Abstract
Some halogen-free acyclic task-specific ionic liquids (TSILs) were synthesized as cheap and recyclable catalysts for synthesis of 1,8-dioxo-octahydroxanthenes in water. This methodology offers several advantages, such as good yields, short reaction times, simple procedure, and mild conditions. The products could simply be separated from the catalyst/water, and the catalyst could be reused at least six times without noticeably decreasing the catalytic activity.
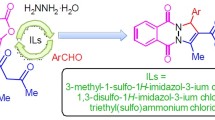
Graphical Abstract
Some recyclable acyclic SO3H-functionalized ionic liquids have been used as catalysts for the synthesis of 1,8-dioxo-octahydroxanthenes. The products could simply be separated from the catalyst/water, and the catalyst could be reused at least six times without noticeably decreasing the catalytic activity.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Xanthene derivatives are important classes of organic compounds of a large number of naturally occurring, as well as synthetic derivatives, and occupy a prominent position in medicinal chemistry [1]. Xanthene-based compounds have also been investigated for agricultural bactericide activity [2], anti-inflammatory effect [3] and antiviral activity [4]. In particularly, xanthenediones constitute a structural unit in a number of natural products [5], and have been used as versatile synthons because of the inherent reactivity of the inbuilt pyran ring [6]. The synthesis of xanthenediones usually condenses appropriate active methylene carbonyl compounds with aldehydes catalyzed mainly by organic and mineral acids, which often suffer from the drawbacks of long reaction times, harsh reaction conditions, toxicity, and difficulty in product separation.
Recently, many synthetic methods for preparing xanthenediones compounds have been reported by the condensation of aromatic aldehydes and active methylene carbonyl compounds in the presence of p-dodecylbenzenesulphonic acid [7], p-toluenesolfonic acid [8], Fe3+-montmorilonite [9], NaHSO4-SiO2 or silica chloride [10], Amberlyst-15 [11], silica sulfuric acid [12], (NH4)2HPO4 [13], Dowex-50 W [14], SbCl3/SiO2 [15], cyanuric chloride [16], and BiCl3 [17] as catalysts, as well as with the assistance of microwave [18] or ultrasound irradiation [19, 20]. However, the search for the new readily available and green catalysts is still being actively pursued.
Ionic liquids have attracted extensive interest as excellent alternatives to organic solvents these years, due to their favorable properties. Brønsted-acidic TSILs, which possess the advantageous characteristics of solid acids and mineral acids, are designed to replace traditional mineral liquid acids, such as sulfuric acid and hydrochloric acid. The use of ionic liquids as reaction medium may offer a convenient solution to both the solvent emission and catalytic recycling problem. Some researchers have already used ionic liquids as solvents/catalysit to synthesis xanthenediones compounds. Fan et al. used InCl3 · 4H2O [21] and Wang et al. used NaHSO4 for the condensation of aromatic aldehydes with 1,3-cyclohexanedione in ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4) [22]. Dabiri et al. [23] used acidic ionic liquid 1-methlyimidazolium triflouroacetate ([Hmim]TFA) as an efficient reusable catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. Srinivasan et al. reported an efficient method promoted by acidic ionic liquid [Hbim]BF4 under ultrasound irradiation. In fact, the use of Brønsted-acidic TSILs as catalysts is an area of ongoing activity; however, the development and exploration of Brønsted-acidic TSILs are currently in the preliminary stage. To date, research of some “greener” halogen-free ionic liquids with phosphate or octyl sulfate anions and the effects of the anion and toxicology have appeared in literature [24–26]. Thus, it is necessary to synthesize less expensive and halogen-free TSILs, which can be used straightforwardly with the simple procedure.
In our previous work some novel and cheap SO3H-functional halogen-free acidic ionic liquids that bear an alkane sulfonic acid group in an acyclic trialkylammonium cation have been synthesized to catalyze some organic reactions [27–30]. In continuation of our work in studying acid-catalyzed reactions in ionic liquids, we report here the synthesis of 1,8-dioxo-octahydroxanthenes via condensation of aldehydes and 1,3-cyclohexanedione in these halogen-free acidic ionic liquids.
2 Experimental
2.1 Materials and Methods
Melting points were determined on X-6 microscope melting apparatus and reported uncorrected. The IR spectra were run on a Nicolete spectrometer and expressed in cm-1 (KBr). 1H NMR spectra were recorded on Bruker DRX300 (300 MHz) and 13C NMR spectra on Bruker DRX300 (75.5 MHz) spectrometer. Elemental analyses were recorded on Perkin Elmer C spectrometer. Mass spectra were obtained with automated FININIGAN Trace Ultra-Trace DSQ GC/MS spectrometer. All chemicals (AR grade) were commercially available and used without further purification.
2.2 Synthesis of Acidic Halogen-Free Acidic Ionic Liquids (TSILs)
All acyclic SO3H-functionalized halogen-free acidic ionic liquids such as [TMPSA]HSO4, [TMBSA]HSO4, were synthesized according to our previous methods [27–30], the pyridine, imidazole-based acidic ionic liquids for comparison were according to reported methods [31]. Their structures were analyzed by 1HNMR, 13CNMR and MS spectral data (Scheme 1).
The selected spectral data for acidic halogen-free TSILs:
N, N, N-trimethyl-N-propanesulfonic acid ammonium hydrogen sulfate [TMPSA]HSO4 1HNMR (300 MHz, D2O): δ 3.22 (t, J = 7.2 Hz, 2H, N–CH2–C–C–SO3), 2.90 (s, 9H, N–CH3), 2.73 (t, J = 7.8 Hz, 2H, N–C–C–CH2–SO3), 1.99 (m, 2H, N–C–CH2–C–SO3). 13CNMR (75.5 MHz, D2O): δ 65.00, 52.51, 47.89, 18.85. MS (m/z): 279.05 (M+), 182.14(100).
N, N, N-trimethyl-N-butanesulfonic acid ammonium hydrogen sulfate [TMBSA]HSO4 1HNMR (300 MHz, D2O): δ 3.24 (t, J = 8.4 Hz, 2H, N–CH2–C–C–C–SO3), 2.99 (s, 9H, N–CH3), 2.85 (t, J = 7.5 Hz, 2H, N–C–C–C–CH2–SO3), 1.82 (m, 2H, N–C–CH2–C–C–SO3), 1.70 (m, 2H, N–C–C–CH2–C–SO3). 13CNMR (75.5 MHz, D2O): δ 66.15, 53.16, 50.31, 21.46, 19.93. MS (m/z): 293.36 (M+), 196.39(100).
N-propanesulfonic acid pyridinium hydrogen sulfate [PyPSA]HSO4 1HNMR (300 MHz, D2O): δ 8.62 (d, J = 6.0 Hz, 2H, H-2, H-6), 8.30 (t, J = 7.8 Hz, 1H, H-4), 7.84 (t, J = 6.9 Hz, 2H, H-3, H-5), 4.51 (t, J = 7.5 Hz, 2H, N–CH2–C–C–SO3), 2.73 (t, J = 7.2 Hz, 2H, N–C–C–C–CH2–SO3), 2.18–2.23 (m, 2H, N–C–CH2–C–SO3). 13CNMR (75.5 MHz, D2O): δ 146.35, 144.70, 128.82, 60.28, 47.48, 26.47.
1-methyl-3-propanesulfonic acid imidazolium hydrogen sulfate [MIMPSA]HSO4 1HNMR (300 MHz, D2O): δ 8.47(s, 1H, CH), 7.24(d, J = 1.5 Hz, 1H, CH), 7.17(d, J = 1.5 Hz, 1H, CH), 4.08(t, J = 6.9 Hz, 2H, N–CH2–C–C–SO3), 3.62(s, 3H, N–CH3), 2.64(t, 2H, J = 7.5 Hz, 2H, N–C–C–CH2–SO3), 2.03 (m, 2H, N–C–CH2–C–SO3). 13CNMR (75.5 MHz, D2O): δ 136.53, 124.32, 122.57, 48.13, 47.66, 36.46, 25.48. MS (m/z): 302.0 (M+), 300.93(100).
1-butyl-3-methylimidazolium hydrogen sulfate [bmim]HSO4 1H NMR (300 MHz, D2O): δ 8.62 (s, 1H, H-2), 7.33–7.38 (m, 2H, H-4, H-5), 4.09 (t, 2H, J = 7.1 Hz), 3.79 (s, 3H), 1.68–1.78 (m, 2H), 1.16–1.24 (m, 2H), 0.79 (t, 3H, J = 7.4 Hz). 13C NMR (75.5 MHz, D2O): δ 136.35, 124.07, 122.80, 49.85, 36.33, 31.80, 19.31, 13.25. m/z: 236(M+), 139(100, M+–HSO4).
2.3 General Procedure for the Synthesis of 1,8-Dioxo-octahydroxanthenes
To a round-bottomed flask charged with aldehyde (5 mmol) 1, 5,5-dimethyl-1,3-cyclohexanedione (dimedone) or 1,3-cyclohexanedione (10 mmol) 2 in 5 mL of water was added acidic ionic liquid (0.5 mmol) under stirring. The mixture was then stirred for a certain time at 100 °C (Scheme. 2). On completion (monitored by TLC), the precipitated crude product was collected by filtration and recrystallized form ethanol (95%) to afford pure 1,8-dioxo-octahydroxanthenes 3. The filtrated containing ionic liquid could be reused directly in the next run without further purification. The products were identified by IR, 1HNMR, and physical data (m.p.) with those reported in the literatures.
3 Results and Discussion
The synthesis procedures of SO3H-functionalized halogen-free acidic ionic liquids were made up of two-step atom-economic reaction. The zwitterionic-type precursors were prepared through a one-step direct sulfonation reaction. The zwitterions acidification was accomplished by mixing of zwitterions with sulfuric acid (98%, aq.) to convert the pendant sulfonate group into SO3H-functionalized halogen-free acidic ionic liquids. The chemical yields for both the zwitterions formation and acidification steps were essentially quantitative since neither reaction produced byproducts. The preparation of [bmim]HSO4 was accomplished by anion metathesis with NaHSO4 in good yield of 96%.
The fresh new TSILs with HSO4 − anion are somewhat viscous colorless liquids at room temperature. All produced TSILs are entirely miscible with water and soluble or partly soluble in organic solvents.
To optimize the reaction conditions, for the beginning of this study, benzaldehyde and dimedone were employed as the model reactants at 100 °C in TSILs for a length of time to compare the catalytic performance of the TSILs (Table 1). As shown in Table 1, no desirable product could be detected when a mixture of benzaldehyde and dimedone was heated at 100 °C for 6 h in the absence of ionic liquid (Table 1, entry 1), which indicated that the catalysts should be absolutely necessary for this condensation. All the eight TSILs proved to be very active, leading to 90–94% yield of 1,8-dioxo-octahydroxanthenes in the presence of 10% TSILs (entries 5, 8–16). In addition, ionic liquids containing the shorter length of alkyl chain are relatively cheaper. Further, the better immiscibility of the resulted product with the shorter length of alkyl chain ionic liquids should facilitate the separation in work-up procedure. Hence, [TMPSA]HSO4 should be the best catalyst for this condensation among these ionic liquids and the optimized reaction conditions went to entry 5 in Table 1.
Condensation reaction in [TMPSA]HSO4/H2O gave a yield of 94%, which was nearly the same as that in organic solvents. The chemical industry is under considerable pressure to replace many of the volatile organic compounds (VOCs) that are currently used as solvents in organic synthesis. As a clean and cheap solvent, it is important to carry out this reaction in water for the environmental and economic reasons.
When optimizing the reaction condition, the recycling performance of [TMPSA] · HSO4 was investigated using the above same model reaction. After the separation of the products, the catalyst-containing filtrate was reused in the next run without further purification. The data listed in Table 2 showed that the [TMPSA] · HSO4 could be reused at least six times without obviously decreasing of the catalytic activity. Compared with the traditional solvents and catalysts, the easy recycling performance is also an attractive property of the ionic liquids for the environmental protection and economic reasons.
Then, the condensation reaction of various aldehydes with dimedone or 1,3-cyclohexanedione in the presence of [TMPSA] · HSO4 as an environmentally benign ionic liquid was explored under the optimized reaction conditions described above and the results are presented in Table 3. It can easily be seen that t in all cases the reactions gave the products in good yields ranged from 88–95%. Aromatic aldehydes carrying either electron-donation or electron-withdrawing substituents afforded good yields of 1,8-dioxo-octahydroxanthenes in high purity.
4 Conclusion
In summary, some acyclic Brønsted-acidic task-specific ionic liquids such as [TMPSA] · HSO4, [TMBSA] · HSO4 etc. were synthesized in an atom-economic procedure. These ionic liquids were found to be efficient catalysts for synthesis of 1,8-dioxo-octahydroxanthenes in aqueous media, offering the practical convenience in the product separation from the ionic liquid system. The merit of this methodology is that it is simple, mild, and efficient. And the raw materials are cheaper than TSILs with imidazole or triphenylphosphine as the cation. Therefore, we believe that the work reported here would have potential application in green chemistry.
References
Wang HK, Morris-Natschke SL, Lee KH (1997) Med Res Rev 17:367
T Hideo (1981) Jpn Tokkyo Koho JP56005480
Poupelin JP, Saint-Ruf G, Foussard-Blanpin O, Narcisse G, Uchida-Ernouf G, Lacroix R (1978) Eur J Med Chem 13:67
Lambert RW, Martin JA, Merrett JH, Parkes KEB, Thomas GJ (1997) PCT Int Appl WO9706178
Hatakeyma S, Ochi N, Numata H, Takano S (1988) J Chem Soc Chem Commun 1202
CNO’Callaghan , McMurry TBH (1995) J Chem Res S:214
Jin TS, Zhang JS, Xiao JC, Wang AQ, Li TS (2004) Synlett 5:866
Khosropour AR, Khodaei MM, Moghannian H (2005) Synlett 955
Song G, Wang B, Luo H, Yang L (2007) Catal Commun 8:673
Das B, Thirupathi P, Mahender I, Reddy KR, Ravikanth B, Nagarapu L (2007) Catal Commun 8:535
Das B, Thirupathi P, Mahender I, Reddy VS, Rao YK (2006) J Mol Catal A Chem 247:233
Seyyedhamzeh M, Mirzaei P, Bazgir A (2007) Dyes Pigments 76:836
Darviche F, Balalaie S, Chadegani F (2007) Synth Commun 37:105
Shakibaei GI, Mirzaei P, Bazgir A (2007) Appl Catal A Gen 325:188
Zhang ZH, Liu YH (2008) Catal Commun 9:1715
Zhang ZH, Tao XY (2008) Aust J Chem 61:77
Li JJ, Tao XY, Zhang ZH (2008) Phosphorus Sulfur Silicon 183:1672
Hua GP, Li TJ, Zhu SL (2005) Chin J Org Chem 25:716
Jin TS, Zhang JS, Wang AQ (2006) Ultrason Sonochem 13:220
Venkatesan K, Pujari SS, Srinivasan KV (2008) Ultrason Sonochem 15:548
Fan X, Hu X, Zhang X, Wang J (2005) Can J Chem 83:16
Ma JJ, Li JC, Tang RX, Zhou X, Wu QH, Wang C, Zhang MM, Li Q (2007) Chin J Org Chem 27:640
Dabiri M, Baghbanzadeh M, Arzroomchilar E (2008) Catal Commun 9:939
Wasserscheid P, Hal R (2002) Green Chem 4:400
Fraga-Dubreuil J, Bourahla K, Rahmouni M, Bazureau JP, Hamelin J (2002) J Catal Commun 3:185
Garcia MT, Gathergood N, Scammells PJ (2005) Green Chem 7:9
Fang D, Luo J, Zhou X, Liu Z (2007) Catal Lett 116:76
Fang D, Luo J, Zhou X, Ye Z, Liu Z (2007) J Mol Catal A Chem 274:208
Fang D, Liu Z, Zhou X (2007) Chin Appl Chem 24:85
Fang D, Cheng J, Gong K, Shi Q, Liu Z (2008) Catal Lett 121:255
Atef A, Jean PB (2005) Org Process Res Dev 9:743
Acknowledgments
This work was financially supported by the Educational Commission of Jiangsu Provinces (07KJD530238), Nanjing University of Science & Technology, (No. 2006001), and Jiangsu Provincial Key Laboratory of Coastal Wetland Bioresources & Environmental Protection (JLCBE 07020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, D., Gong, K. & Liu, ZL. Synthesis of 1,8-Dioxo-octahydroxanthenes Catalyzed by Acidic Ionic Liquids in Aqueous Media. Catal Lett 127, 291–295 (2009). https://doi.org/10.1007/s10562-008-9677-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9677-0