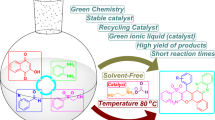
The task-specific room-temperature ionic liquid (TSIL) N, N, N-trimethyl-N-butanesulfonic acid ammonium hydrogen sulfate [TMBSA]HSO4 was synthesized as a cheap and recyclable catalyst for one-pot three-component Mannich reaction in water. Sixteen β-amino carbonyl compounds were obtained in good yields under the mild conditions. The products could simply be separated from the catalyst/water, and the catalyst could be reused at least 7 times without noticeably decreasing the catalytic activity.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Mannich reaction is one of the most important fundamental carbon-carbon bond-forming reactions in organic chemistry for the preparation of secondary and tertiary amine derivatives [1]. The products of Mannich reaction are mainly β-amino carbonyl compounds and 1, 2-amino alcohol derivatives, which are valuable synthetic intermediates for the synthesis of drugs and biologically active compounds. The conventional catalysts for classical three-component Mannich reaction of aldehydes, ketones and amines involve mainly organic and mineral acids, which often suffer from the drawbacks of long reaction times, harsh reaction conditions, toxicity and difficulty in product separation.
Recently, many synthetic methods for preparing these β-amino carbonyl compounds have been reported to improve and modify this reaction. With the assistance of microwave [2] or ultrasound irradiation [3]; using Lewis acids [4], Lewis base [5], Brønsted acids [6], or proline [7] etc. promoters to catalyze Mannich reaction. However, the search for the new readily available and green catalysts is still being actively pursued.
Ionic liquids have attracted extensive interest as excellent alternatives to organic solvents, due to their favorable properties. Brønsted acidic TSILs, which possess the advantageous characteristics of solid acids and mineral acids, are designed to replace traditional mineral liquid acids, such as sulfuric acid and hydrochloric acid [8]. The use of ionic liquids as reaction medium may offer a convenient solution to both the solvent emission and catalytic recycling problem. Some researchers have already used ionic liquids as solvents in Mannich reaction. Akiyama and coworkers reported the Mannich-type reaction of silyl enolates with aldimines in ionic liquids [9]. Yang et al. [10] used ruthenium complexes and Lee et al. [11] used ytterbium (III) triflate to catalyze Mannich reaction in 1-butyl-3-methyl-imidazolium hexafluoro-phosphate (bmim[PF6]). Using Brønsted acidic TSILs as catalysts and solvent for Mannich reaction have also been reported in these years. Zhao and coworkers [12] reported several anion-functional acidic ionic liquids (e.g. 1-methylimidazolium trifluoroacetic acid ([Hmim][TFA])) as catalytically active solvents for three-component Mannich reaction. Li et al. [13] reported the Mannich reaction catalyzed by a cation-functionalized acidic ionic liquid, 1-carboxymethyl-3-methylimidazolium tetrafluoroborate ([cmmim][BF4]) in aqueous 1-butyl-3-methyl-imidazolium tetrafluoroborate ([bmim][BF4]) medium. Quite recently, Sahoo and coworkers [14] reported that Brønsted acidic TSILs bearing triphenyl phosphonium sultone/immidazolium sultone as cation and trifluoroacetic acid (TFA)/p-toluenesulfonic acid (PTSA) as anion could catalyzed Mannich reaction smoothly in excellent yields and less time. However, TSILs with imidazole or triphenylphosphine as the cation are relatively expensive, which hinders their industrial applications. Furthermore, the commonly used dialkylimidazolium ionic liquids (bmimX) showed negligible biodegradability in the Closed Bottle Test (OECD 301 D), and typical ionic liquids consist of halogen containing anions (such as [PF6]−, [BF4]−, [CF3COO]−, [CF3SO3]− or [(CF3SO2)2N]−) which in some regard limits their “greenness” [15]. In addition, many of these procedures need the tedious and energy-consuming vacuum distillation for the recovery of catalytically active ionic liquids. Thus, it is necessary to synthesize less expensive and halogen-free TSILs, which can be used straightforwardly with the simple procedure.
In continuation of our ongoing program to develop environmentally benign methodology with ionic liquids as reaction medium [16], we synthesized a novel acyclic SO3H-functional Brønsted-acidic halogen-free TSIL that bears an butane sulfonic acid group in an acyclic tri-methyl-ammonium cation (scheme 1), and subsequently used as the catalyst for one-pot three-component Mannich reaction. To the best of our knowledge, in the open literatures, this TSIL and its using as the catalyst for Mannich reaction are unprecedented.
Experimental
Materials and methods
Melting points were determined and reported uncorrected. The IR spectra were run on a Nicolete spectrometer and expressed in cm-1 (KBr). 1H NMR spectra were recorded on Bruker DRX300 (300 MHz) and 13C NMR spectra on Bruker DRX300 (75.5 MHz) spectrometer. Elemental analyses were recorded on Perkin Elmer C spectrometer. Mass spectra were obtained with automated FININIGAN Trace Ultra-Trace DSQ GC/MS spectrometer. Thermal analysis was determined by Shimadzu TGA-50(10°C/mim heating rate under nitrogen). All chemicals (AR grade) were commercially available and used without further purification.
Synthesis of tri-methylammonium-butane sulfonate (TMABS)
To a solution of trimethylamine (0.11 mol) in ethanol (25 mL) was added 1, 4-butanesultone (0.10 mol) in portion within 30 mim, and then the mixture was stirred for 1 h at room temperature (25°C). A white precipitate thus formed was cooled to room temperature, and then filtered and washed with petroleum ether. The product was recrystallized from a mixture of water, ethanol and ether, there was obtained 98% yield of white solid product, mp 340−342°C dec with darkening at 300°C.
Synthesis of SO3H-functional Brønsted acidic ionic liquid [TMBSA]HSO4
Equal molar of tri-methylammonium-butane sulfonate (TMABS) and sulfuric acid solutions (96%) were mixed and stirred for 2 h at 80°C. Then, the combined solution was dried in a vacuum at 100°C to remove the water. The produced TSIL was washed repeatedly with diethyl ether to remove unreacted material and dried in a vacuum again, and then the TSIL was obtained quantitatively and in high purity as colorless oil. The TSIL was analyzed by elemental analyses, 1H NMR, 13C NMR and MS spectroscopies, and the spectral data agreed with the structure.
The spectral data for N, N, N-trimetyl-N-butanesulfonic acid ammonium hydrogen sulfate [TMBSA][HSO4]: 1H NMR (D2O,): δ 1.70 (m, 2H, –CH2–), 1.82 (m, 2H, –CH2–), 2.85 (t, J = 7.44 Hz, 2H, –N–CH2–), 2.99 (s, 9H, –CH3), 3.24 (t, J = 8.34 Hz, 2H, –CH2–S). 13C NMR (D2O,): δ 19.93, 21.46, 50.31, 53.16, 66.15. Anal. Calcd. For C7H19NO7S2: C, 28.66; H, 6.53; N, 4.77; Found: C, 28.40; H, 6.51; N, 4.92. MS (m/z): 293.36 (M+), 196.39(100).
General procedure for one-pot three-component Mannich reaction
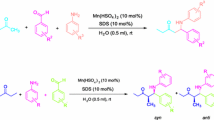
To a round-bottomed flask charged with aldehyde (10 mmol) 1, aniline (10 mmol) 2, ketone (10 mmol) 3 in 10 mL of water was added [TMBSA]HSO4 (1 mmol) under stirring. The mixture was then stirred for a certain time at room temperature (scheme 2). On completion (monitored by TLC), the precipitated crude product was collected by filtration and recrystallized form ethanol–acetone (v/v =1:1) to afford pure Mannich base 4. The filtrated containing [TMBSA]HSO4 could be reused directly in the next run without further purification. The products were identified by IR, 1HNMR, and physical data (m.p.) with those reported in the literatures.
Results and discussion
The synthesis procedure was made up of two-step atom economic reaction. The zwitterionic-type precursor (trimethylammonium butane sulfonate) was prepared through a one-step direct sulfonation reaction of trimethylamine and 1,4-butanesulfone. The zwitterions acidification was accomplished by mixing of zwitterions with sulfuric acid (98%, aq.) to convert the pendant sulfonate group into trimethylbutansulfonic acid ammonium hydrogen sulfate. The chemical yields for both the zwitterions formation and acidification steps were essentially quantitative since neither reaction produced byproducts; the TSIL synthesis was 100% atom-efficient.
The TGA analysis showed that [TMBSA][HSO4]is thermally stable up to 297°C. m.p. −11°C (detected by DSC). This ionic liquid could be entirely miscible with water.
To optimize the reaction conditions, for the beginning of this study, benzaldehyde, aniline and acetophenone were employed as the model aldehyde, anilines and ketone respectively to screen the suitable solvent (table 1). As shown in table 1, Mannich reaction in [TMBSA]HSO4 /H2O gave a yield of 90% (entry 1), which was slightly lower than that in [TMBSA]HSO4 /C2H5OH, [TMBSA]HSO4 / CH3OH and [TMBSA]HSO4 / CH3CN (entries 2, 3, 4), but was higher than that in [TMPSA]HSO4 /CH2Cl2 and [TMBSA]HSO4 /C6H6. The chemical industry is under considerable pressure to replace many of the volatile organic compounds (VOCs) that are currently used as solvents in organic synthesis. As a clean and cheap solvent, it is important to carry out Mannich reaction in water for the environmental and economic reasons.
The effect of the amounts of [TMBSA] · HSO4/H2O on the Mannich reaction was then explored using the same model reaction (table 2).
It showed that no Mannich base could be detected when a mixture of benzaldehyde, aniline and acetophenone was stirred for 24 h in the absence of [TMBSA] · HSO4 (entry 1), which indicated that the catalyst should be absolutely necessary for the Mannich reaction. When the amount of [TMBSA] · HSO4 was increased, a ramp in the yields of Mannich base was clearly observed. The optimal amount of [TMBSA] · HSO4 was 1.0 mmol, the higher amount of the catalyst did not improve the result to a greater extent (entries 8, 9).
When optimizing the reaction condition, the recycling performance of [TMBSA] · HSO4 was investigated using the same model reaction. After the separation of the products, the catalyst-containing filtrate was reused in the next run without further purification. The data listed in table 3 showed that the [TMBSA] · HSO4 could be reused at least 7 times without lowering of the catalytic activity. Compared with the traditional solvents and catalysts, the easy recycling performance is also an attractive property of the [TMBSA] · HSO4 for the environmental protection and economic reasons.
The three-component Mannich reaction of aldehydes, anilines and ketones in the presence of [TMBSA] · HSO4 was accomplished under the optimized reaction conditions described above and the results are presented in table 4. It can easily be seen that the Mannich reaction proceeded smoothly in water and gave reasonable to good yields ranged from 70–92%. Aromatic aldehydes carrying the strong electron-withdrawing substituents (–NO2) did not facilitate Mannich reaction (entries 14, 15, 16), electron-donating substituents on the aromatic ring (entries 11, 12, 13) gave almost the same yields as benzaldehyde (entries 1, 2, 3). In case of anilins, it’s noteworthy that both the electron-donating and weak electron-withdrawing substituents (entries 8, 9) were advantageous to Mannich reaction, but for the strong electron-withdrawing substituents, the lowest yield was obtained (entry 10). In addition, besides the aromatic ketones, aliphatic ketones could also be employed to give good yields. Although [TMBSA] · HSO4 could catalyzed the one-pot three-component Mannich reactions with high chemoselectivity and gave the corresponding products in high yields, the reactions with unsymmetrical acyclic ketones were accomplished with no regioselectivity (ee<1)(entries 7–10).
The results obtained with benzaldehyde, aniline and acetophenone under the optimized conditions were compared with the best ones published so far for this reaction using other catalysts/solvents system, the data listed in table 5 showed that the [TMBSA] · HSO4 was relatively a good catalyst for Mannich reaction.
Conclusion
In summary, an acyclic Brønsted acidic task-specific ionic liquid [TMBSA] · HSO4 was synthesized in an atom-economic procedure. This ionic liquid was found to be an efficient catalyst for one-pot three-component Mannich reaction at room temperature in water, offering the practical convenience in the product separation from the ionic liquid system. The merit of this methodology is that it is simple, mild, and efficient. And the raw materials are cheaper than TSILs with imidazole or triphenylphosphine as the cation. Therefore, we believe that the work reported here would have potential application in green chemistry.
References
For reviews of Mannich-type reaction, see: (a) S.E. Denmark, Nicaise, O.J.-C, in: Comprehensive Asymmetric Catalysis, eds. E.N. Jacobsen, A. Pfaltz and H. Yamamoto, (Springer: Heidelberg, 1999) pp. 923–961; (b) E.F. Kleinmann, in: Comprehensive Organic Synthesis, Vol. 2, ed. B.M. Trost, (Pergamon Press, New York 1991), Ch. 4.1; (c) M. Arend, B. Westermann, N. Risch, Angew. Chem. Int. Ed. 37, (1998) 1044; (d) S. Kobayashi, H. Ishitani, Chem. Rev. 99 (1999) 1069; (e) S. Kobayashi, M. Ueno, in: Comprehensive Asymmetric Catalysis, Supplement Vol. 1, (Springer: Berlin, 2004), pp.143–150; (f) A. Cordova, Acc. Chem. Res. 37 (2004) 102.
(a) N.E. Leadbeater, H.M. Torenius, H. Tye, Mol. Diversity 7 (2003) 135; (b) V. Aberg, H. Almstedt, A. Westermark, F. Almqvist, J. Org. Chem. 69 (2004) 7830.
D.-Y. Hu, B.-A. Song, G.-P. Zhang, S. Yang, W. He, Y.-L. Wu, Y.-P. Hong, L.-H. Jin, G. Liu (2005) Chin. J. Org. Chem. 25:845
(a) M. Shimizu and S. Itohara, Synlett (2000) 1828; (b) S. Kobayashi, T. Busujima and S. Nagayama, Synlett, 5 (1999) 545; (c) T.P. Loh and L.L. Wei, Tetrahedron Lett. 39 (1998) 323; (d). T.- P. Loh and S.- L. Chen, Org. Lett. 4 (2002) 3647; (e) I. Komoto and S. Kobayashi, Chem. Commun. 1842 (2001); (f) T. Ollevier and E. Nadeau, J. Org. Chem. 69 (2004) 9292; (g) M. Shi, S.-C. Cui and Q.-J. Li, Tetrahedron 60 (2004) 6163; (h) W.-B. Yi and C. Cai, J. Flu. Chem. 127 (2006) 1515; (i) L.M. Wang, J.W. Han, J. Sheng, Z.Y. Fan and H. Tian, Chin. J. Org. Chem. 25 (2005) 591; (j) T.J. Meng, L. Xin and J.X. Wang, J. SHANGQIU Teac. Coll. 22 (2006) 101.
Takahashi E., Fujisawa H., Mukaiyama T. (2004) Chem. Lett. 33:936
(a) R.O. Duthaler, Angew. Chem., Int. Ed. 42 (2003) 975; (b) L. Yi, H.S. Lei and J.H. Zou, Synthesis (1991) 717; (c) T. Akiyama, J. Takaya and H. Kagoshima, Adv. Synth. Catal. 344, (2002) 338; (d) S. Iimura, D. Nobutou, K. Manable and S. Kobayashi, Chem. Commun. 1644 (2003); (e) N. Azizi, L. Torkiyan, M.R. Saidi, Org. Lett. 8 (2006) 2079.
(a) B. Rodriguez, C. Bolm, J. Org. Chem. 71 (2006) 2888; (b) B. List, J. Am. Chem. Soc., 122 (2000) 9336; (c) B. List, P. Pojarliev, W.T. Biller, H.J. Martin, J. Am. Chem. Soc. 124 (2002) 827; (d) W. Notz, S.-I. Watanabe, N.S. Chowdari, G. Zhong, J.M. Betancort, F. Tanaka, C.F. Barbas III, Adv. Synth. Catal. 346 (2004) 1131; (e) S. Mitsumori, H. Zhang, P.H.-Y. Cheong, K.N. Houk, F. Tanaka, C.F. Barbas III, J. Am. Chem. Soc. 128 (2006) 1040; (f) I. Ibrahem, J. Casas, A. Córdova, Angew. Chem. Int. Ed. 43 (2004) 6528.
(a) T. Welton, Chem. Rev. 99 (1999) 2071; (b) P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39 (2000) 3773; (c) E.D. Bates, R.D. Mayton, I. Ntai, J.H. Davis, J. Am. Chem. Soc. 124 (2002) 926; (d) A.C. Cole, J.L. Jensen, I. Ntai, T. Tran, K.J. Weaver, D.C. Forbes, J.H. Davis Jr., J. Am. Chem. Soc. 124 (2002) 5962.
T. Akiyama, A. Suzuki, K. Fuchibe, Synlett, (2005) 1024.
Yang X.-F., Wang M., Varma R.S., Li C.-J. (2004) J. Mol. Catal. A: Chem. 214:147
Lee S.-Gi., Park J.H. (2002) Bull. Kor. Chem. Soc. 23:1367
Zhao G., Jiang T., Gao H., Han B., Huang J., Sun D. (2004) Green Chem. 6:75
Li J., Peng Y., Song G. (2005) Catal. Lett. 102:159
Sahoo S., Joseph T., Halligudi S.B. (2006) J. Mol. Catal. A: Chem. 244:179
(a) M.T. Garcia, N. Gathergood, P. J. Scammells, Green Chem. 5 (2005) 9; (b) N. Gathergood, M.T. Garcia, P.J. Scammells, Green Chem. 6 (2004) 166; (c) P. Wasserscheid, R. Hal, A. Bösmann, Green Chem. 4 (2002) 400; (d) J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J.P. Bazureau, J. Hamelin, J. Catal. Commun. 3 (2002) 185
(a). D. Fang, K. Gong, Q. R. Shi, Z. L. Liu, Catal. Commun.(2007) doi: 10.1016/ j.catcom. 2006.12.019; (b). D. Fang, Z. L. Liu, X. L. Zhou, Chin. J. Appl. Chem. 24 (2007) 85
Acknowledgments
The financial supports from the Nanjing University of Science & Technology (N0. 2006001) and Yancheng Teachers College are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, F., Jun, L., Xin-Li, Z. et al. Mannich reaction in water using acidic ionic liquid as recoverable and reusable catalyst. Catal Lett 116, 76–80 (2007). https://doi.org/10.1007/s10562-007-9095-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9095-8