Abstract
A series of nano-structured, mesoporous Au/Ce1 − xZrxO2 type catalysts were synthesized by first synthesizing Ce1−xZrxO2 type of support with different degree of Zr substitutions and following sol–gel route. One weight percent of Au was incorporated on these supports using deposition–precipitation method. These synthesized catalysts were characterized in detail by various techniques, and evaluated for their CO oxidation activity. Effect of SO2 poisoning on catalytic CO oxidation activity was also investigated in detail. It was observed that the catalytic activity for CO oxidation reaction depends on the Ce/Zr molar ratio. The effect of SO2 on CO oxidation was less prominent for the Zr incorporated ‘Au/Ce0.8Zr0.2O2 (ACZ0.2)’ as compared to bare ‘Au/CeO2 (AC)’ catalyst. In case of ACZ0.2, the activity remains quite stable even after 5 h of SO2 exposure, however, after 12 and 18 h, the activity decreases to 62 and 55%, respectively. Whereas AC catalyst shows rapid decline in catalytic activity from the first hour of SO2 exposure, and continue to decline significantly to 35 and 18% after 12 and 18 h of SO2 exposure, respectively. Multiple regeneration studies of both bare and Zr incorporated catalysts show pronounced improvement/regain in catalytic activity.
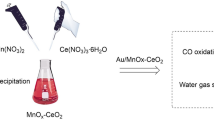
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carbon monoxide (CO) is one of the major air pollutants released from both natural and anthropogenic sources, especially from industries, automobiles and several other combustion sources. Incomplete combustion of fossil fuels and biomass leads to CO emissions via line and point sources, such as engines, oil burners, boilers, and water heaters [1,2,3,4,5]. Since CO is present with different co-existing gases such as N2, CO2, water vapor, and sometimes including poisonous gases like SO2 as well as at different temperatures, hence CO mitigation needs to be source specific. Catalytic CO oxidation is a well-known and commercially exploited process; however, nano-structured catalysis had opened a new gateway for the researchers to further improve the catalysts with respect to efficiency and stability for low temperature CO oxidation. Activity of a catalyst depends on both intrinsic and physical properties, while extensive efforts have also been focused on tailoring the surface area, pore size, morphology, and other physical properties [6,7,8,9,10,11,12,13].
Haruta et al. demonstrated that supported gold nano particles are highly effective for low temperature CO oxidation reactions and they have also discussed the effect of different supports over catalytic activity [14, 15]. Catalytic activity of gold nanoparticles supported catalysts have been extensively studied using various supports such as Fe2O3, CeO2, MnO2, MgO, TiO2, Al2O3, ZnO, Co3O4, ZrO2, SiO2, MCM-48, CuO–CeO2, TiO2–SiO2, etc. [16,17,18,19,20,21,22,23,24,25,26,27]. Bulk gold particles are either inactive or poorly active at low temperature compared to supported gold catalysts. Exceptional activity of supported gold catalysts can be attributed to the metal-support interface as well as reducible active supports. It is generally accepted that the synergy between metal catalysts and oxide based supports, as well as reducibility of such oxide supports are significant aspects governing catalytic activity for many important reactions, including CO, VOCs, and soot oxidation [28,29,30]. Oxide supports are mainly categorized as active or ‘reducible’ and inert or ‘non-reducible’ support according to their redox properties. For instance, Al2O3, MgO, SiO2, etc. are generally non-reducible supports while CeO2, Fe2O3, MnO2, TiO2, etc are considered reducible or active supports because of the presence of mobile oxygen atoms. Reducible active supports enhance the oxygen mobility during the reaction, which is considered as an important criterion for oxidative reactions. As a result, developing low-cost gold catalysts with superior oxidation activity at low temperatures is still a strong area of research interest.
CeO2–ZrO2 mixed oxides have been widely investigated for the three-way catalysis due to their high thermal and chemical stability, these have also been found suitable for water gas shift reactions [31,32,33]. CeO2–ZrO2 based oxide catalysts are found to be more promising due to the presence of defects formed via substitution of the smaller Zr4+ in place of Ce4+ cation into the ceria lattice [34, 35]. The catalytic activity of CeO2–ZrO2 oxide also depends on its surface area, particle size and most importantly on Ce/Zr molar ratio [36,37,38,39,40]. Although catalytic CO oxidation over CeO2–ZrO2 supported catalysts have been studied extensively, the effect of SO2 poisoning and regeneration studies were seldom reported [41,42,43]. Deshmukh et al. have studied the effect of SO2 poisoning over CeO2 and CeO2–ZrO2, and observed the superior tolerance of CeO2–ZrO2 in comparison with CeO2 [41]. Two weight percent of Pd loaded (Ce–Zr) O2 catalysts have been reported for CO oxidation by Ryou et al. although, they have observed good activity even after SO2 exposure and regeneration, the CO conversion was achieved at the temperature below 100 °C was insignificant [43]. Therefore, it is indeed very important to develop a catalyst, which is active at lower temperatures at possibly around room temperature with different feed stream conditions. In the present study, a series of Au/Ce1−xZrxO2 were synthesized by sol gel method, and were evaluated for their catalytic activity toward CO oxidation. The effect of presence of SO2 in the feed stream has been investigated thoroughly as a function of time-on-stream (TOS). Multiple regeneration studies have also been studied on the select catalysts using different conditions. The aim of this research is to understand the importance of physical as well as chemical properties of CeO2 with Zr incorporation for their catalytic activity. The synthesized catalysts were characterized by using different techniques, such as Powder X-ray diffraction (p-XRD), transmission electron microscopy (TEM) and N2 physisorption analysis.
2 Experimental
2.1 Materials
Cerium nitrate, zirconyl nitrate, and citric acid used for the synthesis of Ce1−xZrxO2 were procured from HiMedia, India (analytical grade). Analytical grade HAuCl4 and NaOH were procured from Sigma-Aldrich and Merck India, respectively.
2.2 Synthesis of Ce1−xZrxO2 Support
The citrate sol–gel method was used for the synthesis of a series of Ce1−xZrxO2 (x = 0, 0.1, 0.2, 0.25, 0.4 and 0.5) supports. Stoichiometric amount of cerium nitrate, zirconyl nitrate, and citric acid were dissolved in requisite volume of distilled water to obtain a transparent solution (the molar ratio of total metal nitrate precursors/citric acid was kept as 1:2). The solution was gradually heated at 80 °C (around 2–3 h) to form a viscous solution or gel, and the gel was subsequently heated until a spongy mass was obtained. The spongy mass was thoroughly homogenized by grinding and subsequently calcined at 400 °C for 5 h in a muffle furnace to obtain Ce1−xZrxO2 supports.
2.3 Synthesis of Au/Ce1−xZrxO2
One weight percent of Au deposited Ce1−xZrxO2 samples were prepared by following the deposition precipitation (DP) method. Appropriate amount of the calcined Ce1−xZrxO2 powder was soaked with distilled water, and the pH of the obtained suspension was adjusted to eight by adding 0.1 M NaOH. HAuCl4 solution having pH 8 (adjusted by 0.1 M NaOH solution) was added to the above suspension with continuous stirring. The mixture was stirred at 60 °C for 2 h followed by sonication for 30 min. The product thus obtained was filtered and washed with distilled water for several times to eliminate Cl− ions completely and subsequently dried at 110 °C for 10 h. The Au deposited Ce1−xZrxO2 samples were denoted as ACZx (x = 0.1, 0.2, 0.25, 0.4 and 0.5, where x indicates the mol% of Zr) and AC for x = 0.
2.4 Material Characterization
p-XRD patterns were recorded using Rigaku Miniflex II instrument operated at 30 kV and 15 mA with a monochromator using Cu Kα radiations. The samples were scanned in the 2θ range 10°–80° with a scanning speed of 3° min−1. Diffraction peaks were compared with the standard Joint Committee on Powder Diffraction Standards (JCPDS) database reported by the International Centre for Diffraction Data (ICDD). Crystallite size was also calculated using the Scherrer’s equation. N2 adsorption–desorption isotherms were recorded at −196 °C using a Micromeritics ASAP-2000 instrument. Prior to analysis, the samples were evacuated and pretreated at 200 °C for 6 h. The pore size distribution and surface area were analyzed using the Barrett–Joyner–Halenda (BJH) and Brunauer–Emmett–Teller (BET) method, respectively.
The morphological and structural details of the select ACZ0.2 were studied by TEM. TEM was carried out on a JEOL JEM-3010 microscope operated at 300 kV (LaB6 cathode, point resolution 1.7 Å). For TEM analysis, pinch of catalyst was dispersed in ethanol and ultrasonicated for 15 min. The suspension so obtained was dried at room temperature on a copper grid. Au loading was determined by inductively coupled plasma optical emission spectrometer (ICP–OES) using a Perkin Elmer-Optima 4100 DV instrument.
2.5 Catalytic Activity Measurements
The steady state gas evaluation assembly equipped with a quartz fixed bed type reactor, precise mass flow controllers (Alborg, USA), gas mixing chamber, heating system and gas chromatograph (GC) was used to carry out the CO oxidation reaction at atmospheric pressure. All the catalytic activity experiments were performed using 100 mg of catalysts. The simulated gas consist of 500 ppm CO, 20% O2 balance by He with a total flow rate of 100 SCCM. The reaction was continuously analyzed online by using a Shimadzu GC (GC-2014) equipped with a thermal conductivity detector (TCD). Prior to the evaluations, all the catalysts were subjected to heat treatment in He flow at 100 °C for a period of 0.5 h, to remove any adsorbed gases or contaminant. Effect of SO2 on catalytic CO oxidation was studied by introducing SO2 (10–80 ppm) in the feed stream along with the other gases. The catalytic activity was evaluated in terms of % conversion (X) of CO gas to CO2 according to the below Eq. (1),
3 Results and Discussion
3.1 Characterization Studies
p-XRD analysis results of all the ACZx catalytic materials are given in Fig. 1 and Table 1. Diffraction patterns of all the samples were compared with the standard JCPDS database, which clearly confirms the formation of crystalline cubic fluorite structure (Fm3m space group) of CeO2/Ce1 − xZrxO2 solid solutions with characteristic diffraction peaks corresponding to (111), (200), (220), (311), (222), (400), and (331) planes. The gradual shift of diffraction peaks towards higher 2θ values as well as change in lattice parameters was observed with increasing Zr incorporation. Peak shift as well as change in lattice parameters of cubic structure could be owing to the dissimilar ionic radii of Ce4+ and Zr4+ ions. No characteristic diffraction peak was observed for ZrO2 phase with Zr incorporation up to 50% i.e., ACZ0.5, which evidently confirms the formation of CeO2–ZrO2 solid solutions [44, 45]. In addition, no characteristic diffraction peaks were observed for Au, mainly due to its lower content and high dispersion. The average crystallite size was calculated from line broadening of (111) plane using Scherrer’s equation. The values of lattice parameter, lattice cell volume, and crystallite size are given in Table 1. Decrease in the crystallite size was observed with increase in the Zr/Ce mole ratio. The lattice constant and lattice volume of catalyst also decreases with the increase in Zr/Ce mole ratio. This suggests that unit cell of the cubic phase shrinks in the order AC > ACZ0.1 > ACZ0.2 > ACZ0.25 > ACZ0.4 > ACZ0.5, due to the substitution of smaller Zr4+ ion (0.84 Å) with larger Ce4+ ion (0.97 Å). As given in Table 2, the actual Au content on all ACZx catalysts is found to be almost close to 1% as calculated by ICP–OES analysis.
As shown in Fig. 2, the N2 adsorption—desorption isotherms observed for AC and all ACZx catalysts were type IV indicating the presence of mesopores nature of the structure. However, the area under hysteresis curve in isotherm, as well as pore volume decreases with increasing Zr incorporation. The specific surface area, average pore size, and pore volume were also measured using N2 adsorption isotherms as shown in Table 2. No significant change in surface area was observed with Zr incorporation, whereas the average pore size was observed to decrease with increasing Zr component. However, the better surface area (82 m2 g−1) was observed when the Zr incorporation was 20% i.e., ACZ0.2 catalyst. The morphology and structure of the select ACZ0.2 catalyst was examined by TEM analysis as shown in Fig. 3. TEM images of ACZ0.2 display sheets of mesoporous aggregates of cubic ceria–zirconia solid solution nanocrystals. The particle size of approximately 10 nm was observed for Au nano particles. Electron diffraction pattern of ACZ0.2 catalyst as shown in inset of Fig. 3 confirms the cubic CeO2 structure, which substantiates the result obtained by p-XRD.
3.2 Catalytic Activity
All the synthesized catalysts were evaluated for their catalytic activity toward CO oxidation reaction, as a function of temperature. As shown in Fig. 4, the low-temperature CO oxidation activity of AC improves with 20–25% Zr incorporation. At around 45 °C, complete CO oxidation was observed over AC catalyst, while ACZ0.2 and ACZ0.25 catalyst exhibit 100% conversion at 30 °C. However, the CO oxidation activity slightly decreases with Zr incorporation of either less or more than 20–25%. The complete CO oxidation was achieved over ACZ0.1, ACZ0.4, and ACZ0.5 at around 50, 60, and 80 °C, respectively. The overall good catalytic activity of all these Zr incorporated catalysts could be mainly because of their retained cubic phase structure, whereas the presence of tetragonal ZrO2 phase significantly decreases the catalytic CO oxidation activity [44]. Furthermore, the light-off temperature for all the present catalysts was observed well below the room temperature even under the high space velocity conditions, which clearly explains the practical importance of these catalysts.
3.2.1 Effect of Presence of SO2 in the Feed on CO Oxidation
The effect of SO2 exposure on CO oxidation activity of AC and ACZx catalysts is presented in Fig. 5. The comparative T50, T80, and T100 with the presence and absence of SO2 are also given in Table 3. Noticeable deterioration in CO oxidation activity was observed on all the catalysts after SO2 introduction in the reaction gas feed. Effect of SO2 poisoning was more prominent over AC compared to Zr incorporated ACZx catalysts at lower temperatures. In absence of SO2, the T50 increases in the following order ACZ0.2 ≈ ACZ0.25 ≈ AC > ACZ0.1 > ACZ0.5 > ACZ0.4, whereas it was in the following order ACZ0.2 > AC ≥ ACZ0.25 > ACZ0.5 > ACZ0.4 > ACZ0.1 when 10 ppm SO2 was introduced in the feed. Especially, ACZ0.2 catalyst composition was observed to be optimum with superior resistant towards SO2 in the reaction gas feed stream at lower temperatures. However, T80 and T100 were found to be almost similar for AC and ACZ catalysts with 20–25% Zr incorporation under both the presence and absence of SO2. Turn over frequency (TOF) was calculated as mmol of CO converted per g of catalyst per unit time. Maximum 0.02232 mmol of CO were converted per g of catalyst per min, when AC/ACZ0.2/ACZ0.25 shows the ≈100% CO conversion at room temperature, whereas ACZ0.1/ACZ0.4/ACZ0.5 converts not more than 0.01 mmol of CO at the same temperature. In presence of SO2, the TOF was insignificant at room temperature for all the catalysts except ACZ0.2, which converts maximum of ≈0.006 mmol of CO per g of catalyst per min at room temperature. However, maximum 0.02232 mmol of CO were converted per g of catalyst per min, when AC/ACZ0.2/ACZ0.25 shows the 100% conversion at 100 °C, whereas ACZ0.1, ACZ0.4, and ACZ0.5 convert maximum of 0.008, 0.018, and 0.21 mmol of CO respectively, at 100 °C. These results clearly confirm the noticeable deterioration in CO oxidation activity observed on all the catalysts after SO2 introduction in the reaction gas feed. However, the better TOF was observed over ACZ0.2 catalyst, compared to bare AC and other Zr incorporated catalysts at lower temperature.
3.2.2 Catalyst Stability Studies
The select ACZ0.2 and AC catalysts were subjected to time stream studies at 40 °C to test the catalyst stability in presence and absence of SO2 and the results observed are shown in Fig. 6. The results for comparative activity with SO2 exposure as a function of time and multiple regenerative studies are given in Table 4. The catalyst activity of both AC and ACZ0.2 was found to be quite stable in absence of SO2, whereas the catalytic activity significantly deteriorates over AC as compared to ACZ0.2 catalyst with time in presence of SO2. In case of ACZ0.2, the activity remains quite stable even after 5 h of SO2 exposure, however, after 12 and 18 h, the activity decreases to 62 and 55%, respectively. Whereas for AC catalyst a rapid decline in catalytic activity was observed from the first hour of exposure, and continue to decline significantly with time. The activity decreases to 35 and 18% after 12 and 18 h of SO2 exposure, respectively. The catalytic stability studies therefore, clearly substantiate the sulphur resistant over Zr incorporated catalyst [41, 43]. It was also observed that if the Zr incorporation percentage is higher SO2 poisoning could be more susceptible. As given in Table 4, the activity of ACZ0.5 was found to be completely deteriorated, when it is subjected to longer duration SO2 exposure. This clearly confirms that the 20% Zr incorporation would be optimum for better catalytic performance under SO2 exposure. There are different optimal percentages of Zr incorporation on CeO2 have been reported for better SO2 resistance. Deshmukh et al., studied the effect of SO2 on CeO2 and CeO2–ZrO2 solid solution for CO and C2H6 oxidation, and reported the better SO2 resistance over 25% Zr incorporated CeO2 [41]. Whereas, according to Ryou et al., the better performance was achieved on 42% Zr incorporated CeO2 catalyst, when 2 wt% Pd was dispersed on CeO2–ZrO2 support [43].
Decrease in catalytic activity with SO2 exposure could be because of the adsorption of sulphur species on the catalyst surface. Therefore, multiple regeneration studies of AC and ACZ0.2 catalysts have also been performed to establish the interaction of sulphur species with the catalysts. The pronounced improvement/recovery in the catalytic activity was observed for both the catalysts as shown in Table 4. In the first regeneration step, the catalysts were heated to 150 °C for 2 h under helium flow (after 18 h of SO2 exposure), subsequently evaluated for catalytic activity at 40 °C. After first regeneration, the catalytic activity of AC was improved from 18 to 38%, whereas it was increased from 55 to 76% over ACZ0.2. Afterwards, the catalysts were again subjected to heat treatment under O2 flow for 2 h at 150 °C to remove any easily oxidisable species present on the catalyst surface (second regeneration). The catalysts were then cooled to 40 °C for catalytic CO oxidation test. A better improvement was observed with O2 treatment; around 85% catalytic activity was regained over ACZ0.2 catalyst, whereas on AC catalyst 50% activity was regained. These results clearly prove that most of the sulphur species formed during reaction were present on the surface of catalysts [41], which can be easily removable via heat with air/oxygen treatment. No additional diffraction peaks for SO2 exposed catalysts were observed in comparison with fresh catalysts by p-XRD analysis (not shown), which suggest that no sulfate or other sulfur bearing compound is formed under the SO2 exposure. Only 15% catalytic activity loss was observed over ACZ0.2, while it was almost 50% loss with AC catalyst. This again validates the significance of Zr incorporation with CeO2 for improved resistance to SO2 and higher catalytic activity.
4 Conclusions
In conclusion, nano-structured, mesoporous Au/Ce1−xZrxO2 catalysts were successfully synthesized and subjected to CO oxidation reaction. The CO oxidation activity of Zr incorporated catalysts was found to be dependent on Ce/Zr molar ratio. ACZ0.2 catalyst exhibited the superior catalytic activity compared to bare AC catalyst in presence of SO2 in the feed stream. AC catalyst shows rapid decline in catalytic activity from the first hour of SO2 exposure, and continue to decline significantly to 35 and 18% after 12 and 18 h of SO2 exposures, respectively. Whereas ACZ0.2 shows quite stable activity even after 5 h of SO2 exposure, however, after 12 and 18 h, the activity decreases to 62 and 55%, respectively. These results clearly confirm the sulphur resistance of Zr incorporated catalyst. Catalyst regeneration by heat treatment under O2 atmosphere results in significant improvement/regain of suppressed catalytic activity. After regeneration, merely 15% catalytic activity loss was observed over ACZ0.2, while it was almost 50% loss with AC catalyst. This again confirms the significance of Zr incorporation with CeO2 for improved activity. Regeneration studies also reveal that the interaction between sulphur species and the catalyst was only suprafacial. In this way it appears that Au nano-particles based CO oxidation catalysts shows potential for their practical applications if improved support materials are used.
References
Seyfi B, Baghalha M, Kazemian H (2009) Chem Eng J 148:306–311
Morikawa A, Suzuki T, Kanazawa T et al (2008) Appl Catal B Environ 78:210–221
Morikawa A, Kikuta K, Suda A, Shinjo H (2009) Appl Catal B Environ 88:542–549
Zhang J, Smith KR, Uma R et al (1999) Chemosph Glob Chang Sci 1:353–366
Olivier JGJ, Pieter J, Bloos J et al (1999) Chemosph Glob Chang Sci 1:1–17
Cuenya BR (2010) Thin Solid Films 518:3127–3150
Royer S, Duprez D (2011) ChemCatChem 3:24–65
Min BK, Friend CM (2007) Chem Rev 107:2709–2724
Lu J, Zhang Y, Jiao C et al (2015) Sci Bull 60:1108–1113
Miceli P, Bensaid S, Russo N, Fino D (2014) Nanoscale Res Lett 9:254
Santillo G, Deorsola FA, Bensaid S et al (2012) Chem Eng J 207:322–328
Megarajan SK, Rayalu S, Nishibori M, Labhsetwar N (2015) New J Chem 39:2342–2348
Megarajan SK, Rayalu S, Nishibori M et al (2015) ACS Catal 5:301–309
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Chem Lett 405–408
Haruta M, Yamada N, Kobayashi T, Iijima S (1989) J Catal 115:301–309
Pillai UR, Deevi S (2006) Appl Catal A Gen 299:266–273
Pattrick G, Van Der Lingen E, Corti CW et al (2004) Top Catal 30/31:273–279
Wu KC, Tung YL, Chen YL, Chen YW (2004) Appl Catal B Environ 53:111–116
Bandyopadhyay M, Korsak O, Van Den Berg MWE et al (2006) Microporous Mesoporous Mater 89:158–163
Bond GC, Thompson DT (2000) Gold Bull 33:41–50
Qian K, Jiang Z, Huang W (2007) J Mol Catal A Chem 264:26–32
Menard LD, Xu F, Nuzzo RG, Yang JC (2006) J Catal 243:64–73
Pandey AD, Güttel R, Leoni M et al (2010) J Phys Chem C 114:19386–19394
Haruta M (2003) Chem Rec 3:75–87
Moreau F, Bond GC, van der Linden B et al (2008) Appl Catal A Gen 347:208–215
Tai Y, Murakami J, Tajiri K et al (2004) Appl Catal A Gen 268:183–187
Haider P, Grunwaldt J-D, Seidel R, Baiker A (2007) J Catal 250:313–323
Piumetti M, Bensaid S, Andana T et al (2017) Appl Catal B Environ 205:455–468
Rodriguez JA, Liu P, Hrbek J et al (2007) Angew Chem Int Ed 46:1329–1332
Cargnello M, Doan-nguyen VVT, Gordon TR et al (2013) Science 341:771–774
Ozawa M, Okouchi T, Haneda M (2015) Catal Today 242:329–337
Yang X, Yang L, Lin S, Zhou R (2015) J Hazard Mater 285:182–189
Jeong D-W, Na H-S, Shim J-O et al (2015) Catal Sci Technol 5:3706–3713
Alifanti M, Baps B, Blangenois N et al (2003) Chem Mater 15:395–403
Madier Y, Descorme C, Le Govic AM, Duprez D (1999) J Phys Chem B 103:10999–11006
Dobrosz-Gómez I, Kocemba I, Rynkowski JM (2008) Appl Catal B Environ 83:240–255
Dobrosz-Gómez I, Gómez-García MÁRJ (2010) Kinet Catal 51:823–827
Mastelaro VR, Briois V, de Souza DP, Silva CL (2003) J Eur Ceram Soc 23:273–282
Boaro M, Vicario M, Llorca J et al (2009) Appl Catal B Environ 88:272–282
Letichevsky S, Tellez CA, de Avillez RR et al (2005) Appl Catal B Environ 58:203–210
Deshmukh SS, Zhang M, Kovalchuk VI, D’Itri JL (2003) Appl Catal B Environ 43:135–145
Luo T, Gorte RJ (2004) Appl Catal B Environ 53:77–85
Ryou Y, Lee J, Lee H et al (2015) Catal Today 258:518–524
Ayastuy JL, González-Marcos MP, Gil-Rodríguez A et al (2006) Catal Today 116:391–399
Yu JC, Zhang L, Lin J (2003) J Colloid Interface Sci 260:240–243
Acknowledgements
This research work was carried out under the International Scientific Partnership Programme (through ISPP#0057) of King Saud University, Riyadh, Saudi Arabia for establishing research collaboration between King Saud University and CSIR-NEERI. Thanks are also due to Director CSIR-NEERI for providing research facilities. KRC no-811268512.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valechha, D., Megarajan, S.K., Fakeeha, A.H. et al. Effect of SO2 on Catalytic CO Oxidation Over Nano-Structured, Mesoporous Au/Ce1−xZrxO2 Catalysts. Catal Lett 147, 2893–2900 (2017). https://doi.org/10.1007/s10562-017-2182-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2182-6