Abstract
Skin allografts from cadaver donors are an important resource for treating extensive burns, slow-healing wounds and chronic ulcers. A high level of cell viability of cryopreserved allografts is often required, especially in burn surgery, in Italy. Thus, we aimed to determine which conditions enable procurement of highly viable skin in our Regional Skin Bank of Siena. For this purpose, we assessed cell viability of cryopreserved skin allografts procured between 2011 and 2013 from 127 consecutive skin donors, before and after freezing (at day 15, 180, and 365). For each skin donor, we collected data concerning clinical history (age, sex, smoking, phototype, dyslipidemia, diabetes, cause of death), donation process (multi-tissue or multi-organ) and timing of skin procurement (assessment of intervals such as death-harvesting, harvesting-banking, death-banking). All these variables were analysed in the whole case study (127 donors) and in different groups (e.g. multi-organ donors, non refrigerated multi-tissue donors, refrigerated multi-tissue donors) for correlations with cell viability. Our results indicated that cryopreserved skin allografts with higher cell viability were obtained from female, non smoker, heartbeating donors died of cerebral haemorrhage, and were harvested within 2 h of aortic clamping and banked within 12 h of harvesting (13–14 h from clamping). Age, cause of death and dyslipidaemia or diabetes did not appear to influence cell viability. To maintain acceptable cell viability, our skin bank needs to reduce the time interval between harvesting and banking, especially for refrigerated donors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin substitutes with the same biological properties as viable human skin are not yet available. Autologous skin grafting remains the treatment of choice for deep burns and other types of skin loss (Saffle 2009). However, autologous grafting of burn patients is often impossible due to low availability of skin donor sites (Greenleaf and Hansbrough 1994; Leon-Villapalos et al. 2010). In such cases, human skin allografts are considered the best alternatives for wound coverage as they prevent loss of water, proteins and electrolytes, provide temporary protection from infections, help maintain homeostasis and significantly accelerate re-epithelisation by providing persistent dermal matrix elements (See et al. 2001; Castagnoli et al. 2003; Franchini et al. 2009; Cleland et al. 2014). They also decrease wound pain and help maintain low bacterial loads in contaminated wounds (Verbeken et al. 2012). Human skin allografts are also an effective dressing for chronic venous ulcers and slow-healing wounds, alone or in association with dermal allografts and/or negative pressure therapy (Blackburn et al. 1998). With the phenomenon of ageing populations, the number of people with non-healing wounds is increasing together with the need for human skin allografts. This has prompted a proliferation of skin bank facilities and research to improve processing and storage methods (Bravo et al. 2000; Robb et al. 2001; Pianigiani et al. 2005).
Nowadays, the debate whether cell viability of skin allograft is necessary or not, is ongoing. It is widely accepted that viable, cryopreserved, skin allografts (CSA) are superior to all other dressing materials, and the majority of physicians agree that higher viability is usually associated with better wound bed preparation and graft take (Castagnoli et al. 2003; Franchini et al. 2009; Kua et al. 2012; Gaucher et al. 2012; Pirnay et al. 2012; Cleland et al. 2014; Gaucher and Jarraya 2014).. During the last decade, a great variety of non viable dermal matrix has been proposed, including natural semi-synthetic or synthetic products (Banyard et al. 2015). In particular, non-viable gamma-irradiated or glycerolized skin allografts can be successfully employed if CSA are not available or whether cell-viability is not required for wound treatment (Rooney et al. 2008). It is therefore important for skin banks to assess and certify the viability of CSA before transplant. Nevertheless, we are actually unable to predict the degree of viability of a CSA before procurement from cadaver donors or before skin banking. The aim of this retrospective study was to determine the conditions, if any, that enable procurement of highly viable skin and thus predict CSA viability values after storage. We hence investigated whether specific aspects of donor clinical history (i.e. age, gender, smoking, phototype, dyslipidaemia, diabetes, cause of death), donation type (multi-organ/heartbeating donors or multi-tissue/non heartbeating donors) and timing of skin procurement (death-harvesting, harvesting-banking and death-banking intervals) could influence skin viability.
Materials and methods
Donor selection and data collection
Donor selection was based on medical documentation (clinical records and reports) and serological screening (HIV, hepatitis B and C virus, syphilis, human T-lymphotrophic virus, CMV) (Pianigiani et al. 2013) in line with national guidelines and European directives (Linee guida 2013). Between April 2011 and September 2013, our Skin Bank received skin grafts from 249 consecutive skin donors: skin from 37 donors was processed to obtain dermal allografts only (i.e., de-epidermized dermis), whereas skin from 212 donors was processed for skin allografts. In particular, 85/212 were glyceropreserved and 127/212 skin allografts were cryopreserved. This group of 127 donors processed for cryopreservation represented our case study.
We collected details on gender, age, clinical history (i.e. dyslipidaemia, diabetes, smoking) and phototype. Causes of death were classified into five categories, namely: cardiac (heart failure, myocardial infarction, aortic dissection, hypovolemic shock, haemorrhagic shock, cardiac shock, ventricular fibrillation, arrhythmia), respiratory (acute respiratory failure, pulmonary oedema), post-traumatic (polytrauma, cranial trauma, suicide), cerebral ischaemia (stroke, post-anoxic coma) and cerebral haemorrhage. Donors were also divided into two categories, multi-organ (i.e. heartbeating) and multi-tissue (i.e. non heartbeating) donors. We defined three groups: group A included heartbeating donors (skin harvested after circulation has ceased by aortic clamping); group B was composed of non heartbeating donors who underwent refrigeration within 6 h of death; group C consisted of non heartbeating donors who were not refrigerated. According to Italian national guidelines, skin can be procured up to 24 h after death if the body is refrigerated within 6 h of death (Linee guida 2013). If the donor body is not refrigerated after death, then procurement should be completed within 12 h of death.
Procurement of skin samples
In Italy, skin banks are governed by European directives suggesting the application of Good Manufacturing Practices (GMP) (EU Guidelines GMP 2008) Procurement of skin from cadavers is carried out by a team of authorized medical practitioners on call 24 h a day. The cadaver skin is procured under aseptic conditions after appropriate cleaning with povidone-iodine solution, disinfection with tincture of chlorhexidine and adequate shaving to reduce resident microbial flora that lives primarily in and around hair follicles. Samples 400–800 µm thick are cut by battery-operated dermatome from the posterior trunk and the lower limbs and placed in sterile, sealed containers filled with transport medium (saline solution 0.9 %—Galenica Senese) supplemented with Penicillin (100 IU/ml) and Streptomycin (100 µg/ml). The containers are then transferred in refrigerated tanks (+2 °C/+10 °C) to the skin bank, where data recording and preservation procedures are undertaken in laboratory clean-rooms or specific areas i.e., grade A laminar flow cabinets in a GMP grade B environment, according to national regulations (EU Guidelines GMP 2008; Linee Guida CNT 2013).
Processing and microbiological testing of skin samples
The tissue is processed for antimicrobial treatment according to skin bank protocols (Pianigiani et al. 2010, 2013). As a precautional measure, donor skin contaminated with virulent bacteria and critical pathogens, such as Clostridium spp., is not processed but discarded (Pianigiani et al. 2010). Since processing time for cryopreservation must be brief to ensure cell viability, corrective actions cannot usually be performed before packaging. The pre-processing sample (fragments of at least 5 cm2 from different body areas) for microbiological testing is obtained during procurement in the operating theatre and placed in sterile saline without antibiotics. During processing, the skin undergoes serial steps in normal saline and cryoprotectant solution (D-MEM, 15 % glycerol, P/S, gentamycin sulphate 100 μg/ml and amphotericin B 5 μg/ml). It is then packaged and frozen at −80 °C in a programmable slow-rate freezer (Planer-Kryosave Integra, mod. 750). The post-processing sample (i.e., after packaging and freezing) is sent to the microbiology laboratory, where bacteriological and mycological protocols are applied. In bacterial testing protocol, samples are considered negative if bacteria do not grow for 48, 72 h and 30 days in aerobic, anaerobic and slow-growing cultures, respectively. Cultures are scored positive even for a single bacterial colony identified to genus and species level using selective media and semi-automatic biochemical tests (ATB-BioMerieux). According to the species identified an antibiogram is also performed. Slow-growing bacteria (e.g. mycobacteria) are identified by 16S rRNA sequencing. For mycological protocol, samples are treated as for the previous protocol using Sabouraud agar with chloramphenicol and incubated at +28 °C in air for 21 days. This period enables detection of fast-, medium- and slow-growing mycetes. If no yeast or fungi grow in 21 days the sample is declared negative. If yeast colonies grow their genus and species are determined by means of selective chromogenic substrates (ChromAgar and Sabouraud cycloheximide agar, BioMerieux) and semiautomatic biochemical tests (ATB-BioMerieux). If filamentous fungal colonies grow they are identified by microscopic observation after staining with lactophenol cotton blue or by slide culture.
We previously validated a double-control post-processing strategy that proved to maintain a sensitivity of 100 % and high specificity (77 %), and enable us to reduce the discard rate due to microbiological contamination to 1.2 % (Pianigiani et al. 2013). Indeed, a further post-processing quality control is performed besides microbiological testing of the processed tissues for fast and slow growing aerobic and anaerobic bacteria and fungi on tissue by 21-day skin cultures. This period enables detection of slow-growing microorganisms, which would not be identified by 3- or 7-day skin cultures. If no bacteria, yeasts or fungi grow in 21 days the sample is declared negative (Pianigiani et al. 2010, 2013).
Viability assessment
The literature describes various methods of determining viability, such as proliferation potential in cell culture, histomorphology and biochemistry (e.g. oxygen or glucose consumption assay, vital staining methods, lactate production, etc.). However, those methods give often not comparable results, and are not quantitative but qualitative (e.g. counting Trypan-blue-stained cells in trypan blue test) (Bravo et al. 2000; Castagnoli et al. 2003; Franchini et al. 2009; Hermans 2011). In our Skin Bank, we performed blue test and cell culture tests in the validation phase of the protocol. Then, after a period of data collection and evaluation, we decided not to use culture test for routinary viability testing, because it is a time-consuming, expensive and not homogeneous method. Nevertheless, the MTT metabolic assay (based on tetrazolium salts [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]) is widely used test for cell viability assessment in skin banks, because of its reproducibility and reliability (Bravo et al. 2000; Castagnoli et al. 2003; Franchini et al. 2009). It relies on reduction of water-soluble tetrazolium salts to the corresponding insoluble formazan pigments by mitochondrial enzymes. The pigment produced is dissolved and extracted with an organic solvent and quantified spectrophotometrically, measuring extractant optical density (OD) at a wavelength of 570 nm (ThermoScientific-Evolution 60) to obtain the OD of the positive control. The corrected OD 570nm of each sample is then expressed as the OD of the positive control minus the OD of the negative control (obtained by boiling a sample of the same fragment in distilled water for 30 min): this reflects the cumulative metabolic activity of viable cells (Bravo et al. 2000).
When allografts are received by the laboratory, we routinely obtain five punch-biopsies (6 mm ∅) from each skin donor and five corresponding corrected ODs by the MTT test: the mean corrected OD is the corrected OD 570nm PRE-freezing of each donor. The post-freezing MTT test is performed after 15 days of cryopreservation and thawing at ambient temperature to obtain the corrected OD 570nm POST. We usually calculate the percentage viability loss after 15 days of cryopreservation, expressed as OD RESIDUAL [%], as shown in Table 1. Hence, CSA procured for each skin donor routinely receive 2 MTT tests: before cryopreservation (i.e., time 0) and after 15 days of cryopreservation.
Moreover, in order to consider the impact of specimen weight on the MTT test procedure, we also evaluated the weight (mg) of each of the five disks from a given skin graft in 50 out of 127 CSA.
Cryopreservation
For cryopreservation, skin has to be processed within 72 h of reception. The skin is transferred to sterile containers and washed in sterile saline solution to remove blood and other residues (e.g. sterile Vaseline oil used to facilitate procurement). It is then incubated overnight at +4 °C in cryoprotectant solution (Base–Alchimia with 15 % glycerol). Freezing is performed in a slow rate freezer at −1 to −2 °C per minute, from +10 to −90 °C (Planer-Kryosave Integra, mod. 750). In order to assess skin viability performance after 1 year of storage at −80 °C, we performed a total of 4 MTT tests (i.e., at time 0, day 15, day 180 and day 365 of cryopreservation) in 26 CSA out of 127 donors (approximately 1 every 5 donor).
Histology
Pre- and post-cryopreservation samples of skin specimens (1/10) were processed for histology (Hematoxilin/Eosin—H&E stain). In order to evaluate which cells (epidermal or dermal) mainly metabolize MTT salts, we performed histological examination in 1/40 samples immediately after the MTT test. Cryostat sections (thickness 6 µm) were cut and placed directly on a glass slide. The slides were air-dried for 2 h and then observed by light microscope (Photomicroscope 3, Zeiss ICM 405).
Allograft viability, variables and data grouping
We evaluated how donor-related or method-related parameters can influence cell viability, which was expressed by the three viability indexes (OD 570nm PRE, OD 570nm POST and OD RESIDUAL). Donor-related parameters included: sex, age, clinical history (i.e. dyslipidaemia, diabetes, smoking), phototype and cause of death. Method-related parameters concerned donor type (heartbeating/non-heartbeating) and harvest timing (harvesting-MTT, death-MTT, death-harvesting intervals). For heartbeating donors, time of death coincides with aortic clamping, which stops circulation: we therefore expressed those intervals as clamping-harvesting and clamping-MTT. Possible correlations between donor-related/method-related variables and allograft viability were investigated in the whole statistical sample and in each group (A, B, C). Finally, we compared the three viability formulas between groups as follows: viability of allografts from heartbeating vs. non-heartbeating donors (i.e. group A vs. B + C); viability of CSA from refrigerated vs non-refrigerated donors (i.e. group B vs. A + C); viability of CSA from non-refrigerated and non-heartbeating donors vs. refrigerated heartbeating and non-heartbeating donors (i.e. group C vs. A + B). We also compared viability between single groups: group A vs. B; group B vs. C; group A vs. C.
Statistical analysis
Descriptive statistics, including frequency count, mean and standard deviation for quantitative variables, frequency count and percentage for qualitative variables, were computed. The Kolmogorov–Smirnov test was applied to check all quantitative variables, age and viability index for normal distribution. In cases of normal distribution, the t test and one-way analysis of variance (ANOVA) were used to compare groups, with the Bonferroni post hoc test for pairwise comparisons. For non normal data we used the non parametric tests of Mann–Whitney and Kruskal–Wallis with the Dunn post hoc test for pairwise comparisons. The Pearson correlation coefficient, r, was computed to evaluate correlations between all quantitative variables. A statistical significance of 95 % (p < 0.05) was always considered. SPSS software, release 10, was used for all statistical computations. For statistical analysis of causes of death, we excluded group A (i.e. heartbeating donors), as approximately 90 % of donors in this group died of cerebral haemorrhage (no statistically significant data). Thus, cardiac, respiratory, traumatic and cerebral ischemia were examined as causes of death in non heartbeating donors (group B + C).
Results
Donor selection and data collection
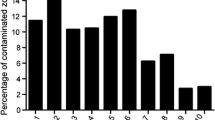
Our database of 127 donors was composed of 82 males and 45 females, aged between 18 and 75 years, with a median age of 59 years. Phototypes were as follows: phototype III—121 donors, phototype IV—3 donors, phototype II—3 donors. Twenty-seven donors had been smokers and 94 non smokers; 21 donors had been dyslipidaemic and 13 had suffered from diabetes mellitus. Group A was composed of 58 donors (30 males, 28 females), group B of 57 donors (45 males, 12 females) and group C of 12 donors (7 males, 5 females). Cardiac disease was the most common cause of death (34 %; Fig. 1).
Harvesting-banking intervals
The duration (in hours) of the different phases of skin procurement (donor death, harvesting, banking) is reported in Table 2 for the whole population (127 donors) and single subgroups.
Cryopreservation
To assess loss of viability due to storage in mechanical freezers, we calculated OD 570nm POST after 6 and 12 months in 26 samples. After 15 days of storage, OD varied from 0.252 to 1.089 (median 0.596); after 6 months 0.013–0.831 nm (median 0.46) and after 1 year 0.004–0.64 (median 0.4091) (Fig. 2). This was a loss of 23 % between 15 days and 6 months, and 10 % between 6 and 12 months. The overall percentage loss of viability in this limited group was 33 % after 1 year of storage at −80 °C.
Histology
Histological observation of 15-day cryopreserved samples showed normal epidermal cell layers with no sign of gross damage (Fig. 3). The stratum corneum was generally not fragmented and the dermal-epidermal junction was on the whole intact. Fibroblasts were still present in the dermis. Histological alterations due to freezing (e.g. epidermal-dermal junction separation, dermal cavitation and fragmentation of collagen or elastic fibres) were rarely observed. Separation of the stratum corneum from the epidermis was sometimes observed when the interval between harvesting and banking was more than 48 h.
Histological examination performed after the MTT test only showed pigment fixation in the epidermal layer, thus confirming the efficacy of the MTT viability assay (Fig. 4a, b).
Histomorphological analysis performed on cryostat tissue sections after MTT assay. The purple formazan pigment (MTT salt) is limited to the epidermal layers, thus marking viable cells only (a). Sporadic fixation is observed within the dermis, due to the presence of fibroblasts and endothelial cells (b)
Viability assessment
The five disks obtained from the same skin allograft proved to have different weights, although they were supposed to be of the same diameter and thickness. The weight of the disks ranged from 0.0106 to 0.0312 g and the correlation between weight and OD 570nm proved to be non linear.
Allograft viability, variables and data grouping
Viability assessment of CSA of the whole statistical sample and the subgroups, expressed as OD 570nm PRE and POST with reference to freezing, is reported in Fig. 5. After 15 days of cryopreservation, cell viability, expressed as OD RESIDUAL, showed a statistically significant (p < 0.05) mean decrease of 51 % (range 18–99 %) in the case study (Table 3). All three viability indices (OD 570nm PRE, OD 570nm POST and OD RESIDUAL) were significantly higher in skin from heartbeating than from non-heartbeating donors (group A vs. B + C). In particular, median OD750 POST of group A was significantly higher (+23 %) than that of B + C (student t test; p < 0.05). We also obtained significantly different medians for OD570POST for the other groups: +21 % between group B (refrigerated donors) and A + C (non refrigerated donors); +24 % between A and B (p < 0.05; Student t-test). Otherwise, we did not find any statistically significant difference between group C (non heartbeating non refrigerated donors) and groups A + B, group B and C, or group A and C (p > 0.05; student t test).
No significant correlation between cell viability and the age of skin donors was observed in the 127 donors or in each group (A,B,C). Similarly, neither dyslipidaemia nor diabetes seemed to influence viability. For non-heartbeating donors (groups B + C), no cause of death (cardiac, respiratory or traumatic causes or cerebral ischemia) was associated with higher cell viability.
When gender was considered, female donors showed higher OD570nm PRE and POST values (+16 %) than male donors (p < 0.05; test t student). Smoker donors had lower values of OD 570nm PRE and POST (−17 %) than non smokers (p < 0.05; test t student); OD 570nm PRE and POST comparison of these two groups is shown as boxplots in Fig. 6.
Boxplot of pre (OD570nmPRE) (a) and post-freezing viability (OD570nmPOST) (b) for smoking and not smoking donors. The central line represents the median value. Box size represents the interquartile range (IQR). Whiskers extend from each end of the box to the most extreme data values within 1.5 times the IQR from the ends of the box. Outliers are data with values beyond the ends of the whiskers and are displayed with a circle
As regards timing of harvesting and banking, Pearson correlation analysis between cell viability before and after cryopreservation and the different phases of skin procurement gave the following results. Considering all groups, cell viability significantly decreased in proportion with increasing harvesting-MTT interval (p < 0.05) (Fig. 7; Table 4). In particular, cell viability of group A donors decayed more rapidly (median OD570nm PRE < 1.5 nm) starting from 13 to 14 h after aortic clamping and 12 h after harvesting. Otherwise, we did not find any significant correlation between death-MTT and death-harvesting intervals in the case study or in individual groups (p > 0.05) (Table 4). For refrigerated donor skin (group B), the duration of death-refrigeration and refrigeration-MTT intervals did not significantly influence cell viability (Table 4). However, cell viability appeared to decay more rapidly (median OD570nm PRE < 1 nm) starting from approximately 19 h after death and 17 h after harvesting.
Discussion
The importance of skin allografts viability has long been debated, and whether a high viability value is really necessary for its function as a biological dressing or even for graft taking has never been fully assessed. Although some authors speculate that it is not an essential factor for the engraftment of burn wound beds (Hermans 2011; Cleland et al. 2014), viable CSA appear to have better transplant performance and to stimulate neovascularisation, accelerate healing, regulate systemic response immunomodulation and reduce mortality risk (Leon-Villapalos et al. 2010; Kua et al. 2012; Cleland et al. 2014). Moreover, when comparing viable cryo-preserved skin allografts (CSA) with unviable glycerol-preserved allografts, many authors assessed reported better clinical outcomes with CSA in the treatment of burned patients, both adults and children (Kua et al. 2012; Verbeken et al. 2012). In our experience, most surgeons found that CSA stimulate granulation of the wound bed in hard-healing ulcers and wounds better than glycerolised non viable skin [unpublished data].
To assess and certify the viability of CSA before transplantation is therefore relevant for a skin bank (Castagnoli et al. 2003; Franchini et al. 2009; Gaucher and Jarraya 2014). However, laboratory assessment of tissue viability has always raised questions about what these measurements really indicate in an in vivo setting. Moreover, findings from different authors cannot always be compared, as different formulae are used to calculate skin viability (Table 5). To consistently express cell viability of CSA, we therefore excluded all possible confounding factors (e.g. weight, fresh skin viability, skin area, normalization to 100 %). Since it is commonly accepted that the relationship of optical density to weight of skin samples has a linear relationship, many authors used weight as denominator in their formulae (Castagnoli et al. 2003; Franchini et al. 2009; Kua et al. 2012). Our results indicate that in each group of five samples, OD did not increase proportionally with weight, confirming a non-linear relationship (data not shown). Like Gaucher and Jarraya (2014), we therefore considered weight a confounding factor. In addition, some authors normalized OD PRE to 100 by setting the OD of fresh skin at 100 % (Castagnoli et al. 2003; Franchini et al. 2009; Gaucher and Jarraya 2014). On the contrary, we think that the initial viability of CSA cannot be expressed as a percentage, as viability is not necessarily 100 % in vivo. To verify this hypothesis, we obtained 50 skin samples (punch biopsy) from 50 living donors, and we performed the MTT test immediately after harvesting. In 2/3 of cases, viability values were comparable to those of skin allografts from cadaver donors [data not shown]. Furthermore, we suggest that expressing viability in terms of corrected OD [nm] could be more appropriate and closer to real data. We previously validated the freezing procedure used in all cryopreserved allografts on 233 samples (data not shown) in order to demonstrate the efficacy of slow freezing.
In testing viability after 6 and 12 months of storage, we observed that cell viability on average decreased with time. Compared with fresh skin viability (pre-freezing), the loss of cell viability was 54.4, 65 and 69 % after 15 days, 6 months and 1 year, respectively. Compared with viability assessment after 15 days of storage, the loss was 23 and 33 % after 6 and 12 months, respectively. Thus, the greatest loss occurs in the first 15 days ofstorage. A similar regression trend was reported by Schiozer et al. (2013), who, however, used the lactate production test for viability assessment of CSA. Castagnoli et al. (2003) also reported that cryopreservation always gave constant results (50 % loss of cell viability), suggesting that final viability mostly depends on the length of the pre-freezing period, irrespective of initial values. Though freezing procedures achieve comparable outcomes, we cannot exclude the possibility that the number of viable cells in CSA depends on other factors, such as initial viability, considering the large differences in PRE-freezing viability. In our study, a median reduction in cell viability of 50 % (range 19–81 %) was observed after 15 days of storage at −80 °C for the whole case study. These results appear to be in line with the literature data (Castagnoli et al. 2003; Gaucher et al. 2012).
Histological analysis and viability assessment (MTT test at 6 and 12 months) confirmed the validity of the cryopreservation methods used in our skin bank. After 15 days of cryopreservation, we did not observed histological alterations due to freezing (e.g. epidermal-dermal junction separation, dermal cavitation or fragmentation of collagen and elastic fibres; Castagnoli et al. 2003; Wood et al. 2014). Fragmentation of the stratum corneum, that largely depended on the harvesting-banking interval, was rarely detected (Kua et al. 2012).
There is consensus that storage temperature can also influence cell viability (Udoh et al. 2000; Robb et al. 2001; Schiozer et al. 2013). Indeed, it has been shown that cadaver skin should be treated with cryoprotectants, cooled gradually and thawed rapidly in order to avoid alterations in cell integrity and achieve viable CSA (Udoh et al. 2000; Schiozer et al. 2013). Various studies evaluated different refrigeration temperatures for short- and long-term storage. Storage of the tissue at +4 °C reduced viability in a few weeks (Robb et al. 2001; Li et al. 2012; Gaucher et al. 2012); similar results were observed with storage at −70 °C (Walcerz and Karow 1996; Schiozer et al. 2013). Other authors used a three-stage cryopreservation protocol (first hour at −20 °C, then −80 °C for at least 24 h, followed by liquid nitrogen until use) to obtain acceptable viability (i.e. 50 % viability loss in the first 2–3 weeks; May and Roberts 1988; Castagnoli et al. 2003). Many authors agree that when allografts are subjected to long-term cryopreservation, they should be stored below −130 °C to avoid ice crystal formation and cell damage (Schiozer et al. 2013; Gaucher et al. 2012). Udoh et al. (2000) estimated similar cell survival rates (89.3 % determined by flow cytometry) for skin grafts cryopreserved at −135 and −80 °C for 1 month. However, after 6 months, viability was significantly higher in the first group (61.7 vs 35.2 %). Similarly, Gaucher et al. (2012) used liquid nitrogen freezers at −150 and −170 °C, with a mean cryopreservation time of 10 days prior to thawing. Liquid nitrogen has some disadvantages: it must be continually replenished, it is difficult to store a large number of specimens, tissue samples must be tightly sealed, and it is more expensive (Udoh et al. 2000; Hermans 2011). We are currently equipping with liquid nitrogen containers to store allografts for burn centres, in order to maintain high viability over time.
Regarding skin procurement timing, it is generally accepted that skin viability is maintained when the delay between harvesting and freezing is brief (Castagnoli et al. 2003; Franchini et al. 2009; Leon-Villapalos et al. 2010; Gaucher and Jarraya 2014). Prior to the present study, there was no data on the effects of death/clamping-harvesting, harvesting-MTT and death/clamping-MTT intervals or on the harvesting-banking process for different types of skin donors; nor had the different phases of the harvesting-banking process (i.e. brain death-aortic clamping, brain death-harvesting, brain death-MTT in heartbeating donors; death-refrigeration and refrigeration-harvesting in non heartbeating donor) been investigated for effects on cell viability in different types of skin donors. In this study, the harvesting-MTT interval proved to significantly influence loss of viability in all groups, while this correlation was not demonstrated for death-harvesting and death-MTT phases. On average, the greatest loss in cell viability decay started 13–14 and 17 h after harvesting in group A and group B donors, respectively. It is therefore important to reduce the post-harvesting phase in order to prevent major cell viability decay in all donors and especially in heartbeating ones. Indeed, an inverse pattern was also observed in group A, albeit with low statistical significance (p = 0.11), possibly due to the small sample size (58 donors) (Table 4).
A series of variables can influence cell viability, directly, as the method-related factors (type of donors, harvesting-banking interval), or indirectly, as donor-related factors. Indeed, group A donors showed the highest viabilities (median OD570nmPRE = 1.5 nm) because skin was procured immediately after death, with a very short post-mortem delay (median 2 h), so that epithelial cells maintained their arterial supply of nutrients for longer than in non-heartbeating donors. The cells of group C donors showed the lowest viability of our statistical sample (median OD570nmPRE = 1.2 nm), although the death-harvesting interval (mean 8.83 h) was much shorter than for group B (mean 13.28 h). These findings suggest that early refrigeration effectively delays post-mortem loss of viability. However, it should be remembered that group C consisted of only 12 donors and differences found were not statistically significant; moreover, these donors proved to have low pre-freezing cell viability (OD570nmPRE). Of the donor-related factors, smoking and gender turned out to be the most important. Indeed, a positive history of smoking and male gender were associated with lower viability (−17 and −16 %, respectively) than non smoking history and female gender. Our data suggest a possible role of smoking in decreasing cell viability. The potential of smoking to accelerate cell senescence (e.g. through oxidative stress, inhibition of antioxidant defences, reduction of cytokine production by human plasmacytoid dendritic cells) has been observed in lung epithelial cells (Wang et al. 2001) and dermal fibroblasts (Yang et al. 2013). However, no data on long-term effects of smoking on skin cell viability have been reported in post-mortem skin samples.
With regard to the influence of the factor gender on cell viability, it is worth considering two aspects: (1) the number of female donors dying of brain haemorrhage (hence the proportion of women in group A for whom the harvesting-banking interval was necessarily briefer); (2) the number of female smoker donors, since smoking is correlated with lower cell viability. Indeed, about 53 % of female donors died of cerebral haemorrhage, accounting for a considerable fraction of all female donors (24 out of 45). Female smoker donors were only 15 % of all females (7 out of 45), while male smoker donors were 23 % of all males (20 out of 85). Interestingly, we did not find significant correlation between donor age and CSA viability (as previously reported by Gaucher and Jarraya 2014). Then we can hypothesize that skin viability is more influenced by smoke-induced ageing than physiological ageing. Regarding phototype, no statistical evaluation was possible because 95.7 % of donors showed phototype III. Dyslipidaemia and diabetes did not seem to influence cell viability.
Based on the results of this preliminary study, some recommendations can be proposed. CSA with higher cell viability can plausibly be obtained from female, non-smoker, heartbeating donors dying of cerebral haemorrhage; the skin should be harvested within 2 h of aortic clamping and banked within 12 h of harvesting (13–14 h after clamping). In general, we recommend reducing the interval between harvesting and banking for all types of CSA, but particular attention should be paid when harvesting from refrigerated donors. Although the guidelines allow procurement within 24 h, and Franchini et al. reported a clear decreasing trend of cell viability when the MTT test is performed more than 24 h after harvesting, our results suggest that skin should be harvested within 19–20 h of death and banked within 17 h of harvesting, in order to maintain acceptable viability.
However, further research on a larger database is required in order to confirm or interpret these results and to improve statistical significance. We are currently assessing additional data, including clinical history, harvesting body sites and therapeutic outcome in patients with burns and hard-to-heal wounds or ulcers (unpublished data).
References
Banyard DA, Bourgeois JM, Widgerow AD, Evans GR (2015) Regenerative biomaterials: a review. Plast Reconstr Surg 135(6):1740–1748
Blackburn JH, Boemi L, Hall WW et al (1998) Negative-pressure dressings as a bolster for skin grafts. Ann Plast Surg 40(5):453–457
Bravo D, Rigley TH, Gibran N, Strong DM, Newman-Gage H (2000) Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns 26(4):367–378
Castagnoli C, Alotto D, Cambieri I et al (2003) Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolium salt assay. Burns 29:759–767
Cleland H, Wasiak J, Dobson H et al (2014) Clinical application and viability of cryopreserved cadaveric skin allografts in severe burns: a retrospective analysis. Burns 40(1):61–66
EU guidelines to good manufacturing practice medicinal products for human and veterinary use—Annex 1, Ed. 2008
Franchini M, Zanini D, Bosinelli A et al (2009) Evaluation of cryopreserved donor skin viability: the experience of the regional tissue bank of Verona. Blood Transfus 7:100–105
Gaucher S, Jarraya M (2014) Cryopreserved human skin allografts: efficacy and viability. Burns 40(3):526–527
Gaucher S, Elie C, Verola O, Jarraya M (2012) Viability of cryopreserved human skin allografts: effects of transport media and cryoprotectants. Cell Tissue Bank 13:147–155
Greenleaf G, Hansbrough JF (1994) Current trends in the use of allograft skin for patients with burns and reflections on the future of skin banking in the United States. J Burn Care Rehabil 15(5):428–431
Hermans MHE (2011) Preservation methods of allografts and their (lack of) influence on clinical results in partial thickness burns. Burns 37(5):873–881
Kua EHJ, Goh CQ, Ting Y et al (2012) Comparing the use of glycerol preserved and cryopreserved allogenic skin for the treatment of severe burns: differences in clinical outcomes and in vitro tissue viability. Cell Tissue Bank 13:269–279
Leon-Villapalos J, Eldardiri M, Dziewulski P et al (2010) The use of human deceased donor skin allograft in burn care. Cell Tissue Bank 11(1):99–104
Li Z, Overend C, Maitz P, Kennedy P (2012) Quality evaluation of meshed split-thickness skin grafts stored at 4 °C in isotonic solutions and nutrient media by cell cultures. Burns 38(6):899–907
Linee guida per il prelievo, la processazione e la distribuzione dei tessuti a scopo di trapianto (2013) Centro Nazionale per i Trapianti (CNT). Linee guida Centro Nazionale Trapianti 10 luglio 2013, LG190607. http://www.trapianti.salute.gov.it
May SR, Roberts DP (1988) Development of a passive device for freezing large amounts of transplantable skin at one time in a −70 °C mechanical refrigerator. Cryobiology 25:186–196
Pianigiani E, Ierardi F, Cherubini F et al (2005) Skin bank organization. Clin Dermatol 23(4):353–356
Pianigiani E, Ierardi F, Cuciti C et al (2010) Processing efficacy in relation to microbial contamination of skin allografts from 723 donors. Burns 36:347–351
Pianigiani E, Ierardi F, Fimiani M (2013) Importance of good manufacturing practices in microbiological monitoring in processing human tissues for transplant. Cell Tissue Bank 14(4):601–607
Pirnay JP, Verween G, Pascual B et al (2012) Evaluation of a microbiological screening and acceptance procedure for cryopreserved skin allografts based on 14 day cultures. Cell Tissue Bank 13(2):287–295
Robb EC, Bechmann N, Plessinger RT et al (2001) Storage media and temperature maintain normal anatomy of cadaveric human skin for transplantation to full-thickness skin wounds. J Burn Care Rehabil 22(6):393–396
Rooney P, Eagle M, Hogg P et al (2008) Sterilisation of skin allograft with gamma irradiation. Burns 34(5):664–673
Saffle JR (2009) Closure of the excised burn wound: temporary skin substitutes. Clin Plast Surg 36(4):627–641
Schiozer WA, Gemperli R, Mühlbauer W et al (2013) An outcome analysis and long-term viability of cryopreserved cultured epidermal allografts. Assessment of the conservation of transplantable human skin allografts. Acta Cirúrgica Brasileira 28(12):824–832
See P, Phan TT, Chua JJ et al (2001) Our clinical experience using cryopreserved cadaveric allograft for the management of severe burns. Cell Tissue Banking 2:113–117
Udoh Y, Yanaga H, Tai Y et al (2000) Long-term viability of cryopreserved cultured epithelial grafts. Burns 26(6):535–542
Verbeken G, Verween G, De Vos D et al (2012) Glycerol treatment as recovery procedure for cryopreserved human skin allografts positive for bacteria and fungi. Cell Tissue Bank 13(1):1–7
Walcerz DB, Karow AM (1996) Cryopreservation of cells for tissue engineering. Tissue Eng 2(2):85–96
Wang H, Liu X, Umino T et al (2001) Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol 25(6):772–779
Wood JM, Soldin M, Shaw TJ, Szarko M (2014) The biomechanical and histological sequelae of common skin banking methods. J Biomech 47(5):1215–1219
Yang GY, Zhang CL, Liu XC et al (2013) Effects of cigarette smoke extracts on the growth and senescence of skin fibroblasts in vitro. Int J Biol Sci 9(6):613–623
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Pianigiani, E., Tognetti, L., Ierardi, F. et al. Assessment of cryopreserved donor skin viability: the experience of the regional tissue bank of Siena. Cell Tissue Bank 17, 241–253 (2016). https://doi.org/10.1007/s10561-016-9550-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-016-9550-0