Abstract
Immobilization of silver nanoparticles (Ag NPs) to improve recyclability is crucial for applications in nanocatalysts. Herein, silver nanoparticles were prepared on copper foil by immersing copper foil in the solution of silver citrate, containing an excess of citric acid. The nanoparticles were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDS), and atomic force microscope (AFM). The catalytic activity of silver nanoparticle on copper was studied in reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in the presence of excess borohydride. The catalyst can be easily recycled and showed excellent reusability as a conversion higher than 95% was achieved after 30 cycles. Thus, the preparation of nanoparticle aggregates on copper foil has been proven a feasible, straightforward, and effective protocol, which would facilitate the applications of Ag NPs in environmental control.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silver nanoparticles have been investigated extensively to understand their physical and chemical properties during recent years, not only for scientific knowledge but also for technological applications. However, their applications usually suffer from difficult recovery and irreversible aggregation due to the high surface energy and large surface area [1, 2]. Immobilization of silver nanoparticles on a variety of supports including semiconductors [3, 4], metal oxides, [5] polymers, [6,7,8,9,10] and carbon materials has been proven capable of addressing these issues, [11, 12] and then broadening the applications within, for example, memory devices, [13, 14] photovoltaic cells, [15] and supercapacitors [16]. Moreover, the immobilization of nanoparticles on the supports can facilitate their reuse as a result of accessible separation from the reaction mixture, [17,18,19,20] and also provide them free of stabilizer. Nevertheless, weak interactions between silver nanoparticles and the supports give rise to the leakage, aggregation, [21] or poor distribution of nanoparticles [22]. In this regard, preparing silver nanoparticles with good stability are crucial.
Manufacturing of many antipyretic and analgesic drugs, such as phenacetin, paracetamol, and so on, needs 4-aminophenol as a potent intermediate. It is also used enormously as a corrosion inhibitor, photographic developer, anticorrosion-lubricant, and hair-dyeing agent [23, 24]. Thus, being a common precursor material for 4-aminophenol, a newer and cheaper method for catalytic hydrogenation of 4-nitrophenol is always in demand. The conventional procedures for hydrogenation of 4-NP involve iron/ acid as a reducing agent [25]. However, treatment with metallic reagents has limitations. Metal oxides are produced in huge amounts as sludge out of these reactions. Therefore, there is a demand for an alternative effective and green method for the reduction of 4-nitrophenol. As a consequence, hydrogenation of 4-nitrophenol has been studied in the presence of various heterogeneous metal catalysts such as Pd, [26] Ni [27, 28], Re, [29] Pt, [30] and TiO2-supported Ni [31] and the reduction of 4-NP by hydrazine in the presence of Raney nickel as a catalyst in ethanol–water have been studied. The reduction of 4-nitrophenol by borohydride in the presence of suitable catalysts has also been accepted as an alternative route [32]. In this respect, catalytic applications of nanosized Ni, [33] Au and Ag, [34,35,36,37] bimetallic Pt-Ni, [38] resin bound silver, [39] and so on could be worth mentioning. In addition, metal nanoparticles synthesized on polymeric matrix such as poly(acrylic acid) and poly (nisopropylacrylamide) have also shown their worth for catalytic reduction of 4-nitrophenol [40, 41].
Here we present a cost-effective and simple method, based on a wet chemical reaction, to produce silver nanoparticles deposited on commercial copper foil in a reliable way. This method involves the galvanic displacement (also known as electroless deposition) of silver on commercial copper foil. It is subsequently demonstrated that the thus-obtained silver structures can be used for reduction of 4-nitrophenol with high recyclability.
2 Experimental Section
2.1 Materials
All the chemicals were analytical reagent grade and used as purchased without further purification. Silver nitrate (AgNO3), citric acid and 4-nitrophenol were obtained from Sigma Alderich Company.
2.2 Deposition of Silver Onto Commercial Copper Foil
For electropolishing, the copper foils were degreased in ethanol and acetone for 5 min, respectively, followed by distilled water rinsing after each step. The copper foils were then immersed in a 0.1 M HNO3 aqueous solution for 2 min with ultrasound in order to remove the oxidized surface layer and then rinsed with distilled water and dried at room temperature for future use. An acidic solution of silver citrate (Ag3C6H5O7, 300 ppm), containing excess of citric acid (H3C6H5O7) were prepared. For deposition of silver, the Cu foil was immersed into distilled water and then the prepared solution of silver citrate was added dropwise to the reaction vessels for 48 h at room temperature (about 298 K), followed by rinsing with a large amount of distilled water for three times. The products were finally dried and stored in for further usage (Fig. 1).
3 Characterization
3.1 XRD, FESEM, and EDX Analyses
The morphology and composition of the of silver nanoparticles were examined with the SEM, AFM, XRD and EDX experiments. Structural and morphological properties and the uniformity of the silver nanoparticles on copper were studied by SEM, XRD and AFM. Scanning electron microscopy (SEM) images show that films exhibit uniform surface morphology with well-defined spherical particles with average size of 10–15 nm without agglomeration (Fig. 2).
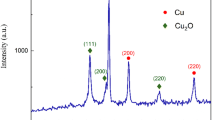
The nature of Ag entities on copper foil was probed using Grazing incidence X-ray diffraction and Energy Dispersive Spectroscopy (EDS). The XRD pattern of silver film on copper (Fig. 3) includes four obvious peaks at 38.16°, 44.32°, 64.50° and 77.4° corresponding to the (111), (200), (220) and (311) planes, which are reflections of face-centered cubic (fcc) silver (JCPDS, silver file 4–0783). No characteristic peaks to indicate impurities such as Ag2O were found in this sample. Thus, pure silver metal with fcc symmetry was synthesized on the commercial copper foil. The sharp diffraction peaks of copper foil appeared at 2θ values of 43.7°, 50.9° and 74.5° for the index (111), (200) and (220) planes of crystalline Cu particles, respectively.
The silver nanoparticles were also confirmed by EDX patterns (Fig. 4). The characteristic peaks related to the silver are located between 2 and 4 keV. In Fig. 4, the intense peaks attributed to elemental silver are observed between 2.9 and 3.6 keV, pertaining to the silver characteristic lines Lα and Lβ. These results confirm those obtained by the other analysis methods.
The Atomic Force Microscopy (AFM) helps in evaluating parameters like amplitude or height parameters, functional or statistical parameters and spatial parameters which describe the surface topography or the roughness. The topographic images of the silver nanoparticles were obtained using a Bruker make multimode-V atomic force microscope (AFM) in contact mode (air) with silicon nitride tip of 10 nm radius of curvature (Fig. 5). As shown in Fig. 5, the AFM results showed that the silver layer is smooth and the maximum height difference of the cross section is negligible.
3.2 Optimization of the Catalyst
To determine the optimum amount of sodium borohydride and the catalyst for the reduction of 4-nitrophenol, reduction studies were carried out with varying amounts of NaBH4 and the catalyst. It can be seen from Table 1 that upon keeping the amount of sodium borohydride constant, the time for completion of the reduction of 4-nitrophenol decreases on increasing surface of the catalyst. Similarly, upon keeping the surface of catalyst constant, the time for completion of the reaction decreases on increasing the amount of borohydride reagent. No reaction occurred either in the absence of the catalyst or sodium borohydride. To optimize the time duration of the reaction as well as the amount of the catalyst and NaBH4 required for the reaction, 220 cm2 surface of the containing 0.02 mol of NaBH4 was selected as the optimum amount for reducing 2.0 × 10−4 mol of 4-nitrophenol. This experiment is important because an optimum time duration once determined will enable one conveniently to follow the reaction kinetics of the reduction of 4-nitrophenol spectrophotometrically, which will be discussed in the next section.
3.3 Catalytic Reduction of 4-nitrophenol
The catalytic activity of the silver nanostructures on copper foil was investigated in the reduction of 4-NP to 4-AP by NaBH4 (Fig. 6). Catalytic reduction of 4-nitrophenol was monitored via Uv/Vis spectrophotometry. In the absence of silver nanostructures on copper foil, it was observed that the absorption band of 4-nitrophenol (λmax = 317 nm) is red shifted to 400 nm, which is attributed to the formation of 4-nitrophenolate ions under alkaline conditions by the introduction of NaBH4, which can be observed by the color change from light yellow to dark yellow after addition of NaBH4. The intensity of the peak remained unaltered at 400 nm with time. However, in the presence of the catalyst the dark yellow color of 4-nitrophenolate ions solution vanished quickly, indicated by the fast intensity decrease in the absorbance of 400 nm. Concomitantly, a new peak appeared at 300 nm, and increased with reduction time, which is assigned to 4-aminophenol, confirming the reduction 4-NP to 4-AP. The experiment shows that the reaction did not happen when no the catalyst was used.
Figure 6 shows the real time changes in the absorbance of both the reactant 4-NP and the product 4-AP in the presence of the catalyst. There is no change in the absorbance of 4-NP within the first 5 s, which we define as the induction period (t0). During this period, the catalyst does not seem to participate in the reduction reaction, and the catalysis reaction is paralyzed until the induction period is finished. After induction period, the reduction starts and the absorbance of 4-nitrophenol decreases. Figure 6 shows the absorption spectrum of the reaction mixture at time interval of 0.5 min. It is seen from the spectrum that the intensity of the absorption peak of 4-NP decreases to almost zero within 12 min.
The reaction can be described as follows: Sodium borohydride reacts with water to generate large amounts of hydrogen molecules and NaBO2, this reaction catalyst by the silver nanostructures, and at the same time, 4-nitrophenol adsorbs onto the silver surface. Here, 4-nitrophenol is reduced to 4-aminophenol via two intermediates, namely 4-nitrosophenol and 4-hydroxyaminophenol, which desorbs afterwards (Scheme 1). At λ = 400 nm, one can see the decrease of the absorption of the nitrophenolate ion, while the increasing peak at around λ = 300 nm indicates the increasing concentration of 4-aminophenol. There are three isosbestic points (280, 320 and 470 nm) in the UV/vis-spectra which are in full agreement with most published data. It should be noted that gas bubbles of H2 may evolve during the runs (Fig. 6). These bubbles would severely impede the optical measurements because their presence would lead to a shift of the UV–vis spectra and a loss of the isosbestic points.
The catalytic reduction of 4-NP to 4-AP is schematically presented in scheme 1. It is a six-electron transfer process. The role of the catalyst in redox reactions can be described by electrochemical current potential. In the present case, electron transfer occurs from BH4− to 4-NP through adsorption of the reactant molecules on to the catalyst surface. The heterogeneous catalysis reaction may occur in four steps: (1) Adsorption is commonly an essential first step in heterogeneous catalysis, (2) formation of the surface complex by diffusion of the molecule to the active site, (3) reaction of the complex to form the adsorbed product, and (4) finally desorption of products from adsorption sites. The mechanism, in the case of heterogeneous catalysis, depends on many factors, namely, the adsorption behavior of the molecules, the concentration, surface coverage, and the nature of the substrate.
If an excess of borohydride is used, the catalytic reduction reaction occurred at the surface of catalytic nanostructures following a monomolecular mechanism.
The reactant A (4-NP) interacted with the active sites on the catalyst surface C (the silver nanoparticles on copper foil) to form the adsorbed species AC, which underwent reaction to form the product P (4-AP). Thus, the kinetics of the reduction can be treated as pseudo-first-order in 4-NP concentration, which simplifies the present analysis. The pseudo-first-order rate constant of the reaction catalyzed by the catalyst was calculated by measuring the disappearance of the characteristic peak, viz., the concentration of 4-NP quantitatively at 400 nm collected at given intervals (Fig. 7). The rate constant can be calculated using the formula
Recyclability of catalysts is a vital factor for green chemistry and their practical application. Reuse cycles of the silver nanoparticles on copper foil catalyst were then examined. The catalyst was separated from the reaction solution after each cycle, and then washed for the next run under identical condition. Moreover, no significant change was observed in the properties, size and morphology of the catalyst even after running of 30 cycles (Fig. 7b). As shown in Fig. 7a, a linear relationship between ln([4NP]t/[4NP]0) and reaction time (t) is obtained for the catalyst in the first cycle and after running 30 cycles, indicating the reaction is first-order with respect to 4-nitrophenol. The rate constants (k) are measured from the slope of the lines and the values are 0.0032 and 0.0025 s−1 for cycle 1 and cycle 30, respectively. A catalyst supported on solid matrix has always been appreciated in the case of expensive catalysts because of their recyclability. In the present experiment the catalyst is good in terms of its recyclable property. A long shelf life of the catalyst was also tested. After storage at room temperature for 12 months, no detectable change was observed in the catalytic activity of the catalyst. Therefore, the as-prepared silver nanolayer catalyst was proven to have excellent catalytic activity, reusability, and long term stability, and thus to be a promising catalyst for the borohydride reduction reaction of 4-nitrophenol.
The catalyst turnover number (TON) and the turnover frequency (TOF) are two important quantities used for comparing catalyst efficiency. In heterogeneous catalysis, the TON is the number of reactant moles that 1 g of catalyst can convert into products. The TOF is simply TON/time. The mass of silver nanoparticles on the copper foil was calculated by mass of silver nitrate used to deposit silver on copper foil. For the catalyst, using 2.0 × 10–4 M concentration of 4-NP and 1.0 g·L−1 catalyst, the TOF was found to be 1.73 × 10–4 mol·g−1·s−1.
4 Conclusion
We show here that simple galvanic displacement from acidic baths can deposit silver nanostructure on copper foil in certain ranges of pH. The particles were characterized by XRD, SEM, AFM and EDS analysis, and they were found to be spherical and crystalline, and with sizes of < 15 nm. The effectiveness of the silver nanostructure on copper foil as catalyst for 4-NP reduction to 4-AP by excess borohydride has been evaluated. The catalysts supported on solid matrix have been appreciated in the case of expensive catalysts because of their recyclability. This makes the process cost-effective.
References
Zhang J, Zhang M, Tang K, Verpoort F (2014) Small 10:32
Kumar KS, Kumar VB, Paik PJ (2013) Nanoparticle 1:1
Zhu BB, Lu DN, Ge J, Liu Z (2011) Acta Biomater 7:2131–2138
Mashhadizadeh MH, Karami Z (2011) J Hazard Mater 190:1023
Ai L, Zeng C, Wang Q (2011) Catal Commun 14:68
Gupta S, Uhlmann P, Agrawal M, Chapuis S, Oertel U, Stamm M (2008) Macromolecules 41:2874
Chen GF, Lu JR, Lam C, Yu Y (2014) Analyst 139:5793
Taheri S, Baier G, Majewski P, Barton M, Forch R, Landfester K, Vasilev K (2014) Nanotechnology 25:305102
Wang RZ, Wang Z, Lin S, Deng C, Li F, Chen Z, He H (2015) RSC Adv 5:40141
Wang S, Zhang J, Yuan P, Sun Q, Jia Y, Yan W, Chen Z, Xu Q (2015) J Mater Sci 50:1323
Qu J-C, Ren C-L, Dong Y-L, Chang Y-P, Zhou M, Chen X-G (2012) Chem Eng J 211–212:412
Huang Q, Wang J, Wei W, Yan Q, Wu C, Zhu X (2015) J Hazard Mater 283:123
Song Y, Cui K, Wang L, Chen S (2009) Nanotechnology 20:105501
Kondo T, Lee SM, Malicki M, Domercq B, Marder SR, Kippelen B (2008) Adv Funct Mater 18:1112
Wang DH, Park KH, Seo JH, Seifter J, Jeon JH, Kim JK, Park JH, Park OO, Heeger AJ (2011) Adv Energy Mater 1:766
Nguyen VH, Shim J-J (2011) Synth Met 161:2078
Zhu J, Zhang Y, Lu D, Zare RN, Ge J, Liu Z (2013) Chem Commun 49:6090
Zhao C, Li X, Li L, Cheng G, Gong X, Zheng J (2013) Langmuir 29:1517
Zhu Z, Guo X, Wu S, Zhang R, Wang J, Li L (2011) Ind Eng Chem Res 50:13848
Xu LQ, Yap BSM, Wang R, Neoh K-G, Kang E-T, Fu GD (2014) Ind Eng Chem Res 53:3116
Zhu H, Ma Z, Clark JC, Pan Z, Overbury SH, Dai S (2007) Appl Catal A 326:89
Lam E, Hrapovic S, Majid E, Chong JH, Luong JH (2012) Nanoscale 4:997
Corbett JF (1999) Dyes Pigm 41:127–136
Rode CV, Vaidya MJ, Chaudhari RV (1999) Org Process Res Dev 3:465–470
Crossley ML (1922) Ind Eng Chem 14:802–804
Lakshmi Kantam M, Chakravarti R, Pal U, Sreedhar B, Bhargava S (2008) Adv Synth Catal 350:822–827
Sugimori A (1961) Bull Chem Soc Jpn 34:407–411
Rizhi C, Yan D, Weihong X, Nanping X (2007) Chin J Chem Eng 15:884–888
Belousov VM, Palchevskaya TA, Bogutskaya LV, Zyuzya LA (1990) J Mol Catal 60:165–172
Vaidya MJ, Kulkarni SM, Chaudhari RV (2003) Org Process Res Dev 7:202–208
Chen R, Du Y, Xing W, Xu N (2006) Chin J Chem Eng 14:665–669
Goswami N, Rahman ML, Huque ME, Qaisuddin M (1984) Chem Technol Biotechnol 34:195–202
Chen R, Wang Q, Du Y, Xing W, Xu N (2009) Chem Eng J 145:371–376
Esumi K, Isono R, Yoshimura T (2004) Langmuir 20:237–243
Panigrahi S, Basu S, Praharaj S, Pande S, Jana S, Pal A, Ghosh SK, Pal T (2007) J Phys Chem C 111:4596–4605
Pradhan N, Pal A, Pal T (2001) Langmuir 17:1800–1802
Praharaj S, Nath S, Ghosh SK, Kundu S, Pal T (2004) Langmuir 20:9889–9892
Ghosh SK, Mandal M, Kundu S, Nath S, Pal T (2004) Appl Catal A 268:61–66
Jana S, Ghosh SK, Nath S, Pande S, Praharaj S, Panigrahi S, Basu S, Endo T, Pal T (2006) Appl Catal A 313:41–48
Lu Y, Mei Y, Schrinner M, Ballauff M, Moller MW, Breu J (2007) J Phys Chem C 111:7676–7681
Lu Y, Mei Y, Ballauff M (2006) J Phys Chem B 110:3930–3937
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azadbakht, R., Menati, S., Amiri Rudbari, H. et al. Deposited Silver Nanoparticles on Commercial Copper by Galvanic Displacement as an Effective Catalyst for the Reduction of 4‑Nitrophenol in Aqueous Solution. Catal Lett 150, 3214–3222 (2020). https://doi.org/10.1007/s10562-020-03219-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03219-7