Abstract
This study deals with the synthesis of N-propylphthalimide substituted Ag(I)–N-heterocyclic carbene (NHC) complexes and N-propylphthalimide substituted Ru(II)–NHC complexes in the transfer hydrogenation of ketones. The Ag(I)–NHC complexes were synthesized from the imidazolium salts and Ag2O in dichloromethane at room temperature. The Ru(II)–NHC complexes have been prepared from Ag(I)–NHC complexes using transmetallation method. The six N-propylphthalimide substituted Ag(I)–NHC complexes and six N-propylphthalimide substituted Ru(II)–NHC complexes have been characterized by spectroscopic techniques and elemental analyses. N-propylphthalimide substituted Ru(II)–NHC complexes have been analyzed as catalysts for the transfer hydrogenation of ketones and exhibit activity in this reaction.
Graphical Abstract
This study deals with the synthesis of N-propylphthalimide substituted Ag(I)-N-heterocyclic carbene (NHC) complexes and N-propylphthalimide substituted Ru(II)–NHC complexes in the transfer hydrogenation of ketones. The Ag(I)–NHC complexes were synthesized from the imidazolium salts and Ag2O in dichloromethane at room temperature. The Ru(II)–NHC complexes have been prepared from Ag(I)–NHC complexes using transmetallation method. The six N-propylphthalimide substituted Ag(I)–NHC complexes and six N-propylphthalimide substituted Ru(II)–NHC complexeshavebeencharacterizedbyspectroscopictechniquesandelementalanalyses. N-propylphthalimide substituted Ru(II)–NHC complexes have been analyzed as catalysts for the transfer hydrogenation of ketones and exhibit activity in this reaction.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carbenes, divalent and six valence electrons containing in shells are neutral carbon species. For many years, carbenes have been known as very reactive and can not be isolated [1]. The majority of carbenes is known as short-lived reagents intermediates [2]. For this reason many chemists have hesitated to use of carbene compounds, especially as precursors ligands in the transition metal chemistry. N-heterocyclic carbenes (NHCs) were first studied by Wanzlick at the beginning of 1960s [3]. The first execution of NHCs as a ligand for metal complexes were separately synthesized by Wanzlick [4] and Öfele [5] in 1968. However, the field of NHCs as ligands in transition metal chemistry haven’t changed much until a report on the extraordinary stability, isolation and storability of crystalline NHC IAd by Arduengo et al. in 1991 [6, 7].
NHC form intriguingly stable bonds with the majority of metals [8–10]. Phosphines contain usually much weaker bonds but for saturated and unsaturated NHCs of comparable steric demand very similar bond dissociation energies have been observed [11]. Consequently, the balance between the free carbene and the carbene metal complex lies more suitable than the case for phosphines. This minimizes the amount of free NHC in solution and thus increases the life-time of the complex as well as its strength against heat, air and moisture.
Ag(I)–NHC complexes have taken considerable attention due to simpler synthetic strategies, fascinating structural diversity, stability and most importantly they have been admitted as effective carbene group transfer agents in the synthesis of many structurally and catalytically important transition metal NHC complexes [12]. In the beginning Ag(I)–NHC complexes demonstrate good precursor for the synthesis of other transition metal–carbene complexes. For example, Au(I), Rh(I), Pd(II), Cu(I), Ru(II), Ru(III), Ir(I), and Pt(II) complexes are synthesized by transmetallation [13].
For the past 20 years Ru complexes have increased application in the field catalysis and organometallic chemistry [14–18]. Recently, the newly synthesized Ru complexes have provided with particularly ligands to maintain a compatible equilibrium between the electronic and steric environment around the metal and to control on their stability, activity and chemoselectivity profiles [19–30]. Typically, most of NHC ligands emerge quite suitability with heteroatom including air, moisture and functional groups, thus expanding areas of application [31] in the many organic transformations. The structural motifs of Ru(II)–NHC complexes have found wide application in catalytic processes [32–34].
Transition metal-catalysed transfer hydrogenation of 2-propanol used as the hydrogen source is an effective method in organic synthesis [35]. In recent years a large number of applications dealing with this issue have been reported [36, 37]. In this process, the reaction conditions, partially contain mild conditions, economic and environmentally friendly is very important. In this reaction commonly used the catalysts of ruthenium(II) complexes. However sometimes rhodium and iridium derivatives have also been used [38].
In our study, illustrates the synthesis of six N-propylphthalimide substituted Ag(I)–NHC complexes, six N-propylphthalimide substituted Ru(II)–NHC complexes and their application in the transfer hydrogenation of aromatic ketones using 2-propanol.
2 Experimental
All synthesis involving Ag(I)–NHC complexes 2a–f and Ru(II)–NHC complexes 3a–f were carried out under an inert atmosphere in flame-dried glassware using standard schlenk techniques. The solvents used were purified by distillation over the drying agents indicated and were transferred under Ar: Et2O (Na/K alloy), CH2Cl2 (P4O10), hexane, toluene (Na).
All other reagents were commercially available by Aldrich Chemical Co. and used without further purification. Melting points were identified in glass capillaries under air with an Electrothermal-9200 melting point apparatus. FT-IR spectra were saved as KBr pellets in the range 400–4,000 cm−1 on a AT, UNICAM 1000 spectrometer. Proton (1H) and Carbon (13C) NMR spectra were recorded using either a Varian AS 400 Merkur spectrometer operating at 400 MHz (1H), 100 MHz (13C) in CDCl3 and DMSO-D6-d6 with tetramethylsilane as an internal reference. All reactions were observed on a Agilent 6890 N GC system by GC-FID with a HP-5 column of 30 m length, 0.32 mm diameter and 0.25 μm film thickness. Column chromatography was performed using silica gel 60 (70–230 mesh). Elemental analyses were performed by Turkish Research Council (Ankara, TURKEY) Microlab.
2.1 Synthesis of Bromo[1-(N-Propylphthalimide)-3-Benzylimidazol-2-Ylidene]Silver(I), 2a
To a solution of 1-(N-propylphthalimide)-3-benzylimidazol bromide (0.514 g, 1.2 mmol) in dichloromethane (30 mL), silver(I)oxide (0.139 g, 0.6 mmol) and activated 4 molecular sieves was added. The reaction mixture was stirred for 24 h at room temperature in dark condition. The reaction mixture was filtered through Celite and the solvent were evaporated under vacuum to afford the product as a white solid. The crude product was recrystallized from dichloromethane/diethyl ether (1:3) at room temperature. Yield: 490 mg, (77 %).
2.1.1 Analytical Data for Bromo[1-(N-Propylphthalimide)-3-Benzylimidazol-2-Ylidene]Silver(I), 2a
1H NMR (300 MHz, DMSO-d6), δ 1.91–1.95 (m, 2H, –CH2CH 2 CH2NC8O2); 2.51 (t, 2H, J: 3.3 Hz –NCH 2 CH2CH2NC8O2); 3.47–3.50 (m, 4H, –NCH 2 CH 2 N–); 3.54–3.58 (m, 2H, –CH2CH 2 NC8O2); 4.66 (s, 2H, –NC H 2 C6H5); 7.25–7.89 (m, 9H, Ar–H). 13C NMR (300 MHz, DMSO-d6), δ22.5 (–CH2CH 2 CH2NC8O2); 27.0 (–NCH 2 CH2NC8O2); 35.4 (–CH2CH 2 NC8O2); 48.6 and 48.9 (–NCH2 CH2N–); 55.4 (–NCH2C6H5); 123.5, 128.1, 128.3, 128.9, 128.9, 129.0, 129.4, 132.1, 132.2, 134.8 and 136.7. (Ar–C); 168.4 (C=O); 204.3 (2-C). m.p.: 308–310 °C; ν(CN): 1,662.5 cm−1. Anal. Calc. for C21H22AgBrN3O2: C: 47.04; H: 4.14; N: 7.84. Found: C: 47.02; H: 4.12; N: 7.83.
2.2 Synthesis of Bromo[1-(N-Propylphthalimide)-3-(2-Methylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2b
The synthesis of 2b was carried out in the same way as that described for 2a, but 1-(N-propylphthalimide)-3-(2-methylbenzyl)imidazol bromide (0.531 g, 1.2 mmol) was used instead of 1-(N-propylphthalimide)-3-benzylimidazol bromide. Yield: 480 mg, (74 %).
2.2.1 Analytical Data for Bromo[1-(N-Propylphthalimide)-3-(2-Methylbenzyl)Imidazol-2-ylidene]Silandr(I), 2b
1H NMR (300 MHz, DMSO-d6); δ 1.91–1.93 (m, 2H, –CH2CH 2 CH2NC8O2); 2.24 (s, 3H, –C6H4CH 3 ); 2.51 (t, 2H, J: 3.6 Hz –NCH 2 CH2CH2NC8O2); 3.46–3.48 (m, 4H, –NCH 2 CH 2 N–); 3.50–3.65 (m, 2H, –CH2CH 2 NC8O2); 4.59 (s, 2H, –NC H 2 C6H4); 7.20–7.86 (m, 8H, Ar–H). 13C NMR (300 MHz, DMSO-d6); δ19.6 (–CH2 CH2CH2NC8O2); 26.7 (–CH2C6H4 CH3); 27.0 (–NCH2CH2CH2NC8O2); 35.4 (–CH2 CH2NC8O2); 48.6 and 48.8 (–NCH2 CH2N–); 52.3 (–NCH2C6H4); 123.5, 126.6, 128.3, 131.0, 132.1, 134.5, 134.8 and 136.7 (Ar–C); 168.4 (C=O); 204.6 (2-CH). m.p.: 295–297 °C; ν(CN): 1,665.8 cm−1. Anal. Calc. for C22H24AgBrN3O2: C: 48.02; H: 4.40; N: 7.64. Found: C: 47.99; H: 4.38; N: 7.62.
2.3 Synthesis of Bromo[1-(N-Propylphthalimide)-3-(3-Methylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2c
The synthesis of 2c was carried out in the same way as that described for 2a, but 1-(N-propylphthalimide)-3-(3-methylbenzyl)imidazol bromide (0.531 g, 1.2 mmol) was used instead of 1-(N-propylphthalimide)-3-benzylimidazol bromide. Yield: 490 mg, (75 %).
2.3.1 Analytical Data for Bromo[1-(N-Propylphthalimide)-3-(3-Methylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2c
1H NMR (300 MHz, DMSO-d6); δ 1.92–1.94 (m, 2H, –CH2CH 2 CH2NC8O2); 2.29 (s, 3H, –C6H4CH 3 ); 2.51 (t, 2H, J: 3.6 Hz –NCH 2 CH2CH2NC8O2); 3.50–3.53 (m, 4H, –NC H 2 CH 2 N–); 3.63–3.65 (m, 2H, –CH2CH 2 NC8O2); 4.60 (s, 2H, –NC H 2 C6H4); 7.08–7.87 (m, 8H, Ar–H). 13C NMR (300 MHz, DMSO-d6), δ21.4 (–CH2 CH2CH2NC8O2); 26.9 (–CH2C6H4 CH3); 28.9 (–NCH2CH2NC8O2); 35.3 (–CH2 CH2NC8O2); 48.5 and 48.8 (–NCH2 CH2N–); 54.3 (–NCH2C6H4); 123.5, 125.2, 128.7, 128.9, 129.1, 132.1, 132.2, 134.8, 136.5 and 138.4. (Ar–C); 168.4 (C=O); 204.3 (2-CH). m.p.: 288–290 °C; ν(CN): 1,665.8 cm−1. Anal. Calc. for C22H24AgBrN3O2: C: 48.02; H: 4.40; N: 7.64. Found: C: 48.00; H: 4.39; N: 7.62.
2.4 Synthesis of Bromo[1-(N-Propylphthalimide)-3-(4-Methylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2d
The synthesis of 2d was carried out in the same way as that described for 2a, but 1-(N-propylphthalimide)-3-(4-methylbenzyl)imidazol bromide (0.531 g, 1.2 mmol) was used instead of 1-(N-propylphthalimide)-3-benzylimidazol bromide. Yield: 510 mg, (78 %).
2.4.1 Analytical Data for Bromo[1-(N-Propylphthalimide)-3-(4-Methylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2d
1H NMR (300 MHz, DMSO-d6), δ 1.90–1.93 (m, 2H, –CH2CH 2 CH2NC8O2); 2.28 (s, 3H, –C6H4CH 3 ); 2.51 (s, 2H, –NCH 2 CH2CH2NC8O2); 3.49–3.54 (m, 4H, –NC H 2 CH 2 N–); 3.59–3.65 (m, 2H, –CH2CH 2 NC8O2); 4.60 (s, 2H, –NC H 2 C6H4); 7.12–7.86 (m, 8H, Ar–H). 13C NMR (300 MHz, DMSO-d6), δ 16.8 (–CH2 CH2CH2NC8O2); 21.2 (–CH2C6H4 CH3); 27.0 (–NCH2CH2NC8O2); 35.4 (–CH2 CH2NC8O2); 48.5 and 48.9 (–NCH2 CH2N–); 54.0 (–NCH2C6H4); 123.5, 128.1, 128.2, 128.4, 129.5, 129.6, 129.7, 132.1, 133.6, 134.8 and 137.5; (2-CH); 168.4 (C=O); (Ar–C); 204.2. m.p.: 310–312 °C; ν(CN): 1,667.8 cm−1. Anal. Calc. for C22H24AgBrN3O2: C: 48.02; H: 4.40; N: 7.64. Found: C: 48.01; H: 4.37; N: 7.62.
2.5 Synthesis of Bromo[1-(N-Propylphthalimide)-3-(2,4,6-Trimethylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2e
The synthesis of 2e was carried out in the same way as that described for 2a, but 1-(N-propylphthalimide)-3-(2,4,6-trimethylbenzyl)imidazol bromide (0.565 g, 1.2 mmol) was used instead of 1-(N-propylphthalimide)-3-benzylimidazol bromide. Yield: 560 mg, (81 %).
2.5.1 Analytical Data for Bromo[1-(N-Propylphthalimide)-3-(2,4,6-Trimethylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2e
1H NMR (300 MHz, DMSO-d6), δ 1.87–1.91 (m, 2H, –CH2CH 2 CH2NC8O2); 2.21 and 2.26 (s, 9H, –CH2C6H2 (CH 3 )3); 2.51 (t, 2H, J: 3.3 Hz –NCH 2 CH2CH2NC8O2); 3.48–3.52 (m, 4H, –NC H 2 CH 2 N–); 3.58–3.62 (m, 2H, –CH2CH 2 NC8O2); 4.52 (s, 2H, –NC H 2 C6H2); 6.87–7.82 (m, 6H, Ar–H). 13C NMR (300 MHz, DMSO-d6), δ16.5 (–CH2 CH2CH2NC8O2); 20.5 (–CH2C6H2 (CH3)2); 21.0 (–C6H2 CH3); 27.1 (–NCH2CH2NC8O2); 35.4 (–CH2 CH2NC8O2); 48.5 and 49.0 (–NCH2 CH2N–); 55.4 (–NCH2C6H2); 123.5, 128.9, 129.7, 132.1, 134.8, 137.6 and 137.8. (Ar–C); 168.3 (C=O); not obserand (2-CH). m.p.: 193–195 °C; ν(CN): 1,666.7 cm−1. Anal. Calc. for C24H28AgBrN3O2: C: 49.85; H: 4.88; N: 7.27. Found: C: 49.83; H: 4.86; N: 7.25.
2.6 Synthesis of Bromo[1-(N-Propylphthalimide)-3-(2,3,5,6-Tetramethylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2f
The synthesis of 2f was carried out in the same way as that described for 2a, but 1-(N-propylphthalimide)-3-(2,3,5,6-tetramethylbenzyl)imidazol bromide (0.582 g, 1.2 mmol) was used instead of 1-(N-propylphthalimide)-3-benzylimidazol bromide. Yield: 580 mg, (82 %).
2.6.1 Analytical Data for Bromo[1-(N-Propylphthalimide)-3-(2,3,5,6-Tetramethylbenzyl)Imidazol-2-Ylidene]Silandr(I), 2f
1H NMR (300 MHz, DMSO-d6), δ 1.86–1.93 (m, 2H, –CH2CH 2 CH2NC8O2); 2.16 and 2.17 (s, 12H, –CH2C6H(CH 3 )4); 2.51 (t, 2H, J: 3.0 Hz –NCH 2 CH2CH2NC8O2); 3.45–3.49 (m, 4H, –NCH 2 CH 2 N–); 3.56–3.58 (m, 2H, –CH2CH 2 NC8O2); 4.53 (s, 2H, –NCH 2 C6H); 6.96–7.81 (m, 5H, Ar–H). 13C NMR (300 MHz, DMSO-d6), δ15.7 (–CH2 CH2CH2NC8O2); 16.3 and 20.7 (–CH2C6H(CH3)4); 27.1 (–NCH2CH2NC8O2); 35.4 (–CH2 CH2NC8O2); 48.8 and 49.2 (–NCH2 CH2N–); 65.4 (–NCH2C6H); 123.5, 131.6, 132.0, 133.7, 134.1 and 134.8. (Ar–C); 168.3 (C=O); 203.3 (2-C). m.p.: 119–120 °C; ν(CN): 1,667.8 cm−1. Anal. Calcd for C25H30AgBrN3O2: C: 50.70; H: 5.11; N: 7.07. Found: C: 50.68; H: 5.09; N: 7.08.
2.7 Synthesis of Dichloro[1-(N-Propylphthalimide)-3-Benzylimidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3a
To a solution of bromo[1-(N-propylphthalimide)-3-benzylimidazol-2-ylidene]silver(I)(0.150 g, 0.28 mmol) in dichloromethane (30 mL), Di-μ-chloro-bis[chloro(η6-1-isopropyl-4-methylbenzene)ruthenium(II)] (0.086 g, 0.14 mmol) was added. The reaction mixture was stirred for 24 h at room temperature in dark condition. The reaction mixture was filtered through Celite and the solvent were evaporated under vacuum to afford the product as a red-brown solid. The crude product was recrystallized from dicloromethane:dietylether (1:3) at room temperature. Yield: 150 mg, (82 %).
2.7.1 Analytical Data for Dichloro[1-(N-Propylphthalimide)-3-Benzylimidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3a
1H NMR (300 MHz, CDCI3); δ 1.19–1.24 (m, 6H, Ru–C6H4CH(C H 3 )2); 1.96–1.99 (m, 2H, –CH2CH 2 CH2NC8O2); 2.18 (s, 3H, Ru–C6H4CH 3 ); 2.85–2.95 (m, 1H, Ru–C6H4CH(CH3)2); 3.13–3.20 (m, 2H, –NCH 2 CH2CH2NC8O2); 3.46–3.52 (m, 2H, –CH2CH 2 NC8O2); 3.70–3.77 (m, 4H, –NCH 2 CH 2 N–); 5.00 (s, 2H, –NC H 2 C6H5); 5.27 and 5.32 (d, 4H, J: 5.4 Hz and 6.6 Hz Ru–Ar–H). 7.25–7.90 (m, 9H, Ar–H); 13C NMR (300 MHz, CDCI3); δ15.3 (–CH2CH 2 CH2NC8O2); 18.9 (C6H4CH(CH3)2); 23.3 (C6H4 CH(CH3)2); 28.5 (–NCH 2 CH2NC8O2);30.5 (Ru–C6H4 CH3); 35.6 (–CH2CH 2 NC8O2); 48.8–50.8 (–NCH2 CH2N–); 55.8 (–NCH2C6H5); 82.2, 85.6, 86.9, 99.4 and 109.3. (Ru–Ar–C); 123.1, 123.5, 127.6, 127.9, 128.6, 131.9, 132.6, 133.8 and 137.0. (Ar–C); 168.8 (C=O); 208.7 (Ru–C carb.). m.p.: 194–197 °C; ν(CN): 1,494.3 cm−1. Anal. Calc. for RuC32H39Cl2N3O2: C: 57.39; H: 5.87; N: 6.27. Found: C: 57.37; H: 5.87; N: 6.26.
2.8 Synthesis of Dichloro[1-(N-Propylphthalimide)-3-(2-Methylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3b
The synthesis of 3b was carried out in the same way as that described for 3a, but bromo[1-(N-propylphthalimide)-3-(2-methylbenzyl)imidazol-2-ylidene]silver(I)(0.154 g, 0.28 mmol) was used instead of bromo[1-(N-propylphthalimide)-3-benzylimidazol-2-ylidene]silver(I). Yield: 160 mg, (86 %).
2.8.1 Analytical Data for Dichloro[1-(N-Propylphthalimide)-3-(2-Methylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3b
1H NMR (300 MHz, CDCI3); δ 1.12–1.19 (m, 6H, Ru–C6H4CH(C H 3 )2); 1.92–1.96 (m, 2H, –CH2CH 2 CH2NC8O2); 2.05 (s, 3H, C6H4CH 3 ); 2.23 (s, 3H, Ru–C6H4CH 3 ); 2.74–2.83 (m, 1H, Ru–C6H4CH(CH3)2); 3.37–3.42 (m, 2H, –NCH 2 CH2CH2NC8O2); 3.44–3.48 (m, 2H, –CH2CH 2 NC8O2); 3.67–3.79 (m, 4H, –NCH 2 CH 2 N–); 4.59 (s, 2H, –NC H 2 C6H4); 5.04 and 5.23 (d, 4H, J: 5.7 Hz and 5.1 Hz Ru–Ar–H); 7.11–7.81 (m, 8H, Ar–H). 13C NMR (300 MHz, CDCI3); δ15.3 (–CH2CH 2 CH2NC8O2); 18.7 (C6H4CH(CH3)2); 21.5 (C6H4 CH3); 23.7 (C6H4 CH(CH3)2); 28.5 (–NCH 2 CH2NC8O2); 30.5 (Ru–C6H4 CH3); 35.6 (–CH2CH 2 NC8O2); 49.3–50.8 (–NCH2 CH2N–); 52.6 (–NCH2C6H5); 82.1, 82.5, 85.1, 87.1, 98.9 and 108.8. (Ru–Ar–C); 123.1, 125.0, 126.1, 127.0, 130.7, 132.5, 133.8 and 135.8. (Ar–C); 168.8 (C=O); 209.7(Ru–C carb.). m.p.: 187–189 °C; ν(CN): 1,496.7 cm−1. Anal. Calc. for RuC33H41Cl2N3O2: C: 57.97; H: 6.04; N: 6.15. Found: C: 57.95; H: 6.03; N: 6.13.
2.9 Synthesis of Dichloro[1-(N-Propylphthalimide)-3-(3-Methylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3c
The synthesis of 3c was carried out in the same way as that described for 3a, but bromo[1-(N-propylphthalimide)-3-(3-methylbenzyl)imidazol-2-ylidene]silver(I)(0.154 g, 0.28 mmol) was used instead of bromo[1-(N-propylphthalimide)-3-benzylimidazol-2-ylidene]silver(I). Yield: 150 mg; (84 %).
2.9.1 Analytical Data for Dichloro[1-(N-Propylphthalimide)-3-(3-Methylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3c
1H NMR (300 MHz, CDCI3); δ 1.21–1.26 (m, 6H, Ru–C6H4CH(C H 3 )2); 1.98–2.01 (m, 2H, –CH2CH 2 CH2NC8O2); 2.18 (s, 3H, Ru–C6H4CH 3 ); 2.34 (s, 3H, C6H4CH 3 ); 2.87–2.96 (m, 1H, Ru–C6H4CH(CH3)2); 3.12–3.18 (m, 2H, –NCH 2 CH2CH2NC8O2); 3.50–3.54 (m, 2H, –CH2CH 2 NC8O2); 3.70–3.77 and 3.85–3.91 (m, 4H, –NCH 2 CH 2 N–); 4.80 (s, 2H, –NC H 2 C6H5); 5.26 and 5.33 (d, 4H, J: 5.7 Hz and 4.8 Hz Ru–Ar–H); 7.07–7.90 (m, 8H, Ar–H). 13C NMR (300 MHz, CDCI3); δ 18.9 (C6H4CH(CH3)2); 21.5 (–CH2CH 2 CH2NC8O2); 21.8 (C6H4 CH3); 23.5 (C6H4 CH(CH3)2); 28.5 (–NCH 2 CH2NC8O2); 30.5(Ru–C6H4 CH3); 35.6 (–CH2CH 2 NC8O2); 48.8–50.8 (–NCH2 CH2N–); 55.7 (–NCH2C6H5); 82.0, 82.3, 85.5, 86.9, 99.5 and 109.3. (Ru–Ar–C); 123.1, 124.8, 128.3, 128.5, 128.6, 132.6, 133.8, 137.0 and 138.4. (Ar–C); 168.9 (C=O); 208.7 (Ru–C carb.). m.p.: 208–210 °C; ν(CN): 1,501.3 cm−1. Anal. Calc. for RuC33H41Cl2N3O2: C: 57.97; H: 6.04; N: 6.15. Found: C: 57.96; H: 6.03; N: 6.13.
2.10 Synthesis of Dichloro[1-(N-Propylphthalimide)-3-(4-Methylbenzyl)Imidazolidin-2-ylidene](p-Cymene)Ruthenium(II), 3d
The synthesis of 3d was carried out in the same way as that described for 3a, but bromo[1-(N-propylphthalimide)-3-(4-methylbenzyl)imidazol-2-ylidene]silver(I)(0.154 g, 0.28 mmol) was used instead of bromo[1-(N-propylphthalimide)-3-benzylimidazol-2-ylidene]silver(I). Yield: 170 mg, (90 %).
2.10.1 Analytical Data for Dichloro[1-(N-Propylphthalimide)-3-(4-Methylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3d
1H NMR (300 MHz, CDCI3); δ 1.22–1.29 (m, 6H, Ru–C6H4CH(C H 3 )2); 1.97–1.99 (m, 2H, –CH2CH 2 CH2NC8O2); 2.18 (s, 3H, Ru–C6H4CH 3 ); 2.34 (s, 3H, C6H4CH 3 ); 2.86–2.95 (m, 1H, Ru–C6H4CH(CH3)2); 3.15–3.18 (m, 2H,–NCH 2 CH2CH2NC8O2); 3.38–3.46 (m, 2H, –CH2CH 2 NC8O2); 3.75–3.85 (m, 4H, –NCH 2 CH 2 N–); 5.03 (s, 2H, –NC H 2 C6H5); 5.24 and 5.32 (d, 4H, J: 7.0 Hz and 7.0 Hz Ru–Ar–H); 7.12–7.89 (m, 8H, Ar–H). 13C NMR (300 MHz, CDCI3); δ15.5 (–CH2CH 2 CH2NC8O2);18.9 (C6H4CH(CH3)2); 23.4 (C6H4 CH(CH3)2); 21.9 (C6H4 CH3); 28.5 (–NCH 2 CH2NC8O2); 30.5 (Ru–C6H4 CH3); 35.6 (–CH2CH 2 NC8O2); 48.8–50.8 (–NCH2 CH2N–); 55.5 (–NCH2C6H4); 82.2, 82.3, 85.5, 86.9, 99.4 and 109.3. (Ru–Ar–C); 123.1, 127.8, 129.3, 132.6, 133.8, 133.9 and 137.2. (Ar–C); 168.8 (C=O); 208.5 (Ru–C carb.). m.p.: 191–193 °C; ν(CN): 1,493.1 cm−1. Anal. Calc. for RuC33H41Cl2N3O2: C: 57.97; H: 6.04; N: 6.15. Found: C: 57.95; H: 6.02; N: 6.14.
2.11 Synthesis of Dichloro[1-(N-Propylphthalimide)-3-(2,4,6-Trimethylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3e
The synthesis of 3e was carried out in the same way as that described for 3a, but bromo[1-(N-propylphthalimide)-3-(2,4,6-trimethylbenzyl)imidazol-2-ylidene]silver(I)(0.162 g, 0.28 mmol) was used instead of bromo[1-(N-propylphthalimide)-3-benzylimidazol-2-ylidene]silver(I). Yield: 170 mg, (87 %).
2.11.1 Analytical Data for Dichloro[1-(N-Propylphthalimide)-3-(2,4,6-Trimethylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3e
1H NMR (300 MHz, CDCI3); δ 1.20–1.23 (m, 6H, Ru–C6H4CH(C H 3 )2); 1.88–1.92 (m, 2H, –CH2CH 2 CH2NC8O2); 2.14 (s, 3H, Ru–C6H4CH 3 ); 2.18 and 2.24 (s, 9H, C6H2(CH 3 )3); 2.87–2.91 (m, 1H, Ru–C6H4CH(CH3)2); 3.02–3.10 (m, 4H, –NCH 2 CH2CH 2 NC8O2); 3.56–3.76 (m, 4H, –NC H 2 CH 2 N–); 4.65 (s, 2H, –NC H 2 C6H5); 5.25 and 5.32 (d, 4H, J: 5.7 Hz and 6.2 Hz Ru–Ar–H); 6.76–7.80 (m, 6H, Ar–H). 13C NMR (300 MHz, CDCI3); δ15.3 (–CH2CH 2 CH2NC8O2); 18.9 (C6H4CH(CH3)2); 20.1 and 20.7 (C6H2(CH3)3); 24.1 (C6H4 CH(CH3)2); 28.5 (–NCH 2 CH2NC8O2); 30.6 (Ru–C6H4 CH3); 35.5 (–CH2CH 2 NC8O2); 48.3–48.8(–NCH2 CH2N–); 51.0 (–NCH2C6H5); 81.6, 83.3, 85.3, 86.4, 100.2 and 108.8. (Ru–Ar–C); 123.0, 129.2, 129.6, 132.6, 133.8, 137.6 and 138.6. (Ar–C); 168.3 (C=O); 208.3 (Ru–C carb.). m.p.: 156–158 °C; ν(CN): 1,494.1 cm−1. Anal. Calc. for RuC35H44Cl2N3O2: C: 59.06; H: 6.37; N: 5.90. Found: C: 59.04; H: 6.36; N: 5.88.
2.12 Synthesis of Dichloro[1-(N-Propylphthalimide)-3-(2,3,5,6-Tetramethylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3f
The synthesis of 3f was carried out in the same way as that described for 3a, but bromo[1-(N-propylphthalimide)-3-(2,3,5,6-tetramethylbenzyl)imidazol-2-ylidene]silver(I)(0.166 g, 0.28 mmol) was used instead of bromo[1-(N-propylphthalimide)-3-benzylimidazol-2-ylidene]silver(I). Yield: 170 mg, (85 %).
2.12.1 Analytical Data for Dichloro[1-(N-Propylphthalimide)-3-(2,3,5,6-Tetramethylbenzyl)Imidazolidin-2-Ylidene](p-Cymene)Ruthenium(II), 3f
1H NMR (300 MHz, CDCI3); δ 1.25–1.30 (m, 6H, Ru–C6H4CH(C H 3 )2); 1.82–1.90 (m, 2H, –CH2CH 2 CH2NC8O2); 2.18 and 2.22 (s, 12H, C6H(CH 3 )4); 2.29 (s, 3H, Ru–C6H4CH 3 ); 2.87–2.91 (m, 1H, Ru–C6H4CH(CH3)2); 2.93–3.10(m, 4H, –NCH 2 CH2CH 2 NC8O2); 3.42–3.50 (m, 4H, –NC H 2 CH 2 N–); 4.50 (s, 2H, –NC H 2 C6H5); 5.35 and 5.49 (d, 4H, J:5.7 Hz and 6.0 Hz Ru–Ar–H); 6.94–7.87 (m, 6H, Ar–H). 13C NMR (300 MHz, CDCI3); δ15.4 (–CH2CH 2 CH2NC8O2); 17.9 (C6H4CH(CH3)2); 19.4 (C6H(CH3)4); 23.3 (C6H4 CH(CH3)2); 27.5 (–NCH 2 CH2NC8O2); 29.6 (Ru–C6H4 CH3); 34.5 (–CH2CH 2 NC8O2); 47.4–48.3 (–NCH2 CH2N–); 50.0 (–NCH2C6H); 80.7, 82.8, 84.0, 85.0, 99.3 and 107.6. (Ru–Ar–C); 122.0, 130.6, 131.4, 131.6, 132.7 and 133.0. (Ar–C); 167.8 (C=O); 207.0 (Ru–C carb.). m.p.: 191–193 °C; ν(CN): 1,496.4 cm−1. Anal. Calc. for RuC33H41Cl2N3O2: C: 59.58; H: 6.53; N: 5.79. Found: C: 59.56; H: 6.52; N: 5.77.
2.13 General Method for the Transfer Hydrogenation of Ketones
The catalytic hydrogen transfer reactions were carried out in a closed Schlenk flask under argon atmosphere. Substrate ketone (1 mmol), catalyst Ru(II)–NHC complex 3a–f (0.01 mmol) and KOH (4 mmol) was heated to reflux in 10 mL of i-PrOH for 1 h. The solvent was then removed under vacuum. At the conclusion of the reaction, the mixture was cooled, extracted with ethyl acetate/hexane (1:5), filtered through a pad of silicagel with copious washings, concentrated, and purified by flash chromatography on silicagel. The product distribution was determined by 1H NMR spectroscopy, GC and GC–MS.
3 Results and Discussion
3.1 Synthesis of Ag(I)–NHC Complexes 2a–f
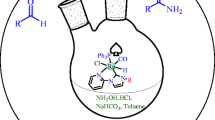
The synthetic route for unsymmetrically N-propylphthalimide substituted Ag(I)–NHC complexes and their corresponding Ru(II)–NHC complexes defined in this study illustrated in Schemes 1 and 2. The unsymmetrically substituted Ag(I)–NHC complexes 2a–f were prepared by stirring 1-(N-propylphthalimide)-3-alkylimidazolidinium salts with 0.5 equivalents of Ag2O in dichloromethane at room temperature for 24 h. The Ag(I)–NHC complexes as off white solid in 74–82 % yield. The Ag(I)–NHC complexes were soluble in halogenated solvent and insoluble in non-polar solvents. The complexes were characterized by spectroscopic techniques (1H, 13C NMR, IR) and elemental analysis. 1H and 13C NMR spectra are consistent with the proposed formulate. In the 1H NMR and 13C NMR spectra in d-DMSO-D6, loss of signals for the imidazolium proton (NCHN) (8.65–9.65 ppm) and imidazolium carbon (NCHN) at (157.5–158.4 ppm) showed the formation of the expected silver complexes. The 13C NMR spectra, resonances of the carbene carbon atoms of complexes appeared in the range δ203.3–204.6 ppm respectively for 2a–f. These signals are shifted downfield compared to the carbene precursors which further demonstrates the formation of expected Ag(I)–NHC complexes. The IR data for Ag(I)–NHC complexes emerge a characteristic ν(C=N) band at 1,503.6–1,508.2 respectively, for 2a–f. The NMR and FT-IR values are similar to results of other Ag(I)–NHC complexes.
3.2 Synthesis of Ru(II)–NHC Complexes 3a–f
The N-propylphthalimide substituted Ru(II)–NHC complexes 3a–f were prepared from synthesized Ag(I)–NHC complexes via transmetallation method (Scheme 2). The air and moisture stable Ru(II)–NHC complexes were soluble in solvents such as chloroform, toluene, dichloromethane. The Ru(II)–NHC complexes 3a–f were prepared by stirring bromo[1-(N-propylphthalimide)-3-alkylimidazol-2-ylidene]silver(I) with 0.5 equivalents of [RuCl2(pcym)]2 in dichloromethane at room temperature for 24 h. The Ru(II)–NHC complexes as a red–brown solid in 82–90 % yield. The Ru(II)–NHC complexes were soluble in halogenated solvent and insoluble in non-polar solvents. The Ru(II)–NHC complexes have been characterized by analytical and spectroscopic techniques. In the 1H NMR spectra, resonances for the isopropyl and methyl prothons of the p-cymene group of complexes 3a–f in the range 1.19–1.30 (methyl of izopropyl group), 2.85–3.10 (methyl of izopropyl group) and 2.18–2.29 ppm (p-methyl of p-cymene group) respectively showed the formation of the NHC–ruthenium complexes. The 13C NMR spectra, resonances of the carbene carbon atoms of complexes appeared in the range δ207.0–209.7 ppm respectively for 3a–f. These signals are shifted downfield compared to corresponding Ag(I)–NHC complexes of Ru(II)–NHC complexes carbene carbons signal at the range 203.3–204.6 ppm respectively showed the formation of the expected Ru(II)–NHC complexes. The IR data for Ru(II)–NHC complexes exhibit a characteristic ν(C=N) band at 1,493.1–1,501.3 respectively, for 3a–f. The NMR and FT-IR values are similar to results of other Ru(II)–NHC complexes.
3.3 Catalytic Transfer Hydrogenation of Ketones
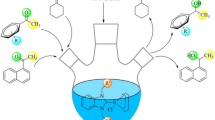
Ruthenium complexes have been used as active catalysts for transfer hydrogenation using 2-propanol as a hydrogen source. The reaction conditions for this transformation are economic, partly mild and environmentally benign friendly. 2-propanol using as a hydrogen source is a popular reactive solvent for the catalytic transfer hydrogenation since it is easy to handle and is relatively non-toxic, environmentally benign, and inexpensive. The volatile acetone by-product can also be easily removed.
We have investigated and compared the catalytic properties of N-propylphthalimide substituted Ru(II)–NHC complexes 3a–f in the transfer hydrogenation of various methyl aryl ketones. The reduction of acetophenone with 2-propanol to 1-phenylethanol was chosen as a model reaction. The catalytic transfer hydrogenations of ketones were carried out using ruthenium (II) precatalyst (0.01 mmol), KOH (4 mmol) and substrate ketone (1.00 mmol) in 2-propanol at 80 °C. The conversion was monitored by GC and NMR. It is well known that catalytic transfer hydrogenation is sensitive to the nature of the base. We surveyed K2CO3, Cs2CO3, NaOH, KOH, t-BuOK and NaOAc for the choice of base. The highest rate was observed when KOH was employed. A variety of ketones by 2-propanol were transformed to the corresponding secondary alcohols. Typical results are summarized in Table 1.

When compared with similar studies, particularly in terms of reaction time [39–43], all complexes 3a–f seem to be reasonably active in transfer hydrogenation reactions. Generally, all complexes 3a–f are seen to be reasonably active in hydrogen transfer reactions. Under the reaction conditions complex 3c turned out to be the active catalyst in comparison with 3a, 3b, 3d, 3e and 3f. The reduction of acetophenone with 3c was completed within 1 h reaching 80 %. In contrast, acetophenone was reduced within 1 h using 3a, 3b, 3d, 3e and 3f with 65, 77, 65, 78 and 63 % conversion, respectively (Table 1).
A variety of ketones were converted to be corresponding secondary alcohols. Typical results is illustrated in Table 1. Under those conditions p-metoxyacetophenone and p-fluoroacetophenone react neatly and in good yields with 2-propanol (Table 1). The existence of electron withdrawing (F) or electron donating (OCH3) substituents on acetophenone (Table 1) has effect on the reduction of major of ketones to their corresponding alcohols. The more conversion of p-flouroacetophenone to secondary alcohol was obtained at a time 1 h (Table 1).
The transformation of ketones with bulky substituents was not shown or mildly decreased. We tried this reaction with benzophenone at 1 h. But, we have achieved low yields. Therefore, we have extended the duration of experiments for benzophenone to 2 h. The benzophenone was reduced within 1 h using 3a and 3b with 18 and 20 % conversion, respectively. However, the yields lower than 2 h, for example the reduction of benzophenone with 3a and 3b was completed within 70 and 98 % respectively (Table 1).
4 Conclusions
As a result, we reported the synthesis of the six N-propylphthalimide substituted Ag(I)–NHC complexes 2a–f and six N-propylphthalimide substituted Ru(II)–NHC complexes 3a–f. The Ru(II)–NHC complexes were prepared via the Ag(I)–NHC complexes transmetallation route. Catalytic activities of Ru(II)–NHC complexes were readily accessible and are effective catalyst precursors for the transfer hydrogenation of ketones. The catalytic activities of these six N-propylphthalimide substituted Ru(II)–NHC complexes have been examined for the transfer hydrogenation of ketones and exhibited excellent activity in this reaction.
References
Grubbs RH (2003) Handbook of Metathesis. Wiley, Weinhei
Scholl M, Trnka TM, Morgan JP, Grubbs RH (1999) Tetrahedron Lett 40:2247
Huang J, Stevens ED, Nolan SP, Peterson JL (1999) J Am Chem Soc 121:2674
Wanzlick HW, Schönherr HJ (1968) Angew Chem Int Ed Engl 7:141
Öfele K (1968) J Organomet Chem 12:42
Arduengo III AJ, Harlow RL, Kline M (1991) J Am Chem Soc 113:361
Arduengo III AJ, Krafczyk R (1998) Chem Unserer Zeit 32:6
Hong SH, Day MW, Grubbs RH (2004) J Am Chem Soc 126:7414
Chatterjee AK, Morgan JP, Scholl M, Grubbs RH (2000) J Am Chem Soc 122:3783
Austin JF, Kim SG, Sinz CJ, Xiao W-J, MacMillan DWC (2004) Proc Natl Acad Sci 101:548
Chatterjee AK, Morgan JP, Scholl M, Grubbs RH (2000) J Am Chem Soc 122:3783
JB Lin Ivan, CS Vasam, (2007) Coord Chem Rev 251:642
JB Lin Ivan, CS Vasam, (2004) Inorganic Chem 25:75
Murahashi S-I, Takaya H, Naota T (2002) Pure Appl Chem 74:19
Naota T, Takaya H, Murahashi S-I (1998) Chem Rev 98:2599
Bruneau C, Dixneuf PH (1999) Acc Chem Res 32:311
Selegue JP (2004) Coord Chem Rev 248:1543
Trost BM, Frederiksen MU, Rudd MT (2005) Angew Chem Int Ed 44:6030
Tfouni E, Ferreira KQ, Doro FG, DaSilva RS, DaRocha ZN (2005) Coord Chem Rev 249:405
Distefano AJ, Nishart JF, Isied SS (2005) Coord Chem Rev 249:507
Demonceau A, Diaz EA, Lemoine CA, Stumpf AV, Pietraszuk C, Gulinski J, Marciniec B (1995) Tetrahedron Lett 36:3519
Lee HM, Bianchini C, Jia G, Barbara P (1999) Organometallics 18:1961
Zaja M, Connon SJ, Dunne AM, Rivard M, Buschmann N, Jiricek J, Blechert S (2003) Tetrahedron 59:6545
Ung T, Hejl A, Grubbs RH, Schrodi Y (2004) Organometallics 23:5399
Wong C-Y, Chan MCW, Zhu N, Che C-M (2004) Organometallics 23:2363
Dragutan V et al (2007) Coordination Chem Rev 251:765
Iwasa S, Tsushima S, Nishiyma K, Tsuchiya Y, Takesawa F, Nishiyama H (2003) Tetrahedron Asymmetry 14:855
Priya S, Balakrishna MS, Mobin SM, McDonald R (2003) J Organomet Chem 688:227
Dragutan V, Dragutan I, Verpoort F (2005) Platin Met Rev 49:33
Dragutan I, Dragutan V (2006) Platin Met Rev 50:81
Schwab P, Grubbs RH, Ziller JW (1996) J Am Chem Soc 118:100
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Perry MC, Burgess K (2003) Tetrahedron Assymetry 14:951
Mata JA, Poyatos M, Peris E (2009) Coord Chem Rev 109:3677
Ojima I (2000) Catalytic Asymmetric Synthesis, 2nd edn. Wiley, New York
Cheng Y, Lu X-Y, Xu H-J, Li Y-Z, Chen X-T, Xue Z-L (2010) Inorg Chim Acta 363:430
Ding N, Hor TSA (2010) Dalton Trans 39:10179
Nishibayasni Y, Takei I, Uemnura S, Hidai M (1999) Organometallics 18:2291
Özdemir İ, Yaşar S (2005) Transit Met Chem 30:831
Demir S, Özdemir İ, Çetinkaya B, Şahin E, Arici C (2011) J Coord Chem 64:2565
Yiğit B, Yiğit M, Özdemir İ, Çetinkay E (2012) Transit Met Chem 37:297
Fernández FE, Puerta MC, Valerga P (2012) Organometallics 31:6868
DePasquale J, White NJ, Ennis EJ, Zeller M, Foley JP, Papish ET (2013) Polyhedron 58:162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aktaş, A., Gök, Y. N-Propylphthalimide-Substituted Silver(I) N-Heterocyclic Carbene Complexes and Ruthenium(II) N-Heterocyclic Carbene Complexes: Synthesis and Transfer Hydrogenation of Ketones. Catal Lett 145, 631–639 (2015). https://doi.org/10.1007/s10562-014-1453-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1453-8