Abstract
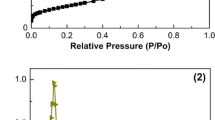
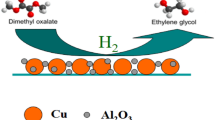
Ordered mesoporous Cu–Mg–Al composite oxides were synthesized via the one-pot evaporation-induced self-assembly strategy. Using this method, copper was first homogeneously incorporated into the ordered mesoporous spinel matrix. After H2 reduction treatment, according to X-ray diffraction (XRD) and transmission electron microscopy (TEM) results, copper existed as metallic nanoparticles with the size of 6–10 nm that well decorated the parent mesoporous skeleton. The metallic nanoparticles were then re-oxidized to copper oxide when exposed to air or during CO oxidation reaction at low temperatures. Thus, copper migrated from bulk spinel phase to the surface after the reduction–oxidation treatment. Moreover, the copper on the surface was re-incorporated into the bulk spinel phase by further thermal treatment at much higher temperature in the presence of air. The correlation between the state of copper in the mesoporous composite oxides and the catalytic performance toward CO oxidation was studied. It was found that copper existed as oxide nanoparticles on the surface of mesoporous Mg–Al skeleton is much more active than that existed as lattice Cu ions in spinel phase.






Similar content being viewed by others
References
Gamarra D, Belver C, Fernández-García M et al (2007) A selective CO oxidation in excess H2 over copper-ceria catalysts: identification of active entities/species. J Am Chem Soc 129:12064–12065
Jiang HQ, Cao ZW, Schirrmeister S et al (2010) A coupling strategy to produce hydrogen and ethylene in a membrane reactor. Angew Chem Int Ed 49:5656–5660
Jiang HQ, Wang HH, Liang FY et al (2009) Direct decomposition of nitrous oxide to nitrogen by in situ oxygen removal with a perovskite membrane. Angew Chem Int Ed 48:2983–2986
Remediakis IN, Lopez N, Nørskov JK (2005) CO oxidation on rutile-supported Au nanoparticles. Angew Chem Int Ed 44:1824–1826
Zhu YY, Wang XD, Huang YQ et al (2012) Identification of the crystallographic sites of Ir in BaIr0.2FeAl10.8O19 hexaaluminate. J Phys Chem C 116:24487–24495
Zhang Y, Wang XD, Zhu YY et al (2013) Stabilization mechanism and crystallographic sites of Ru in Fe-promoted barium hexaaluminate under high-temperature condition for N2O decomposition. Appl Catal B 129:382–393
Burch R (1996) Low NO x options in catalytic combustion and emission control. Pure Appl Chem 68:377–385
Burch R (1997) Low NO x options in catalytic combustion and emission control. Catal Today 35:27–36
Qian JC, Chen ZG, Liu CB et al (2014) Biotemplated fabrication of hierarchical mesoporous CeO2 derived from diatom and its application for catalytic oxidation of CO. Chin Sci Bull 59:3260–3265
Zhang WD, Lu XF, Zhou WL et al (2014) Mesoporous iron oxide-silica supported gold catalysts for low-temperature CO oxidation. Chin Sci Bull 59:4008–4013
Gu D, Schüth F (2014) Synthesis of non-siliceous mesoporous oxides. Chem Soc Rev 43:313–344
Zhao DY, Huo QS, Feng JL et al (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 120:6024–6036
Fukuoka A, Araki H, Kimura JI et al (2004) Template synthesis of nanoparticle arrays of gold, platinum and palladium in mesoporous silica films and powders. J Mater Chem 14:752–756
Li L, Shi JL, Yan JN (2004) A highly efficient heterogeneous catalytic system for heck reactions with a palladium colloid layer reduced in situ in the channel of mesoporous silica materials. Chem Commun 17:1990–1991
Zhao RH, Li CP, Guo F et al (2007) Scale-up preparation of organized mesoporous alumina in a rotating packed bed. Ind Eng Chem Res 46:3317–3320
Wu ZX, Li Q, Feng D et al (2010) Ordered mesoporous crystalline γ-Al2O3 with variable architecture and porosity from a single hard template. J Am Chem Soc 132:12042–12050
Huo QS, Margolese DI, Ciesla U et al (1994) Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 368:317–321
Bai P, Wu PP, Yan ZF et al (2009) Cation-anion double hydrolysis derived mesoporous γ-Al2O3 as an environmentally friendly and efficient aldol reaction catalyst. J Mater Chem 19:1554–1563
Yuan Q, Yin AX, Luo C et al (2008) Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability. J Am Chem Soc 130:3465–3472
Morris SM, Fulvio PF, Jaroniec M (2008) Ordered mesoporous alumina-supported metal oxides. J Am Chem Soc 130:15210–15216
Yuan Q, Duan HH, Li LL et al (2010) Homogeneously dispersed ceria nanocatalyst stabilized with ordered mesoporous alumina. Adv Mater 22:1475–1478
Cai WK, Yu J, Anand C et al (2011) Facile synthesis of ordered mesoporous alumina and alumina-supported metal oxides with tailored adsorption and framework properties. Chem Mater 23:1147–1157
Jiang HQ, Bongard H, Schmidt W et al (2012) One-pot synthesis of mesoporous Cu-γ-Al2O3 as bifunctional catalyst for direct dimethyl ether synthesis. Microporous Mesoporous Mater 164:3–8
Sun LB, Tian WH, Liu XQ (2009) Magnesia-incorporated mesoporous alumina with crystalline frameworks: a solid strong base derived from direct synthesis. J Phys Chem C 113:19172–19178
Wang N, Shen K, Huang LH et al (2013) Facile route for synthesizing ordered mesoporous Ni-Ce-Al oxide materials and their catalytic performance for methane dry reforming to hydrogen and syngas. ACS Catal 3:1638–1651
Gu D, Jia CJ, Bongard H et al (2014) Ordered mesoporous Cu–Ce–O catalysts for CO preferential oxidation in H2-rich gases: influence of copper content and pretreatment conditions. Appl Catal B 152:11–18
Yang BL, Chan SF, Chang WS et al (1991) Surface enrichment in mixed oxides of Cu, Co and Mn, and its effect on CO oxidation. J Catal 130:52–61
Liu W, Flytzanistephanopoulos M (1995) Total oxidation of carbon monoxide and methane over transition metal fluorite oxide composite catalysts: I. Catalyst composition and activity. J Catal 153:304–316
Liu W, Flytzanistephanopoulos M (1995) Total oxidation of carbon-monoxide and methane over transition metal fluorite oxide composite catalysts: II. Catalyst characterization and reaction-kinetics. J Catal 153:317–332
Avgouropoulos G, Ioannides T (2003) Selective CO oxidation over CuO–CeO2 catalysts prepared via the urea-nitrate combustion method. Appl Catal A 244:155–167
Kawabata T, Matsuoka H, Shishido T et al (2006) Steam reforming of dimethyl ether over ZSM-5 coupled with Cu/ZnO/Al2O3 catalyst prepared by homogeneous precipitation. Appl Catal A 308:82–90
Xing Y, Liu ZX, Suib SL (2007) Inorganic synthesis for the stabilization of nanoparticles: application to Cu/Al2O3 nanocomposite materials. Chem Mater 19:4820–4826
Behrens M, Kasatkin I, Kuhl S et al (2010) Phase-pure Cu, Zn, Al hydrotalcite-like materials as precursors for copper rich Cu/ZnO/Al2O3 catalysts. Chem Mater 22:386–397
Luo MF, Zhong YJ, Yuan XX et al (1997) TPR and TPD studies of CuO/CeO2 catalysts for low temperature CO oxidation. Appl Catal A 162:121–131
Luo MF, Zheng XM (1998) Redox behaviour of CeO2 and Ce0.5Zr0.5O2 supported CuO catalysts for CO oxidation. Acta Chem Scand 52:1183–1187
Acknowledgments
This work was supported by the Recruitment Program of Global Youth Experts of China, the National Natural Science Foundation of China (21403267, 21450110410), and Shandong Postdoctoral Innovation Program (201303065).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jingting Lu and Yan Zhang contributed equally to this work.
About this article
Cite this article
Lu, J., Zhang, Y., Jiao, C. et al. Effect of reduction–oxidation treatment on structure and catalytic properties of ordered mesoporous Cu–Mg–Al composite oxides. Sci. Bull. 60, 1108–1113 (2015). https://doi.org/10.1007/s11434-015-0805-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-015-0805-0