabric functionalized with biomass tannic acid and phytic acid. J. Mater. Sci. 57, 14528–14542 (2022). https://doi.org/10.1007/s10853-022-07536-7
X. Zhou, Y.K. Yin, Z.Y. Huang, L. Fu, L.X. Wang, S.H. Chen, H. Wang, Chemical reaction intumescent flame retardant cotton fabric with flame retardancy and UV resistance prepared from phytic acid, tannic acid and diethylenetriamine. Int. J. Cloth. Sci. Technol. 34, 716–731 (2022). https://doi.org/10.1108/IJCST-09-2021-0134
Acknowledgements
This work was supported by the Natural Science Foundation of Anhui Province (No. 1908085QE225), the Key Research and Development Project of Anhui Province (No. 202004a06020023), the Innovation Team Project of Anhui Polytechnic University and the Young and middle-aged Top Talent Project of Anhui Polytechnic University.
Funding
This article is funded by Natural Science Foundation of Anhui Province, 1908085QE225, Yinchun Fang, Key Research and Development Project of Anhui Province, 202004a06020023, Xinhua Liu, Innovation Team Project of Anhui Polytechnic University, No, Xinhua Liu, Young and middle-aged Top Talent Project of Anhui Polytechnic University, No, Yinchun Fang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, C., Fang, Y. & Liu, X. Durable Multifunctional Modification of Nylon Fabric with Excellent Flame Retardancy, Antibacterial and UV-Resistant Properties. Fibers Polym 25, 515–523 (2024). https://doi.org/10.1007/s12221-023-00442-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00442-y