Abstract
A kind of 2D/2D heterojunction photocatalysts was constructed by a facile in situ hydrothermal method that a tetragonal CuInS2 nanosheets was horizontal loaded in ultrathin hexagonal SnS2 nanosheets. Contrasted to pure CuInS2 and SnS2 nanosheets, the CuInS2/SnS2 nano-heterojunctions revealed a noteworthy improvement photocatalytic activity under visible light irradiation for the degradation of organic dyes, which should be attributed to the narrowest band gap and the efficient charge separation and transfer improving the contact region in heterojunction interface. Especially, with 0.5 wt% addition of CuInS2, the heterojunction nanosheets shows a supreme visible-light photocatalytic activity and a superb degradation efficiency which reached 99% in 60 min with the solution of methyl orange. Moreover, CuInS2/SnS2 nano-heterojunctions deliver excellent reusability in five degradation–regeneration cycles. The results indicated that CuInS2/SnS2 has a promising prospect in organic pollutant treatment due to high efficiency and excellent stability.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A new wave of research activities, the efficient utilization of solar energy, has stimulated by the ever-increasing global demand for energy. Visible-light-driven semiconductor photocatalysts, a promising prospective application in the treatment of environmental pollutants in water, have created widespread interest in the scientific and technology communities [1,2,3,4,5]. However, solar energy conversion efficiency still requires much improvement, although various strategies including bandgap engineering, crystal phase control, and microstructure construction have been employed [6, 7]. Recently, developing heterostructured materials by carefully selecting and coupling two different semiconductors has yielded novel photocatalysts with high activity [8,9,10,11].
Metal sulfides are considered promising materials for coupling with other semiconductors, due to their relatively narrow band gap. Among them, tin sulfide (SnS2) has been attracting increasing attention for its cheap, abundant, and its low band gap (1.91–2.35 eV) as well as it can be designed as an environmental friendly visible-light photocatalyst [12,13,14,15]. However, reports showed that its photodegradation performance should be improved [14, 15]. In contrast with single semiconductor, the photocatalysts performance of the two different semiconductors composite can be successful and higher improved, which owns to the narrowest band gap and the efficient charge separation and transfer improving the contact region in heterojunction interface [16,17,18]. For example, SnS2/BiOBr heterojunction nanoplates showed higher photocatalytic activity than BiOBr and SnS2 for the degradation of rhodamine B under visible light irradiation [18]. Recently, Gao et al. achievement illustrated that the photocatalytic activity of SnS2-based heterojunction could be obviously enhanced by way of extending the photoresponding range and increasing the charge separation efficiency [19]. Besides, Zhang et al. demonstrated that it is the RhB photodegradation apparent rate constant that under visible light is higher than that of pure g-C3N4 and SnS2 nanosheets by a factor of 4 and 8, respectively [20].
The remarkable properties of narrow band gap, high stability, and strong visible-light absorption made ternary chalcogenide compounds gained tremendous attraction, and a notable candidate for the application of photocatalyst in recent years [21]. Taking Copper indium disulfide (CuInS2) for example, it is an universal ternary chalcogenide semiconductor that exhibits an excellent photocatalytic property [22,23,24]. However, the small specific surface area and fast electron–hole recombination have brought a huge obstacle to single CuInS2 semiconductors for the application of photocatalyst. Besides, the truth that subdued photocatalytic activity would incredible decrease its photocatalytic efficiency during recycling process. Moreover, it can be observed that, among those SnS2 and copper indium disulfide (CuInS2), the energy levels of the valence band (VB) and conduction band (CB) can match well between the two, which plays a synergistic effect on and benefits to the transmission of photoinduced charge carriers. Therefore, it can be theoretically find out that the CuInS2/SnS2 heterostructure composites can exhibit excellent photocatalytic activity to remove organic pollutant under visible light.
Herein, a 2D/2D heterojunction photocatalysts was constructed by a facile and simple one-step in situ hydrothermal method, through coupling the tetragonal CuInS2 nanosheets with ultrathin hexagonal SnS2 nanosheets. As such, the as-fabricated CuInS2/SnS2 heterojunction nanosheets exhibit significant enhancement on the photocatalytic activities for the degradation of organic dyes under visible light irradiation as compared to pure CuInS2 and SnS2 nanosheets. The morphology, structure, optical and electrochemical properties were characterized, and the photocatalytic activity of CuInS2/SnS2 heterojunctions was tested by photodegrading methyl orange under visible-light irradiation, and the reusability of CuInS2/SnS2 was evaluated by five consecutive photocatalytic runs. The relationship between its structure and performance, and the possible photocatalytic mechanism were also proposed.
2 Experimental
2.1 Materials and Chemicals
Tin(IV) chloride pentahydrate (SnCl4·5H2O) was purchased from Shanghai Fengxian Chemistry Co., Ltd. (Shanghai, China). Thioacetamide (TAA) and cuprous chloride (CuCl) were attained from Tianjin Chemical Reagent Plant (Tianjin, China). Indium chloride (InCl3·4H2O) and methyl orange (MO) were received from Aladdin Chemistry Co. Ltd. (Shanghai, China). Anhydrous ethanol, sodium sulfate (Na2SO4) and iso-propyl alcohol (IPA) were obtained from Shantou Xilong Chemical Co., Ltd. (Shantou, China). p-Benzoquinone (BQ) was supplied by Shanghai Crystal Pure Reagent Co., Ltd. (Shanghai, China). Triethanolamine (TEOA) was acquired from Shanghai Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals and solvents were of analytical grade and used without further purifcation.
2.2 Preparation of CuInS2/SnS2 Nano-heterojunction
First, the SnS2 nanosheets were synthesized by a hydrothermal method. Briefly, 0.02 mmol of SnCl4·5H2O and 0.04 mol TAA were added in 40 mL of deionized water. After stirring for 30 min, the above mixture was transferred into a Teflon-lined stainless steel autoclave with a capacity of 50 mL. The autoclave was sealed and heated at heated at 180 °C for 20 h and then was naturally cooled to room temperature. The precipitate filtered and washed with distilled water and ethanol for several times, and then dried in a vacuum oven at 100 °C for 5 h.
CuInS2/SnS2 nano-heterojunction was fabricated. First, 0.02 mol of SnCl4·5H2O, 0.04 mol TAA were added in 40 mL of deionized water with magnetic stirring marked as solution (A) Then a certain amount CuInS2 (Cu:In:S = 1:1:2) were added in 20 mL of deionized water under magnetic stirring marked as solution (B) Finally, adding the solution B to the solution A under magnetic stirring drop by drop. After stirring for 30 min, the above mixture transferred into a Teflon-lined stainless steel autoclave with a capacity of 100 mL. The autoclave was sealed and heated at heated at 180 °C for 20 h and then was naturally cooled to room temperature. After that the product was washed with deionized water and ethanol several times, and then was obtained in a vacuum oven at 100 °C for 5 h. Samples mass ratio of CuInS2 to SnS2 were denoted as CuInS2/SnS2 (0.1%), CuInS2/SnS2 (0.5%), CuInS2/SnS2 (1%), CuInS2/SnS2 (3%) and CuInS2/SnS2 (5%), respectively.
2.3 Characterization Methods
Morphologies of CuInS2, SnS2 and CuInS2/SnS2 nano-heterojunction were examined by scanning electron microscopy (SEM, KYKY-EM3900M) and transmission electron microscope (TEM, JEM-2010F) and high-resolution transmission electron microscope (HRTEM, JEM-2010F). The crystal phase of the samples was analyzed by X-ray diffraction (XRD). BET surface areas and pore structures were analyzed using a GEMINI VII 2390 surface area and porosity analyzer (Micromeritics, USA). The reflectance spectra of the samples were obtained from a U-3900H scan UV–Vis spectrophotometer (Hitachi, Japan). The photoluminescence (PL) spectra were measured using F-7000 fluorescence spectrophotometer (Hitachi, Japan) with an excitation wavelength of 325 nm, scanning speed of 1200 nm min−1, and the photomultipliers voltage of 700 V.
2.4 Electrochemical Measurement
The photocurrent of pure CuInS2, SnS2 and 0.5% CuInS2/SnS2 nano-heterojunction were determined by using a Chenhua CHI660C Electrochemical Workstation (Shanghai, China) with a standard three-electrode cell. A standard three-electrode system was composed of CuInS2, SnS2 or CuInS2/SnS2 film fabricated on FTO conductive glass (an effective area of 1 cm2) as working electrode, KCl-saturated calomel electrode (SCE) as reference electrode, platinum plate as counter electrode, and 0.5 mol L−1 Na2SO4 as supporting electrolyte. The applied voltage was 0 V for the FTO working electrode. A 300 W xenon lamp (PLS-SXE300, Beijing Trusttech Co., Ltd. China) with a 400 nm cut off filter (400 nm < λ < 780 nm) was used as the source of visible light.
The flat band potentials of pure CuInS2, SnS2 and 0.5% CuInS2/SnS2 nano-heterojunction were determined by Mott–Schottky plots using the same standard three-electrode system as the photocurrent test, and the applied potential ranges between − 1 and 1 V with the scan frequency of 10 kHz.
Electrochemical impedance spectroscopy (EIS) was measured as the same standard three-electrode system and electrochemical workstation as Mott–schottky test, but the electrolyte solution is the mixture of 0.5 mol L−1 Na2SO4, 2.5 mmol L−1 potassium hexacyanoferrate(III) (K3[Fe(CN)6]), and 2.5 mmol L−1 potassium ferrocyanide (K4Fe(CN)6·3H2O).
2.5 Photocatalytic Activity Measurement
In this set of experiments, 0.03 g of pure CuInS2, SnS2 or CuInS2/SnS2 nano-heterojunction were added in aqueous 100 mL of methyl orange (MO) solution (10 mg L−1). Before the reaction, the suspension was magnetically stirred for 30 min in the dark to reach adsorption–desorption equilibrium. Then the suspension was irradiated by 300 W xenon lamps with a 400 nm cut off filter under magnetic stirring. Every 10 min intervals, 3 mL aliquots were collected from the suspension and immediately centrifuged, and the concentration of MO was analyzed using a 754 UV–Vis spectrometer at 465 nm.
2.6 Regeneration and Reuse of Spent CuInS2/SnS2 Nano-heterojunction Photocatalyst
In order to evaluate the stability of pure SnS2 and 0.5% CuInS2/SnS2 nano-heterojunction catalyst, the catalytic reaction was performed for five times using the same sample. After each cycle, the catalyst separated through centrifugation and washed with deionized water and ethanol several times, then dried at about 100 °C for 4 h. The recovered photocatalyst was used in the next cycle under the same condition as above.
3 Results and Discussion
3.1 XRD Analysis
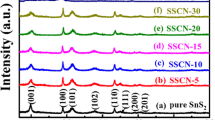
As is shown in Fig. 1, the XRD patterns demonstrated the characterization of CuInS2, SnS2 and CuInS2/SnS2 composites. It can be observed that the CuInS2/SnS2 composite XRD pattern is consistent with those of pure SnS2 (JGPDS No. 023-0677). The main diffraction peaks at about 2θ = 15.03°, 28.20°, 32.12°, 49.96° and 52.50° could be indexed to the (001), (100), (101), (110) and (111) crystalline planes of tetragonal phase of SnS2 respectively. While, observing no typical diffraction peak of CuInS2 in the XRD pattern of CuInS2/SnS2 composite, it is possibly owe to the low loading content of CuInS2.
3.2 Morphology of Samples
The morphologies of pure SnS2 and 0.5% CuInS2/SnS2 nano-heterojunction were characterized by SEM (Fig. 2). Pure SnS2 was composed of hierarchically porous and flower-like spheres, and these spheres are constructed by many bending 2D sheets which are densely interconnected with each other to form an entangled architecture with a quantity of pores. Non-uniform network-like spheres with an average diameter of 5–8 µm were clearly observed from the low-magnification SEM image of pure SnS2 (Fig. 2a). The CuInS2/SnS2 composite shows a rough surface and regular hexahedral shape with a size of 50–70 nm (Fig. 2b).
TEM and HRTEM measurements were performed to further characterize the morphology of 0.5% CuInS2/SnS2. As shown in Fig. 3a, b, the hexagonal SnS2 and tetragonal CuInS2 nano-materials were observed. In addition, as can be seen in Fig. 3c, the measured interplanar spacing is 0.32 nm, it maybe belong to the CuInS2 (112) plane or SnS2 (100) plane. Besides, the SnS2 (100) and (001) plane interplanar spacing is 0.32 and 0.59 nm, respectively, which is corresponded to the XRD results.
3.3 XPS Analysis
XPS is an important technique for the detection of variations in chemical composition and oxidation state for materials. Figure 4a shows the survey spectrum of 0.5% CuInS2/SnS2 heterojunction. Cu, In, Sn and S elements are present in the survey spectrum of CuInS2/SnS2. In addition, the element O (O 1s, 533.14 eV) and C (C 1s, 285 eV) were also obverved in the survey spectrum of CuInS2/SnS2 due to the organic sulfur precursor and the adventitious hydrocarbon from the XPS instrument itself. Figure 4b–e presented the spectra of Sn 3d, S 2p, Cu 2p and In 3d for CuInS2/SnS2 heterojunction. In contrast, the Sn 3d5/2 and Sn 3d3/2 peaks of SnS2 nanosheets which appeared at 486.9 and 495.4 eV [25, 26] respectively, are higher than the corresponding binding energy of CuInS2/SnS2 heterojunction (Fig. 4b). And, two weak peaks which attributed to the Sn–In bonds are found at the Sn 3d core-level of heterojunction nanosheets [27, 28], further indicating the possible bondin configuration of Sn–In on the heterojunction interface. Meanwhile, the S 2p peaks of SnS2 nanosheets, reflecting to S–Sn bond [25, 26], are also changed to the high binding energy side, when coupling with CuInS2 nanosheets. Compared with pure SnS2, the Sn 3d states of CuInS2/SnS2 heterojunction shifted to lower-energy region (Fig. 4b), and the S 2p states of CuInS2/SnS2 heterojunction shifted to higher-energy region (Fig. 4c), which indicated the migration of electrons from CuInS2 to SnS2 in the CuInS2/SnS2 heterojunction [29, 30]. In the CuInS2/SnS2 heterojunction, the binding energy of Cu 2p3/2, Cu 2p1/2, In 3d5/2 and In 3d3/2 located at 932.1, 952.1, 445.1 and 452.5 eV, indicating that the oxidation state of copper and indium are + I and + III, respectively (Fig. 4d, e) [31, 32]. According to the XPS analysis, it can be concluded that CuInS2/SnS2 heterojunctions have been successfully fabricated.
3.4 Brunauer–Emmett–Teller Surface Area and Pore Structure
As is depicted in Fig. 5, it was characterized that the N2 adsorption–desorption isotherms curves of pure SnS2, CuInS2 and CuInS2/SnS2 nano-heterojunction with different CuInS2 content. The results demonstrates that it belongs to type IV and H3 hysteresis loop, indicating the existence of mesopores and macro-porous in nano-heterojunction. All the samples showed broad and bimodal pore size distribution with small mesopores (2–4 nm) and larger ones (5–35 nm), further confirming the existence of mesopores. The BET surface area of SnS2 is 67.9 m2 g−1. With increasing CuInS2 content from 0.1 to 5%, the specific surface area was greatly increased, the BET surface area of CuInS2/SnS2 is much higher than that of SnS2. The main parameters of the samples were listed in Table 1.
3.5 UV–Vis Diffuse Reflectance Spectra
As is shown in Fig. 6, the UV–Vis diffuse reflectance spectra of pure CuInS2, pure SnS2 and CuInS2/SnS2 heterojunctions with different mass ratio are depicted. Contrasted with pure CuInS2 and pure SnS2, the CuInS2/SnS2 heterojunctions absorption intensity was enhanced, with the absorption edge shifting to longer wavelength. Moreover, the band gap energies (Eg) of pure CuInS2, pure SnS2, and CuInS2/SnS2 heterojunctions were calculated by Tauc’s equation:
where α is absorption coefficient, h is planck’s constant, ν is light frequency, A is a constant and E g is band gap energy, and n = 2 for direct-band-gap semiconductor and n = 1/2 for indirect-band-gap semiconductor. The plots of \({(\alpha hv)^2}\) versus \(hv\) were shown in Fig. 6b, and Eg values were obtained by estimating the intercept of the tangent to the plots. The Eg of pure CuInS2, pure SnS2, 0.1% CuInS2/SnS2, 0.5% CuInS2/SnS2, 1% CuInS2/SnS2, 3% CuInS2/SnS2, and 5% CuInS2/SnS2 heterojunctions were estimated to be about 1.57, 2.1, 1.85, 1.91, 1.81, 1.83 and 1.87 eV, respectively.
3.6 Mott–Schottky Analysis
The flat band potentials (V fb ) of CuInS2, SnS2 and CuInS2/SnS2 were calculated according to the Mott–Schottky equation (Fig. 7).
where C = interfacial capacitance, A = the electrode surface, E = applied potential, ε = dielectric constant of the semiconductor, ε 0 = permittivity of free space, T = absolute temperature, e = electronic charge, k B = Boltzmann constant, and N A = carrier concentration [33]. Hence, V fb values can be obtained when a plot of \({1 \mathord{\left/ {\vphantom {1 {{C^2}}}} \right. \kern-0pt} {{C^2}}}\) against potential yields a straight line. The V fb values of CuInS2, SnS2, 0.5% CuInS2/SnS2 are − 0.538, − 0.48, and − 0.43 eV versus the saturated calomel electrode (SCE), respectively. The positive shift of the flat band potentials is consistent with their photocatalytic activities. Moreover, the slope of Mott–Schottky lines is positive, indicating CuInS2, SnS2 and CuInS2/SnS2 heterojunctions with n-type semiconductor properties.
3.7 Photocurrent Transient and PL Emission Spectra Analysis
The photocurrent transient response test of pure CuInS2, pure SnS2 and 0.5% CuInS2/SnS2 heterojunction upon visible-light irradiation was further carried out and the result is shown in Fig. 8. From the Fig. 8a, with light switching on and off, the photocurrent density exhibits a rise and decrease promptly, respectively. Obviously, the photocurrent density of 0.5 wt% CuInS2/SnS2 heterojunction was higher than that of pure SnS2 and CuInS2, which suggests that the CuInS2/SnS2 heterojunction is contribute to rapid separation of photogenerated electrons and holes and agreements with its highest photocatalytic activity. For further detecting the photogenerated electrons and holes recombination, PL emission spectra is usually employed. As Fig. 8b manifested, The PL intensity of 0.5 wt% CuInS2/SnS2 heterojunction was lower than that of pure SnS2 and pure CuInS2. The lowest PL intensity of 0.5 wt% CuInS2/SnS2 indicates the most efficient separation of photogenerated electrons and holes. Both PL and photocurrent results confirmed that the outstanding improvement of charge separation in CuInS2/SnS2 heterojunction photocatalysts.
3.8 Nyquist Impedance Analysis
To evaluate the probability photogenic electron and holes compound, Nyquist impedance analysis was further carried out (Fig. 9). The alternating current (AC) impedance spectra are obtained by equivalent circuit simulation as shown in Fig. 9b, where C = electric double layer capacitor, Zw = Warburg impedance, Rct = charge transfer resistance and Rs = electrolyte resistance, respectively. The total resistance of pure CuInS2 and SnS2 is 50 and 296 Ohm, respectively. The total resistance of CuInS2/SnS2 is only 37 Ohm. It is clear to see that the formation of CuInS2/SnS2 heterojunction is able to lead to dramatic decrease in the semicycle arc as compared to pure CuInS2 and SnS2, suggesting a higher charge transfer rate occurred in CuInS2/SnS2 heterojunction electrode. It is consistent with the PL analysis and photocurrent orders.
3.9 The Evaluation of Photocatalytic Activity
3.9.1 Photocatalytic Performance
The visible-light photocacalytic performance of pure CuInS2, SnS2 and CuInS2/SnS2 heterojunction is displayed in Fig. 10. It was clearly to see from Fig. 10 that the photocatalytic activity of CuInS2/SnS2 heterojunction was higher than that of pure CuInS2. As the amount of CuInS2 increases, the photocatalytic activity increases gradually and then decreases, the order of degradation rate is 0.5 wt% CuInS2/SnS2 > 0.1 wt% CuInS2/SnS2 > 1 wt% CuInS2/SnS2 > 3 wt% CuInS2/SnS2 > SnS2 > 5 wt% CuInS2/SnS2 > CuInS2. The 0.5% CuInS2/SnS2 exhibits the highest photocatalytic activity, which could be attributed to the optimum CuInS2 content resulting in more efficient generation and transfer of photo-generated electrons and holes at interfaces, but excess CuInS2 can result in the recombination of photo-generated electrons and holes as well as hinder light absorption.
3.9.2 Photocatalytic Mechanism
In this part, a tentative photocatalytic reaction mechanism was explored, with IPA (·OH quencher), TEOA (h+ quencher) and BQ (·O2− quencher) beening used and added to the MO solution as scavengers during the MO photodegration process (Fig. 11). As no quenchers added, the MO degradation efficiency reached 99%; while adding TEOA and BQ separately, the degradation efficiency significantly decreased to 23.38% and 15.68%, respectively. Therefore, it can be concluded that the active species (·O2− and h+) have a vital influence on the degradation of MO based on the above results, and the possible photocatalytic mechanism of CuInS2/SnS2 heterojunction is illustrated in Scheme 1. Under visible-light irradiation, both SnS2 and CuInS2 can be excited to generate photogenerated electrons (e−) and holes (h+). The electrons that excited form CuInS2 conduction band (CB) can inject into CB of SnS2; besides, the photogenerated holes in VB of SnS2 have access to transfer easily to the valence band (VB) of CuInS2. Therefore, the separation efficiency of photoinduced charge carriers was remarkably improved by the photosynergistic effect of CuInS2/SnS2 heterojunction through the photoinduced interfacial charge transfer reducing the recombination of photogenerated e− and h+. Since the CB levels of CuInS2 and SnS2 were more negative than the reduction potential of oxygen E (O2/·O2−) (− 0.046 V vs. NHE) [34, 35], thus, the photo-generated e− can react with adsorbed O2 on the surface of the catalyst to produce ·O2− radicals. Compared with the potential of ·OH/H2O (2.68 V vs. NHE) and ·OH/OH− (1.99 V vs. NHE) [36], the remained h+ on the VB of CuInS2 could not react with OH− or H2O generating ·OH radicals because of the lower VB level of CuInS2 (1.28 V vs. NHE). Subsequently, the highly reactive radical species of ·O2− and h+ participated in the photodegration of the MO aqueous solution. The active species trapping experiment results also confirmed that ·O2− and h+ were the main reactive species, with ·OH beening the less critical one for the degradation of MO.
3.10 Photostability of CuInS2/SnS2
The reusability is an important property for practical application. Hence, the photostability of 0.5% CuInS2/SnS2 heterojunction was tested by recycling the photocatalyst for the degradation of MO under visible-light irradiation (Fig. 12). For comparison, the photostability of SnS2 was also investigated. There is no obviously decline in the photocatalytic degradation efficiency of MO after five degradation–regeneration cycles.
4 Conclusions
CuInS2/SnS2 heterojunctions were constructed by a facile in situ one step hydrothermal method. The visible-light photocatalytic activity of CuInS2/SnS2 heterojunctions was influenced by different CuInS2 mass ratio. The optimal 0.5% CuInS2/SnS2 heterojunctions demonstrated the highest visible-light photocatalytic activity, which is almost two times of pure CuInS2. The 0.5% CuInS2/SnS2 showed the most efficient charge separation and transfer, leading to the highest visible-light photocatalytic activity with 99% degradation efficiency of MO in 60 min. Moreover, CuInS2/SnS2 heterojunctions show high catalytic stability after five consecutive cycles, which indicating it has a promising prospect in degradation organic pollutant.
References
Pulkka S, Martikainen M, Bhatnagar A, Sillanpää M (2014) Electrochemical methods for the removal of anionic contaminants from water-a review. Sep Purif Technol 132:252–271
Chong MN, Jin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44:2997–3027
Lachheb H, Puzenat E, Houas A, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2002) Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl Catal B 39:75–90
Yao Y, Qin J, Chen H, Wei F, Liu X, Wang J, Wang S (2015) Graphitic carbon nitride based nanoheterojunctions: a review, one-pot approach for synthesis of N-doped TiO2/ZnFe2O4 hybrid as anefficient photocatalyst for degradation of aqueous organic pollutants. J Hazard Mater 291:28–37
Tian J, Hao P, Wei N, Cui H, Liu H (2015) 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure: enhanced photocatalytic activities and photoelectochemistry performance. ACS Catal 5:4530–4536
Jin J, Yu J, Guo D, Cui C, Ho W (2015) A hierarchical Z-scheme CdS–WO3 photocatalyst with enhanced CO2 reduction activity. Small 11:5262–5271
Tian J, Liu RY, Liu Z, Yu CL, Liu MC (2017) Boosting the photocatalytic performance of Ag2CO3 crystals in phenol degradation via coupling with trace N-CQDs. Chin J Catal 38:1999–2008
Yu CL, Zhou WQ, Zhu LH, Li G, Yang K, Jin RC (2016) Integrating plasmonic Au nanorods with dendritic like α-Bi2O3/Bi2O2CO3 heterostructures for superior visible-light-driven photocatalysis. Appl Catal B 184:1–11
Yu CL, Zhou WQ, Yu JC, Liu H, Wei LF (2014) Design and fabrication of heterojunction photocatalysts for energy conversion and pollutant degradation. Chin J Catal 35:1609–1618
Yu CL, Li G, Kumar S, Yang K, Jin RC (2014) Phase transformation synthesis of novel Ag2O/Ag2CO3 heterostructures with high visible light efficiency in photocatalytic degradation of pollutants. Adv Mater 26:892–898
Mao LY, Li JJ, Xie YL, Zhong YJ, Hu Y (2014) Controllable growth of SnS2/SnO2 heterostructured nanoplates via a hydrothermalassisted self-hydrolysis process and their visiblelight-driven photocatalytic reduction of Cr(VI). RSC Adv 4:29698–29701
Mondal C, Ganguly M, Pal J, Roy A, Jana J, Pal T (2014) Morphology controlled synthesis of SnS2 nanomaterial for promoting photocatalytic reduction of aqueous Cr(VI) under visible light. Langmuir 30:4157–4164
Zhang ZY, Shao CL, Li XH, Sun YY, Zhang MY, Mu JB, Zhang P, Guo ZC, Liu YC (2013) Hierarchical assembly of ultrathin hexagonal SnS2 nanosheets onto electrospun TiO2 nanofibers: enhanced photocatalytic activity based on photoinduced interfacial charge transfer. Nanoscale 5:606–618
Zhang YC, Li J, Xu HY (2012) One-step in situ solvothermal synthesis of SnS2/TiO2 nanocomposites with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI), Appl Catal B 123–124:18–26
Khanchandani S, Srivastava PK, Kumar S, Ghosh S, Ganguli AK (2014) Band gap engineering of ZnO using core/shell morphology with environmentally benign Ag2S sensitizer for efficient light harvesting and enhanced visible-light photocatalysis. Inorg Chem 53:8902–8912
Xu QQ, Feng JT, Li LC, Xiao QS, Wang J (2015) Hollow ZnFe2O4/TiO2 composites: high-performance and recyclable visible-light photocatalyst. J Alloy Compd 641:110–118
Xu Y, Zhang WD (2015) CdS/g-C3N4 Hybrids with improved photostability and visible light photocatalytic activity, Eur J Inorg Chem 2015:1744–1751
Qiu FZ, Li WJ, Wang FZ, Li HD, Liu XT, Sun JY (2017) In-situ synthesis of novel Z-scheme SnS2/BiOBr photocatalysts with superior photocatalytic efficiency under visible light. J Colloid Interface Sci 493:1–9
Gao XM, Huang GB, Gao HH, Pan C, Wang H, Yan J, Liu Y, Qiu HX, Ma N, Gao JP (2016) Facile fabrication of Bi2S3/SnS2 heterojunction photocatalysts with efficient photocatalytic activity under visible light. J Alloy Compd 674:98–108
Zhang ZY, Huang JD, Zhang MY, Yuan Q, Dong B (2015) Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl Catal B 163:298–305
Deng F, Lu XY, Zhao LN, Luo YT, Pei XL, Luo XB, Luo SL (2016) Facile low-temperature co-precipitation method to synthesize hierarchical network-like g-C3N4/SnIn4S8 with superior photocatalytic performance. J Mater Sci 51:6998–7007
Yu C, Zhang LL, Tian L, Liu D, Chen FL, Wang C (2014) Synthesis and formation mechanism of CuInS2 nanocrystals with a tunable phase. CrystEngComm 16:9596–9602
Siemer K, Klaer J, Luck IK, Bruns J, Klenk R, Bräunig D (2001) Efficient CuInS2 solar cells from a rapid thermal process (RTP). Sol Energy Mater Sol Cells 67:159–166
Yuan YJ, Chen DQ, Huang YW, Yu ZT, Zhong JS, Chen TT, Tu WG, Guan ZJ, Cao DP, Zou ZG (2016) MoS2 nanosheet-modified CuInS2 photocatalyst for visible light-driven hydrogen production from water. ChemSusChem 9:1003–1009
Zhang Z, Shao C, Li X, Sun Y, Zhang M, Mu J, Zhang P, Guo Z, Liu Y (2013) Hierarchical assembly of ultrathin hexagonal SnS2 nanosheets onto electrospun TiO2 nanofibers: enhanced photocatalytic activity based on photoinduced interfacial charge transfer. Nanoscale 5:606–618
Zhang YC, Du ZN, Li KW, Zhang M, Dionysiou DD (2011) High-performance visible-light-driven SnS2/SnO2 nanocomposite photocatalyst prepared via in situ hydrothermal oxidation of SnS2 nanoparticles. ACS Appl Mater Interfaces 3:1528–1537
Wu Y, Liu H, Zhang J, Chen F (2009) Enhanced photocatalytic activity of nitrogen-doped titania by deposited with gold. J Phys Chem C 113:14689–14695
Zhou B, Dong S, Zhao H, Liu Y, Wu P (2014) Ferromagnetic spin-order in p-type N-doped SnO2 films prepared by thermal oxidation of SnNx. J Magn Magn Mater 362:14–19
Li XH, Xu HY, Zhang XT, Liu YC, Sun JW, Lu YM (2009) Local chemical states and thermal stabilities of nitrogen dopants in ZnO film studied by temperature-dependent X-ray photoelectron spectroscopy. Appl Phys Lett 95:191903(1)–191903(3)
Zhang Z, Shao C, Li X, Wang C, Zhang M, Liu Y (2010) Electrospun nanofibers of p-type NiO/n-type ZnO heterojunctions with enhanced photocatalytic activity. ACS Appl Mater Interfaces 2:2915–2923
Meng WL, Zhou X, Qiu ZL, Liu CW, Chen JW, J.Yue W, Wang MT, Bi H (2016) Reduced graphene oxide-supported aggregates of CuInS2 quantum dots as an effective hybrid electron acceptor for polymer-based solar cells. Carbon 96:532–540
Xu FY, Zhang JJ, Zhu BC, Yu JG, Xu JS (2018) CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl Catal B 230:194–202
Lim J, Murugan P, Lakshminarasimhan N, Kim JY, Lee JS, Lee S, Choi W (2014) Synergic photocatalytic effects of nitrogen and niobium co-doping in TiO2 for the redox conversion of aquatic pollutants under visible light. J Catal 310:91–99
Chai B, Peng TY, Mao J, Li K, Zan L (2012) Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys Chem Chem Phys 14:16745–16752
Hao RR, Wang GH, Tang H, Sun LL, Xu C, Han DY (2016) Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl Catal B 187:47–58
He YM, Zhang LH, Teng BT, Fan MH (2015) New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ Sci Technol 49:649–656
Acknowledgements
This work supported by the scientific research key project item of Hubei province education office, China (No. D20172904).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, K., Zhang, Y. & Zhang, W. Ultrathin Hexagonal SnS2 Nanosheets Coupled with Tetragonal CuInS2 Nanosheets as 2D/2D Heterojunction Photocatalysts Toward High Visible-Light Photocatalytic Activity and Stability. Catal Lett 148, 1990–2000 (2018). https://doi.org/10.1007/s10562-018-2413-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2413-5