Abstract
A series of dihydropyrimidin-2(1H)-one (DHPM) belongs to one of the important class of therapeutic and pharmacological active compound, were synthesized through the multicomponent reactions (MCRs) of aldehydes, ethyl acetoacetate and urea, followed by the heterogeneous catalyzed Biginelli reaction. In the present endeavour, medium (ZSM-5) and large pore zeolites (Y, BEA and MOR) as well as dealuminated zeolites BEA, were studied as catalysts. An excellent activity for DHPMs synthesis is achieved by optimizing accessibility of the reactants to the active sites and the surface polarity of zeolite catalysts. Moreover, the mechanism of Biginelli reaction was studied by means of GAUSSVIEW energy calculations of adsorbed acylimine intermediate on zeolite by using the density functional method (DFT).
Graphical Abstract
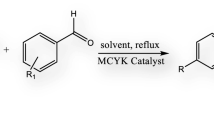
Zeolite assisted heterogeneous Biginelli reaction

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dihydropyrimidin-2(1H)-one (DHPM) and their derivatives have attracted considerable attention because of their diverse therapeutic and pharmacological profile which include calcium channel blockers, antihypertensive and anti-inflammatory agents [1]. Additionally, their particular structure has been found in natural marine alkaloid Batzelladine A and B which are the first low molecular weight natural products reported in the literature to inhibit the binding of HIV gp-120 to CD4 cells, so disclosing a new field towards the development of AIDS therapy [2]. The original procedure for the preparation of DHPMs was reported by Biginelli [3], involving one-pot condensation of ethylacetoacetate, benzaldehyde and urea under strongly acidic conditions. One major drawback of this so called Biginelli reaction, however is the low to moderate yield that is often encountered when substituted aromatic (20–60%) or aliphatic aldehydes (10%) is used [4].
Report concerning this catalytic one pot multicomponent reactions (MCRs), which has been carried out both in solvent and solvent free condition, are available [3–11]. The catalysts consist of Lewis acids such as BF3·OEt2 [5], triflates [6, 7] etc. as well as protic acids such as H2SO4, HOAc, Conc. HCl [8] as promoters. Many other methods including microwave irradiations, ionic liquids and ultrasonic mediated [9–11] are also reported. However, many of these methods are associated with expensive and toxic reagents, stoichiometric amount of catalyst, reaction time, unsatisfactory yields, incompatibility with other functional groups and involve difficult product isolation procedures.
Reported solid acid catalysts for the synthesis of DHPMs are polyoxometalate [12], heteropoly acids [13, 14], silica sulfuric acid [15] and ammonium chloride [16]. Recently, some molecular sieves such as zeolite H-Y, H-ZSM-5, MCM-41, Ersorb-4 (E4) and Heulandite (HEU) type natural zeolites [17–19] have been also reported as efficient heterogeneous catalysts for the synthesis of DHPMs. According to these reports, zeolite showed high catalytic activity in Biginelli reaction. However, all above protocols seems to aim mainly at enhancing the yields of the reaction. So far, not much attention has been paid to the role of surface acidity and pore structure of solid acid catalysts in the mechanism of the Biginelli reaction.
In the present endeavour, we have shown that with proper choice of topology, acidity and adsorption characteristic of zeolite catalyst, it is possible to obtain series of DHPMs with excellent yield through Biginelli reaction. Because bulky molecules or reaction intermediates are involved in the synthesis of DHPMs, large pore zeolite BEA, dealuminated BEA, H-Y and MOR topologies were chosen in our investigation (Scheme 1).
2 Experimental
2.1 Catalyst Preparation and Characterization
The zeolites Na-beta (BEA(12); Si/Al = 12), H-Mordenite (H-MOR(11); Si/Al = 11), Na-Y (Si/Al = 2.43), Na-ZSM-5 (Si/Al = 15) zeolites were obtained from Sud-Chemie India Pvt Ltd., INDIA. The H-form of zeolite were prepared by ion exchange of the Na-form samples with aqueous solution of NH4NO3 (1 M), followed by drying and calcination at 823 K. Zeolite BEA with different Si/Al ratio were prepared by dealumination of these parent zeolite with HNO3 according to the method reported in the literature [20].
The phase purity and crystallinity of the zeolites were analyzed by XRD (D8 Advanced Brucker AXS, Germany) with Cu Kα radiation and nickel filter. Surface area measurement (BET method) was carried out on Micromeritics Gemini at −196 °C using nitrogen adsorption isotherms. Acidity of zeolites were determined on Micromeritics Chemisorb 2720, by a temperature programmed desorption (TPD) of ammonia. Ammonia was chemisorbed at 120 °C and then desorption was carried out up to 700 °C at heating rate of 10 °C/min. The solvents were distilled before use. All reagent used were of analytical grade.
2.2 Typical Procedure for the Biginelli Reaction
All zeolites were activated, by heating at higher temperature of 773–823 K for 3–4 h, before loading into the reactor.
All the reactions were carried out in a round bottom flask attached to a condenser and equipped with a magnetic stirrer under heating in an oil bath. In a typical reaction, to a solution of urea (1 mol) and ethyl acetoacetate (1 mol) in toluene, appropriate amount of aldehyde (1 mol) and zeolite (2 wt%) were added. The reaction mixture was refluxed for 6 and 10 h with zeolite H-BEA and H-MOR, respectively. After completion of the reaction indicated by TLC, the spent catalysts were collected by filtration and then washed with ethanol. Crude product was recovered by evaporating the solvent under reduced pressure. This product was purified by recrystallization with ethanol to afford pure 5-ethoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2-one having melting point 201 °C (reported, 202 °C) in 85% yield with zeolite H-BEA and 60% yield with H-MOR.
The desire product, 5-ethoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2-one (4a) was characterized by comparison of their physical data with those of known compound [17–19]. The spectral data of representative compound including novel DHPM (Table 3, entry 4l) synthesized using 2-chloro-3-formylquinoline [21] as a new aldehyde functionality are given below.
2.2.1 5-Ethoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2-one (4a)
mp 202–204 °C; 1H-NMR (DMSO-d 6 ): 1.1 (t, J = 7.2 Hz, 3H, CH3CH2O), 2.24 (s, 3H, CH3), 4.00 (q, J = 7.2 Hz, 2H, OCH2), 5.16 (s, 1H, CH), 7.18–7.30 (m, 5H, arom CH), 7.78 (s, 1H, NH), 9.25 (s, 1H, NH); IR (KBr): 3244, 1724, 1639 cm−1; Anal. calcd. for C14H16N2O3: C, 64.60; H, 6.20; N, 10.79. Found: C, 64.69; H, 6.28; N, 10.90.
2.2.2 5-Ethoxycarbonyl-6-methyl-4-(2-chloroquinolin-3-yl)-3,4-dihydropyrimidin-2-one (4l)
mp 248–250 °C; 1H-NMR (DMSO-d 6 ): 1.069 (t, J = 7.2 Hz, 3H, CH3CH2O), 2.34 (s, 3H, CH3), 3.99 (q, J = 7.2 Hz, 2H, OCH2), 5.37 (s, 1H, CH), 7.10–7.18 (m, 1H, arom CH), 7.23 (s, 1H, arom CH), 7.32 (d, J = 7.5 Hz, 1H, arom CH), 7.46–7.49 (m, 1H, arom CH), 7.57 (s, 1H, NH), 7.70 (d, J = 7.5 Hz, 1H, arom CH) 9.22 (s, 1H, NH); 13C-NMR (DMSO-d 6 ): 16.74, 20.53, 41.94, 61.73, 98.71, 117.47, 152.76, 154.80, 167.84; IR (KBr): 3294, 3217, 1660, 1434, 1566, 754 cm−1; MS: m/z = 349 (M + 2); Anal. Calcd. for C17H16ClN3O3: C, 59.05; H, 4.66; N, 12.15. Found: C, 59.25; H, 4.62; N, 12.18.
In order to investigate reusability, catalyst was separated from the reaction mixture by filtration, washed with ethanol, dried overnight at 333 K and re-activated by heating in a stream of air at 673–773 K for 4 h. This procedure was followed for each recycle study of the catalyst.
3 Results and Discussion
3.1 Catalysts Characterizations
Physicochemical characterization data provided in the Table 1 reveal that the surface area of the zeolite BEA and dealuminated BEA is higher than the rest of the zeolites and the concentration of acid centre found for zeolites MOR is higher than the rest of the zeolites. Moreover, the XRD patterns of all the dealuminated BEA were found to be similar to the parent zeolite. Moreover, upon dealumination, pore volume and surface area of zeolite is slightly increases and total acidity of zeolite decreases as increase in Si/Al ratio.
3.2 Synthesis of DHPMs
Zeolites with different topologies as solid acid catalysts were used to elucidate the role of the zeolite channel system on their activity and selectivity in the Biginelli reaction. It is observed that, Biginelli reaction did not occur in the absence of zeolite catalyst. The Biginelli reaction of DHPM was carried out with 12-member-ring (MR) tridirectional zeolites (H-Y and H-BEA), a 12-MR monodirectional zeolite (H-MOR) and 10-MR bidirectional zeolite (ZSM-5) in the toluene as a solvent. Table 2 shows the effect of various structural features such as, geometry (pore structure and dimension), acidity and Si/Al ratio of zeolites on the synthesis of DHPM. H-BEA with 3-dimensional 12-membered ring (large pore) showed the high yield of 85% (run 1, Table 2) in considerably shorten reaction time as compared to other zeolites indicating that higher acid strength of the acid centre. Thus, the preferential order to yield DHPM was found to be: H-BEA > H-Y > H-MOR > H-ZSM-5.
3.3 Effect of Geometry and Surface Acidity
The observed low activity of medium pore zeolite, ZSM-5, is probably ascribed to the diffusional limitation of the pores towards bulkier reactant molecules and geometrical constraints for the formation of intermediates inside the pores. Whereas, MOR exhibits largest number of acid sites compare to other zeolites (Table 1) but it seems that the unidirectional channels system causes either inherent diffusional limitations or pore blocking owing to strong adsorption of the reactant or products. Further, in agreement with existing literature [19] it can also be concluded that, existence of strong acid sites in ZSM-5 and MOR does not favor the Biginelli reaction due to the possibility of denseness of the active sites. Moreover, the surface area of all BEA zeolites is higher than other zeolites. Hence, it is concluded that low to moderate acidity and higher surface area of zeolite BEA are prime factors for higher catalytic activity in the Biginelli reaction.
The most surprising observation was the low activity shown by H-Y and H-MOR compared to H-BEA (Table 2). It should be noted in this case that, there is no geometrical constraints for the diffusion of reactant through the pores but the strong adsorption of reactants and/or products in the pores of the catalyst that poisons or block the active sites and channels. The higher activity of H-BEA may also ascribed to higher Si/Al ratio compared to rest of the zeolites consequently leads to higher hydrophobicity. Furthermore, it appears that we may favor the product desorption by controlling the parameters such as polarity of reactant, solvent and catalyst as well. Thus, apart from acidity and geometry; one should also take into account the adsorption properties (hydrophobicity and hydrophilicity) of the zeolites [22].
3.4 Effect of Surface Polarity (Si/Al Ratio) of Zeolites
It is known that, with increasing framework Si/Al ratio, the catalyst becomes more hydrophobic, the concentration of acid sites decreases and strength of remaining sites increases [23]. According to the results and conclusions presented above, we could expect that the less polar zeolite should favor the desorption of adsorbed polar product. Therefore, in order to study the effect of polarity of zeolites, we have carried out Biginelli reaction of benzaldehyde (1a), ethylacetoacetate and urea using H-BEA of varied Si/Al ratio (12, 15, 25 and 34) under various reaction conditions as depicted in Fig. 1. Zeolite BEA with different Si/Al ratio were prepared by dealumination of these parent zeolite BEA(12).
The results from Fig. 1 show a maximum in activity for a sample with Si/Al ratio 15 which presents a much lower concentration of acid sites than sample with low Si/Al ratio (Si/Al = 12) (see Table 1). From these results, it seems that the larger number of acid sites does not guaranty a higher catalytic activity due to the inhibiting adsorption effect of reactant and product. Thus, in the present case, a less polar zeolite with lower concentration of acid sites performs better. In fact, an 85% yield of 4a was obtained with 2 wt% of BEA(15) after 6 h. Moreover, the solvent may also affect the reactivity of the reaction intermediate, it may also adsorbed strongly and poison the active sites, cause substrate exclusion from the surface and increase the resistance to diffusion of the reactants inside the channels. Therefore, the less polar solvent toluene (dielectric constant = 2.38) is more suitable compared to the ethanol, ACN and DMF (dielectric constant = 24.5, 37.5 and 38 respectively).
The similar behavior was observed when the Biginelli reaction of p-hydroxy benzaldehyde (1b), ethylacetoacetate and urea was carried out with the BEA(15) (Fig. 2). However, the decrease in activity of Si/Al curve towards more hydrophobic surface was occurred due to the presence of OH group at para position of the aromatic ring which increases the polarity of substrate 1b as compared to 1a, and therefore in the adsorption/desorption characteristics.
In general, the higher activity of BEA zeolite is due to the higher hydrophobicity and dealumination increases the diffusivity of the reactants and/or products thereby enhances accessibility of the acid sites to the reactant molecules by creating a mesopores in the crystallites.
3.5 Synthesis of Substituted DHPMs
In order to optimized the catalyst concentration, the Biginelli condensation of benzaldehyde, ethyl acetoacetate, and urea was carried out with varied amount of BEA(15) (0.5, 1, 2 up to 5 wt%) in toluene as a solvent. The best result was obtained by carrying out the reaction with 1:1:1 molar ratios of aldehyde, ethyl acetoacetate, urea and 2 wt% of zeolites BEA(15) under reflux condition.
Using the optimized reaction conditions, the performance and efficiency of these procedures were explored for the synthesis of a wide range of substituted DHPMs. The results are summarized in Table 3. It is observed that, aromatic aldehydes with both electron withdrawing and electron-donating substituents reacted efficiently with urea and ethyl acetoacetate in the presence of catalytic amount of H-BEA (2 wt%) under reflux condition to give the corresponding DHPMs without the formation of any side products and in high to moderate yields. The aliphatic aldehydes, which are known to be less reactive under conventional Biginelli reaction conditions also exhibited excellent reactivity to afford high yield (Table 3, entry 4j and 4k). However, bulkier heterocyclic aldehyde gave poor yield due to the geometrical constraints for the diffusion of reactant through the pores of zeolite H-BEA (Table 3, entry 4l).
The suggested mechanism of the zeolite catalyzed transformation is shown in Scheme 2. The first step in the mechanism is the condensation between the aldehyde and urea, with some similarities to Mannich condensation. The acylimine intermediate generated acts as an electrophile for the nucleophilic addition of the keto enol ether, and the carbonyl ketone of the resulting adduct undergoes condensation with the urea NH2 to give the cyclized Biginelli product.
The catalyst H-BEA expected to adsorb the acylimine intermediate prior to the addition reaction. Herein, we implemented GAUSSVIEW energy calculations [24] as proposed by Blasco and his coworkers [25, 26] by using the density functional UB3LYP/STO-3G* method to propose the possible adsorption model. Figure 3a shows the various proposed models that results from the interaction of acylimine intermediate with the Si(OsiH3)3OH and Al(OsiH3)2(OH)2(SiH3)2 clusters, as models of the silanol group and Bronsted acid sites, respectively. The main distances obtained from the optimization of the geometry are also indicated in Fig. 3a. When silanol groups are considered (Models A and B), the model B was found to be relatively more stable than C (−4.83 kcal/mol) where the hydrogen-atom of the silanol interacts with the carbonyl-oxygen and imine-nitrogen atom of acylimine intermediate. The calculated bond length inferred possible hydrogen atom transfer occurs from the zeolite hydroxyl group. Inspection of the relative energy calculated for model C and D indicate that the former is more stable (−2.88 kcal/mol) and no hydrogen-atom transfer occurs in this case. According to the theoretical study, the adsorption of acylimine to zeolite can be proposed through carbonyl-oxygen and imine-nitrogen atoms (Fig. 3b).
3.6 Recyclability of Catalyst
The XRD patterns of zeolites before and after using revealed that the zeolite retained its crystallinity throughout and thus can be reused. The reusability of the catalyst was also confirmed by carrying out the reaction of benzaldehyde, ethyl acetoacetate, and urea in the presence of 2 wt% H-BEA under toluene reflux condition. The catalyst can be separated from the reaction mixture by addition of ethanol followed by simple filtration. The results indicate that the catalyst can be used five times without any loss of its activity (Fig. 4).
4 Concluding Remarks
The synthesis of biological active DHPM over various large pore zeolites (H-Y, H-MOR and H-BEA) was studied. Zeolite presents cleaner green technology over the conventional homogeneous acid catalysts used for the synthesis of DHPMs.
It has been found that the adsorption properties (polarity) of zeolites can be more significant than the total number of acid sites when the polar reactants and solvents are involved.
Zeolite BEA and dealuminated BEA (BEA(15) and BEA(25)) showed higher catalytic activity compared to the other zeolites. This can be attributed to the higher Si/Al ratio of zeolites which consequently results in higher hydrophobicity and thereby the acid strength of the catalyst. Moreover, dealumination increases the diffusivity of the reactants and/or products thereby enhance the accessibility of the acid sites to the reactant molecules by creating a mesopores in the crystallites.
References
Kappe CO (1993) Tetrahedron 49:6963
Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, Brosse CD, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM (1995) J Org Chem 60:1188
Biginelli P (1893) Gazz Chim Ital 23:416
Folkers K, Harwood HJ, Johnson TB (1932) J Am Chem Soc 54:3751
Hu EH, Silder DR, Dolling UHJ (1998) J Org Chem 63:3457
Adapa SR, Alam MM, Varala R (2003) Synlett 1:70
Paraskar AS, Dewkar GK, Sudailal A (2003) Tetrahedron Lett 44:3308
Bussolar JC, McDonnell PA (2000) J Org Chem 65:6779
Khabazzadeh H, Saidi K, Sheibani H (2008) Bioorg Med Chem Lett 18:280
Chen X, Peng Y (2008) Catal Lett 122:313
Yadav JS, Reddy BVS, Reddy KB, Raj KS, Prasad AR (2001) J Chem Soc Perkin Trans 1:1941
Fazaeli R, Tangestaninejad S, Aliyan H, Moghadam M (2006) Appl Catal A Gen 309(1):51
Rafiee E, Shahbazi F (2006) J Mol Catal A Chem 250(1–2):61
Rafiee E, Jafari H (2006) Bioorg Med Chem Lett 16(9):2466
Salehi P, Dabiri M, Zolfigol MA, Bodaghi Fard MA (2003) Tetrahedron Lett 44(14):2891
Shaabani A, Bazgir A, Teimouri F (2003) Tetrahedron Lett 44(4):859
Radha Rani V, Srinivas N, Radhakishan M, Kulkarni SJ, Raghavan KV (2001) Green Chem 3:306
Hegedus A, Hell Z, Vigh I (2006) Synth Commun 36:136
Tajbakhsh M, Mohajerani B, Heravi MM, Ahmadi AN (2005) J Mol Catal A Chem 236(1–2):219
Wagholikar SG, Mayadevi S, Jacob NE, Sivasanker S (2006) Microporous Mesoporous Mater 95:16
Srivastava A, Singh RM (2005) Ind J Chem B 44B:1868
Climent MJ, Corma A, Velty A (2004) Appl Catal A 263:155
Barthomeuf D (1987) Mater Chem Phys 17:49
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford
Fernandez AB, Boronat M, Blasco T, Corma A (2005) Angew Chem Int Ed 44:2373
Fernandez AB, Lezcano-Gonzalez I, Boronat M, Blasco T, Corma A (2009) Phys Chem Chem Phys 11:5141
Acknowledgment
The authors are thankful to the Director, SVNIT, Surat, for providing research and financial assistance. The authors would also like to thank Sud-Chemie India Pvt. Ltd., India, for characterization and gift of samples of zeolites.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mistry, S.R., Joshi, R.S., Sahoo, S.K. et al. Synthesis of Dihydropyrimidinones Using Large Pore Zeolites. Catal Lett 141, 1541–1547 (2011). https://doi.org/10.1007/s10562-011-0639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0639-6