Abstract
Purpose
To determine the effect of major antihypertensive classes on erectile function (EF) in patients with or at high risk of cardiovascular disease.
Methods
We performed a systematic review and frequentist network meta-analysis of randomized controlled trials assessing the effect of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, and thiazide diuretics on EF compared to each other and to placebo (PROSPERO: CRD42020189529). Similarly, we performed a network meta-analysis to explore the effect of different β-blockers on erectile function (nebivolol, other vasodilating and non-vasodilating β-blockers, placebo). Records were identified through search of PubMed, Cochrane Library, and Scopus databases and sources of grey literature until September 2020.
Results
We included 25 studies (7784 patients) in the qualitative and 16 studies in the quantitative synthesis. The risk of bias was concerning or high in the majority of studies, and inconsistency was also high. No significant differences in EF were demonstrated in the pairwise comparisons between major antihypertensive classes. Similarly, when placebo was set as the reference treatment group, no treatment strategy yielded significant effects on EF. In the β-blockers analysis, nebivolol contributed a beneficial effect on EF only when compared to non-vasodilatory β-blockers (OR 2.92, 95%CI 1.3–6.5) and not when compared to placebo (OR 2.87, 95%CI 0.75–11.04) or to other vasodilatory β-blockers (OR 2.15, 95%CI 0.6–7.77).
Conclusion
All antihypertensive medication classes seem to exert neutral or insignificant effects on EF. Further high-quality studies are needed to better explore the effects of antihypertensive medication on EF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erectile dysfunction (ED) is a disease, highly prevalent in the general population, and its prevalence increases with age [1, 2]. ED not only exerts a negative influence on the patients’ quality of life, but it is also considered a marker of increased incidence of cardiovascular events [3, 4]. Furthermore, ED clusters with other cardiovascular risk factors, a finding indicating that ED is a manifestation of a systemic vascular disorder [5]. In particular, ED is twice as prevalent and more severe in the hypertensive compared to the general population [6].
To complicate things further, accumulated evidence suggests that antihypertensive agents often exert unfavourable outcomes on erectile function, thus compromising medication adherence, a factor crucial for hypertension management [7, 8]. Hypertension societies have issued recommendations and consensus papers on ED and its association with antihypertensive medications [6, 9]. Based on existing data, such documents suggest that among major antihypertensive classes, thiazide diuretics and β-blockers possess the worst profile regarding erectile function, while angiotensin receptor blockers (ARBs) the most favourable [6, 7, 10]. Still, recommendations do not comprehensively address this matter as they are mostly based on scarce data or evidence from expert opinions [11]. The latter is also reflected in the insufficient knowledge of the effects of cardiovascular medication on sexual function among physicians [12]. Of importance, contrary to other antihypertensive classes, β-blockers display substantial within-class heterogeneity in terms of effectiveness and adverse cardiac and metabolic profile [13]. In particular, experimental and clinical studies suggest that, unlike other β-blockers, nebivolol is beneficial in terms of erectile function preservation [13].
Within this framework, we aimed to systematically synthesize the available evidence and generate a network meta-analysis, aiming to determine the comparative effects of major classes of antihypertensive medications on erectile function. Due to within-class heterogeneity among β-blockers, we also generated a network meta-analysis exploring the effects of different β-blockers on erectile function.
Methods
Search Strategy
The aims and methods of this systematic review and network meta-analysis were documented in a protocol registered at PROSPERO (ID: CRD42020189529). We reported this study according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement for Network Meta-analyses (PRISMA-NMA) [14].
Two independent authors (IF, NP) systematically searched PubMed, Cochrane Library and Scopus databases for RCTs exploring the effects of antihypertensive agents on erectile function from database inception to September 2020. We conducted a targeted search of the grey literature, including abstracts from conferences organized by relevant scientific associations, published in international journals. EudraCT and Clinicaltrials.gov were also perused for ongoing relevant studies. We also scanned the reference lists of all identified studies for additional eligible trials. The detailed search syntax is available in Data Supplement 1.
Search Eligibility Criteria
We included RCTs on adult male subjects with or at high-risk of cardiovascular disease, studying the effects of orally administered major antihypertensive agents [angiotensin-converting enzyme inhibitors (ACE-i), ARBs, β-blockers, calcium channel blockers (CCBs) and thiazide diuretics]. We considered studies published in any language that assessed erectile function with validated questionnaires or questionnaires developed by the authors of each study. All included trials evaluated erectile function both before and after antihypertensive treatment. Moreover, we encompassed RCTs that compared the effects of an antihypertensive agent belonging in a major antihypertensive class with another or placebo.
On the contrary, we excluded single-arm, phase I and non-randomized or observational studies. When multiple records with potential overlapping populations were identified, the most recent study was included.
Data Extraction and Quality Assessment
Two authors (IF, NP) screened for eligibility all identified records. Any disagreements or discrepancies were resolved by consensus. Data extraction was performed independently in Microsoft Excel spreadsheets, based on relevant templates from the Cochrane Handbook for Systematic Reviews of Interventions. For each included record, we retrieved information about study and participant characteristics, interventions and outcomes. To ensure coherence between the reviewers, we conducted a pilot test. Established methods, recommended by the Cochrane Collaboration, were also used to extract data from full-text articles, summary tables and figures [15]. In trials assessing erectile function at multiple time points, only data concerning the baseline and last evaluation were extracted. In case of missing data, study authors were directly contacted for further information.
The quality of included studies was assessed by two authors independently. We estimated the risk of bias in each study with the revised Cochrane risk-of-bias tool for randomized studies (RoB2), examining sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias [16]. Any discrepancies were resolved by consensus. Accordingly, we evaluated the risk of bias across studies (publication bias) via visual assessment of funnel plot asymmetry and the Egger’s test [17].
Data Synthesis and Statistical Analysis
We performed a network meta-analysis estimating the effect of major antihypertensive classes (ACE-i, ARBs, β-blockers, CCBs and thiazide diuretics) on erectile function compared to each other and to placebo. Since β-blockers are considered a heterogeneous antihypertensive medication class [13], we performed a network meta-analysis to explore the result of different β-blockers on erectile function, by dividing them into vasodilatory (carvedilol and nebivolol) and non-vasodilatory (acebutolol, atenolol, bisoprolol and metoprolol). Moreover, given that nebivolol may exert a favourable effect on erectile function [6], we undertook an additional analysis comparing the role of nebivolol versus other vasodilating and non-vasodilating β-blockers, as well as placebo.
We used the frequentist approach with a random-effects model to produce direct and indirect effect estimates for patients with ED at baseline and at the end of each trial’s follow-up using odds ratios (ORs) throughout all analyses. For all analyses, higher ORs indicated higher odds for improved erectile function after treatment. The included trials assessed erectile function with different tools such as the IIEF-5, KEED and SSDI or miscellaneous questionnaires developed by study authors [18,19,20]. Accordingly, some studies reported the number of participants with ED before and after antihypertensive treatment in a dichotomous (yes/no) way, based on the responses of each questionnaire, while others reported the degree of ED in a continuous way, based on the total score of each questionnaire. To account for these discrepancies in the estimation of erectile function, in studies reporting outcomes in a continuous way, we calculated the mean difference of the ED score before and after the intervention for each treatment arm. Subsequently, we estimated the standardized mean difference (SMD) and converted it to OR using the “smd2or” function of the “meta” package (R software, version 3.6.3). This transformation was imperative in order to incorporate in the same quantifying analysis studies that reported ED in a continuous way and studies that reported ED in a categorical way. Moreover, to classify the major antihypertensive classes in terms of erectile function deterioration, we used the P-score metric, which ranges from 0 to 1, to rank treatments. Overall, the closer a treatment was ranked to 1, the more harmful to erectile function it was considered, while the opposite applied for values close to 0.
To assess for inconsistency, we used both global approaches, e.g. computed the I2 statistic (a value >50% was considered high) and local approaches, e.g. we assessed for consistency between direct and indirect sources of evidence with the node-splitting method. For all estimations, 95% confidence intervals (CIs) which did not include the unit value and p values lower than 0.05 were considered statistically significant. All analyses were performed with R software (version 3.6.3) using the “meta” and “netmeta” packages.
Grading of Evidence
We determined the overall strength of evidence for the effect of major antihypertensive agents as well as different β-blockers on erectile function using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [21] and implementing the Confidence in Network Meta-Analysis (CINeMA) web application as proposed by Salanti and colleagues [22]. Two reviewers (IF, NP) graded risk of bias, inconsistency, indirectness, imprecision and publication bias among included trials.
Results
Search Results and Quality Assessment
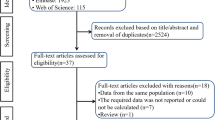
The literature search yielded 4997 relevant records, resulting in 78 eligible articles after screening all titles and abstracts. Ultimately, 25 trials were included in the qualitative synthesis [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47], twelve in the quantitative synthesis of the major antihypertensive classes [23,24,25,26,27,28,29,30,31,32,33,34] and eight in the quantitative synthesis of the β-blockers [31,32,33,34,35,36,37,38]. Three studies were excluded from the quantitative analysis because they were involving only combination antihypertensive treatment [39,40,41] and six studies because they reported insufficient data [42,43,44,45,46,47]. The selection process is illustrated in Fig. 1 and Data Supplement 2.
Employing the RoB2 tool, the risk of bias was considered low in 9, with some concerns in 6 and high in 11 studies (Data Supplement 3).
Study Characteristics
Α total of 7784 participants with a mean age of 56.2 ± 9.6 years were included in our study. The duration of treatment and follow-up ranged from 8 weeks to 5.8 years. Across trials reporting relevant data, 2456 patients reported ED at baseline, and the prevalence of ED was 37.5%. Similarly, 15.9% of participants had concomitant diabetes mellitus type 2, and 38.3 were smokers. Overall, we included 5 studies with at least one ACE-i arm [23, 25, 26, 29, 33], 8 studies with at least one ARB arm [23, 24, 27, 30, 32, 39,40,41], 19 studies with at least one b-blocker arm [25,26,27, 29,30,31,32,33,34,35,36,37,38, 41, 42, 44,45,46,47], 5 studies with at least one CCB arm [29, 33, 40, 41, 46], 9 studies with at least one thiazide arm [25, 28, 29, 33, 34, 39, 42, 43, 47] and 12 studies with at least one placebo arm [23, 27, 28, 31,32,33,34, 39, 43,44,45, 47]. Six trials compared β-blockers with each other [35,36,37,38, 42, 47], and six studies included at least one arm where a combination of antihypertensive treatment was administered [23, 25, 39,40,41,42]. Overall, four studies included patients with coronary artery disease or heart failure [23, 35, 37, 44]. Characteristics of all individual studies are depicted in Table 1.
Network Meta-analysis of Major Antihypertensive Agents Compared to Each Other and to Placebo
A total of twelve studies contributed to the erectile function assessment outcome (33 treatment arms and 2957 total patients analysed). The network graph of interventions is presented in Fig. 2. When placebo was set as the reference treatment group, none of the major antihypertensive agents significantly deteriorated erectile function (Fig. 3). Heterogeneity and inconsistency were deemed high in the model (Q-statistic p value=0.004, I2=55.8%, tau2=0.81).
Network graph of interventions. Each node represents an antihypertensive class and each line the antihypertensive classes directly compared based on the included studies. The digits and the thickness of lines reflect the number of arms from available studies evaluating each direct comparison. *ACE-i angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CCB calcium channel blocker
With regard to pairwise comparisons of the five major antihypertensive classes, no significant differences were evident (Table 2). Of note, there was no direct comparison of the ARB group versus the CCB group and the ARB group versus the thiazide group.
Node splitting method detected significant disagreement between direct and indirect evidence for the CCB group versus the thiazide group, while no other significant disagreements were detected (Data supplement 4). Egger’s regression test did not demonstrate any publication bias (Data supplement 5).
Ranking of Antihypertensive Drug Classes with Regard to their Effect on Erectile Function
The thiazide group ranked as the most detrimental antihypertensive medication class for erectile function (P-score=0.91), followed by the β-blocker group (P-score=0.60) and the CCB group (P-score=0.58). On the other hand, ARBs (P-score=0.27) were ranked as the least detrimental antihypertensive agent for erectile function followed by ACE-i (P-score=0.37).
Grading of Evidence
Overall, the level of evidence was deemed low or very low, due to the high risk of bias of the majority of included trials, as well as to the substantial level of heterogeneity across studies. The grading of the pairwise comparisons is illustrated in Table 2.
Effects of β-Blocker Agents on Erectile Function
We included a total of eight studies (1046 patients) in the quantitative synthesis of β-blockers. Relevant outcomes were available for vasodilatory (nebivolol, carvedilol) and non-vasodilatory β-blockers (acebutolol, atenolol, bisoprolol, metoprolol) as described in the “Methods” section. The network graph of β-blockers, generated by the studies which included at least two arms of different β-blockers or placebo, can be seen in Data supplement 6. Compared to placebo, neither vasodilatory (OR 2.07, 95% CI −0.6–7.1) nor non-vasodilatory β-blockers (OR 0.96, 95% CI 0.33–2.82) significantly improved or deteriorated erectile function. Across the pairwise comparisons, vasodilatory β-blockers seemed to have a significant beneficial effect on erectile function compared to non-vasodilatory β-blockers (OR 2.17, 95% CI 1.15–4) (Data supplement 6).
When nebivolol was assessed separately from the rest of vasodilatory β-blockers group (essentially carvedilol), it did not show any significant beneficial effect on erectile function compared to placebo (OR 2.87, 95% CI 0.75–11.04) or to carvedilol (OR 2.15, 95% CI 0.6–7.77) (Fig. 4). However, nebivolol contributed a significant beneficial effect on erectile function compared to non-vasodilatory β-blockers (OR 2.92, 95% CI 1.3–6.5), while no difference between carvedilol and non-vasodilatory β-blockers was demonstrated (OR 1.36, 95% CI 0.5–3.69) (Data supplement 7). In terms of treatment raking, nebivolol ranked as the least detrimental β-blocker for erectile function (P-score=0.06), followed by vasodilatory β-blockers (P-score=0.5) and placebo (P-score=0.69). On the contrary, non-vasodilatory β-blockers ranked as the most detrimental β-blocker for erectile function (P-score=0.74). Still, in the GRADE assessment, evidence on the matter was rated as low or very low (Table 3).
Discussion
This systematic review and network meta-analysis suggests that there is insufficient evidence to support that any of the main antihypertensive classes exert significant detrimental or beneficial effects on erectile function when compared to each other or to placebo. On the comparative leg of the analysis, on a low strength of evidence, all major antihypertensive classes seem to exert a neutral effect on erectile function. Focusing on β-blockers, nebivolol may provide some beneficial effects on erectile function compared to non-vasodilatory β-blockers, on a low strength of evidence. However, compared to placebo or to other vasodilatory β-blockers, nebivolol did not show any significant beneficial effect on erectile function.
The guidelines for the management of arterial hypertension from the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) state that sexual dysfunction in men may be induced or aggravated by thiazide diuretics and β-blockers, while ACE-i, ARBs, CCBs and vasodilating β-blockers may present neutral or even beneficial effects on erectile function [9]. These recommendations mostly derive from systematic reviews of observational or interventional studies and from expert opinions [7, 8, 10, 48]. A brief meta-analysis of RCTs for the role of ARBs on ED demonstrated that ARBs exert beneficial effects on erectile function when compared with other treatment modalities [49]. However, this effect was almost exclusively driven by a non-randomized study of ARB-treated patients (n=1899) versus control (n=27) [50]. Based on our network meta-analysis of RCTs, there is no such evidence that ARBs exert a beneficial effect on erectile function as none of the major antihypertensive classes may aggravate or improve erectile function. Accordingly, the ESH Working Group on erectile function implies that nebivolol diverges from other β-blockers in terms of erectile function impairment [6]. This recommendation derives predominantly from translational data suggesting that nebivolol facilitates penile artery dilatation by enhancing nitric oxide signalling of the corpora cavernosa [51, 52]. Still, based on our analysis, no such beneficial effect of nebivolol on erectile function was proven in humans. Only a tendency for the beneficial effects of nebivolol compared to placebo on EF is being observed; however, the confidence interval of the comparison is too wide, thus implying that deriving such a conclusion from our results is imprecise, and our analysis may be underpowered to detect such a difference.
Patients’ perception on the adverse events related potential of drugs is important for medication adherence in the setting of arterial hypertension [53]. It has been postulated that being prejudiced for potential adverse events causes the so-called Hawthorne effect that further inhibits sexual function [54, 55]. Upon adverse events development, like ED, which cannot be objectively and extensively assessed by physicians, the presence of such side effects is often exaggerated [56]. Therefore, healthcare providers should promptly offer concise advice and information on the interplay of antihypertensive treatment and ED and must ensure proper medication adherence. Still, in patients reporting ED deterioration, phosphodiesterase type-5 inhibitors may not only be beneficial in treating ED, but they also have additive effects on the lowering of blood pressure and improved medication adherence [57, 58].
Perspectives
In a field of research, where review articles and expert commentary far exceed hard data [59], future prospective studies are needed to thoroughly address the role of major antihypertensive classes on erectile function. Ideally, a carefully designed, large, multi-arm RCT with standardized interventions and erectile function outcomes is necessary to better understand the effects of antihypertensive medications on erectile function and make recommendations for this common encounter. Last but not least, given that combination therapy is now recommended for the achievement of the blood pressure target and that dozens of different combinations exist, there is a paucity of data regarding potential interactions between antihypertensive agents and effectiveness of combinational therapies in erectile function. Without this level of evidence, it should not be stated that an antihypertensive drug class improves or deteriorates erectile function.
Strengths and Limitations
Our systematic review and network meta-analysis presents important strengths. To our knowledge, this is the first study to assess, in a holistic approach, the effects of antihypertensive medication on erectile function by including specifically RCTs and using data synthesis and meta-analysis techniques. In this scope, we generated a network meta-analysis to assess for direct and indirect sources of evidence in a field of research that is alive with multiple interventions and heterogeneously designed studies. Since β-blockers are considered a high heterogeneous drug class in terms of erectile function exacerbation, we provided a separate analysis exploring the within-class different effects of β-blockers. Furthermore, our results contest previously published qualitative analyses and highlight the need for higher quality of evidence to suggest that any antihypertensive treatment exerts beneficial or detrimental effects on erectile function.
The findings of our study should be interpreted in the context of limitations relevant to the significant heterogeneity among the included trials. Across studies, important differences in design, population and sample size were observed. Indeed, our synthesis comprised individuals with normal erectile function or ED, participants with hypertension and/or concomitant cardiovascular comorbidities, patients previously treated for hypertension as well as treatment-naive males. Based on the previous notion, we could not adjust for important moderators of ED such as age, diabetes and smoking. Of note, none of the included trials standardized the effect of different antihypertensive agents on erectile function by assessing in the form of a subgroup analysis the degree of blood pressure lowering leading to erectile function deterioration. Additionally, most included trials were relatively old and raised methodological concerns as they did not strictly abide to the consolidated standards of reporting and performing RCTs. Accordingly, due to inadequacy or lack of relevant data, more than half of the included trials were excluded from the quantitative analysis. Therefore, the network meta-analysis of both major antihypertensive agents and β-blockers was performed with a relatively small number of patients, raising issues of power in terms of its ability to detect any differences among antihypertensive medication classes, if they exist. It should also be stressed that estimates of erectile function displayed significant variety among available trials, as study authors employed different validated and non-validated questionnaires to assess erectile function. To account for such discrepancies, we calculated SMDs and converted continuously reported outcomes to ORs to achieve a uniform effect measure for analysis. Still, this transformation, although described in the Cochrane Collaboration Handbook, may be regarded as an approximation and should be interpreted with caution. All in all, the plethora of limitations of the available body of literature demonstrated that there is insufficient evidence to support that any of the main antihypertensive classes exerts significant detrimental or beneficial effects on erectile function.
Conclusion
Our systematic review and network meta-analysis suggests that all antihypertensive drugs seem to exert a neutral or insignificant effect on erectile function compared to each other or to placebo. Given that evidence is still weak on the matter, our analysis does not support the current ESC/ESH guidelines statement that ED may be induced or aggravated by thiazide diuretics and β-blockers, while ACE-i, ARBs, CCBs, and vasodilating β-blockers may present neutral or even beneficial effects on erectile function. Therefore, carefully designed, large RCTs with standardized interventions and outcomes are needed to better explore the effects of antihypertensive medication on erectile function.
Data Availability
All data are available upon request.
References
Impotence: NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90.
Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–65.
Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61.
Vlachopoulos C, Jackson G, Stefanadis C, Montorsi P. Erectile dysfunction in the cardiovascular patient. n.d.;34:2034–46.
Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–78.
Viigimaa M, Vlachopoulos C, Doumas M, Wolf J, Imprialos K, Terentes-Printzios D, et al. Update of the position paper on arterial hypertension and erectile dysfunction. J Hypertens. 2020;38:1220–34.
Nicolai MPJ, Liem SS, Both S, Pelger RCM, Putter H, Schalij MJ, et al. A review of the positive and negative effects of cardiovascular drugs on sexual function: a proposed table for use in clinical practice. Neth Hear J. 2014;22:11–9.
La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2015;49:3–13.
Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Eur Heart J. 2018;39:3021–104.
Baumhäkel M, Schlimmer N, Kratz M, Hacket G, Jackson G, Böhm M. Cardiovascular risk, drugs and erectile function - a systematic analysis. Int J Clin Pract. 2011;65:289–98.
Al Khaja KAJ, Sequeira RP, Alkhaja AK, Damanhori AHH. Antihypertensive drugs and male sexual dysfunction: a review of adult hypertension guideline recommendations. J Cardiovasc Pharmacol Ther. 2016;21:233–44.
Nicolai MPJ, Liem SS, Both S, Pelger RCM, Putter H, Schalij MJ, et al. What do cardiologists know about the effects of cardiovascular agents on sexual function? A survey among Dutch cardiologists. Part I. Neth Hear J. 2013;21:540–4.
Manolis A, Doumas M, Ferri C, Mancia G. Erectile dysfunction and adherence to antihypertensive therapy: focus on β-blockers. Eur J Intern Med. 2020;81:1–6.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJWV. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: Wiley; 2019.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res. 2000;12:305–11.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26.
Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, et al. The effects of antihypertensive therapy on the quality of lifE. N Engl J Med. 1986;314:1657–64.
Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Giovane CD, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082.
Böhm M, Baumhäkel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: the ongoing telmisartan alone and in combination with ramipril global endpoint trial/telmisartan randomized assessment study in ace intoler. Circulation. 2010;121:1454–5.
Chen Y, Cui S, Lin H, Xu Z, Zhu W, Shi L, et al. Losartan improves erectile dysfunction in diabetic patients: a clinical trial. Int J Impot Res. 2012;24:217–20.
Croog SH, Levine S, Sudilovsky A, Baume RM, Clive J. Sexual symptoms in hypertensive patients: a clinical trial of antihypertensive medications. Arch Intern Med. 1988;148:788–94.
Fogari R, Zoppi A, Corradi L, Mugellini A, Poletti L, Lusardi P. Sexual function in hypertensive males treated with lisinopril or atenolol: a cross-over study. Am J Hypertens. 1998;11:1244–7.
Fogari R, Preti P, Derosa G, Marasi G, Zoppi A, Rinaldi A, et al. Effect of antihypertensive treatment with valsartan or atenolol on sexual activity and plasma testosterone in hypertensive men. Eur J Clin Pharmacol. 2002;58:177–80.
Scharf MB, Mayleben DW. Comparative effects of prazosin and hydrochlorothiazide on sexual function in hypertensive men. Am J Med. 1989;86:110–2.
Suzuki H, Tominaga T, Kumagai H, Saruta T. Effects of first-line antihypertensive agents on sexual function and sex hormones. J Hypertens. 1988;6:S649–51.
Van Bortel LM, Bulpitt CJ, Fici F. Quality of life and antihypertensive effect with nebivolol and losartan. Am J Hypertens. 2005;18:1060–6.
Broekman CP, Haensel SM, Van de Ven LL, Slob AK, Matthijs Broekman CP, Haensel SM, et al. Bisoprolol and hypertension: effects on sexual functioning in men. TGO - Tijdschr Voor Ther Geneesm En Onderz. 1992;17:79–82+83.
Fogari R, Zoppi A, Poletti L, Marasi G, Mugellini A, Corradi L. Sexual activity in hypertensive men treated with valsartan or carvedilol: a crossover study. Am J Hypertens. 2001;14:27–31.
Grimm RH, Grandits GA, Prineas RJ, McDonald RH, Lewis CE, Flack JM, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women: Treatment of Mild Hypertension Study (TOMHS). Hypertension. 1997;29:8–14.
Wassertheil-Smoller S, Blaufox MD, Oberman A, Davis BR, Swencionis C, Knerr MO, et al. Effect of antihypertensives on sexual function and quality of life: The TAIM study. Ann Intern Med. 1991;114:613–20.
Aldemir M, Keleş İ, Karalar M, Tecer E, Adalı F, Pektaş MB, et al. Nebivolol compared with metoprolol for erectile function in males undergoing coronary artery bypass graft. Anatol J Cardiol. 2016;16:131–6.
Brixius K, Middeke M, Lichtenthal A, Jahn E, Schwinger RHG. Nitric oxide, erectile dysfunction and beta-blocker treatment (MR NOED study): benefit of nebivolol versus metoprolol in hypertensive men. Clin Exp Pharmacol Physiol. 2007;34:327–31.
Gür Ö, Gurkan S, Yumun G, Turker P, Gur O, Gurkan S, et al. The comparison of the effects of nebivolol and metoprolol on erectile dysfunction in the cases with coronary artery bypass surgery. Ann Thorac Cardiovasc Surg. 2017;23:91–5.
Martsevich SY, Tolpigina SN, Galiavich AS, Volkova EG, Malishevsky MV, Matiushin GV, et al. Comparison of the influence of long-term treatment based on carvedilol or bisoprolol on metabolic parameters and erectile function in hypertensive patients with overweight or obesity. Results of the randomized open-label parallel-groups stepped trial CABR. Ration Pharmacother Cardiol. 2012;8:626–35.
Joseph P, Lonn E, Bosch J, Lopez P, Zhu J, Keltai M, et al. Long-term effects of statins, blood pressure-lowering, and both on erectile function in persons at intermediate risk for cardiovascular disease: a substudy of the Heart Outcomes Prevention Evaluation-3 (HOPE-3) Randomized controlled trial. Can J Cardiol. 2018;34:38–44.
Xiaoma D, Jianli C, Xin S. Study on the effects of felodipine sustained release tablets joint irbesartan tablets on erectile function of male patients with hypertension. Chin J Androl. 2014;28:43–6.
Yang L, Yu J, Ma R, Zhao F, Lin X, Liu P, et al. The effect of combined antihypertensive treatment (felodipine with either irbesartan or metoprolol) on erectile function: a randomized controlled trial. Cardiol Switz. 2013;125:235–41.
Boydak B, Nalbantgil S, Fici F, Nalbantgil I, Zoghi M, Ozerkan F, et al. A randomised comparison of the effects of nebivolol and atenolol with and without chlorthalidone on the sexual function of hypertensive men. Clin Drug Investig. 2005;25:409–16.
Chang SW, Fine R, Siegel D, Chesney M, Black D, Hulley SB. The impact of diuretic therapy on reported sexual function. Arch Intern Med. 1991;151:2402–8.
Franzen D, Metha A, Seifert N, Braun M, Höpp HW. Effects of beta-blockers on sexual performance in men with coronary heart disease. A prospective, randomized and double blinded study. Int J Impot Res. 2001;13:348–51.
Kostis JB, Rosen RC, Brondolo E, Taska L, Smith DE, Wilson AC. Superiority of nonpharmacologic therapy compared to propranolol and placebo in men with mild hypertension: a randomized, prospective trial. Am Heart J. 1992;123:466–74.
Morrissette DL, Skinner MH, Hoffman BB, Levine RE, Davidson JM. Effects of antihypertensive drugs atenolol and nifedipine on sexual function in older men: a placebo-controlled, crossover study. Arch Sex Behav. 1993;22:99–109.
Rosen RC, Kostis JB, Jekelis A, Taska LS. Sexual sequelae of antihypertensive drugs: treatment effects on self-report and physiological measures in middle-aged male hypertensives. Arch Sex Behav. 1994;23:135–52.
Doumas M, Douma S. The effect of antihypertensive drugs on erectile function: a proposed management algorithm. J Clin Hypertens Greenwich Conn. 2006;8:359–64.
Ismail SB, Noor NM, Hussain NHN, Sulaiman Z, Shamsudin MA, Irfan M. Angiotensin receptor blockers for erectile dysfunction in hypertensive men: a brief meta-analysis of randomized control trials. Am J Mens Health. 2019;13:155798831989273.
Della Chiesa A, Pfiffner D, Meier B, Hess OM. Sexual activity in hypertensive men. J Hum Hypertens. 2003;17:515–21.
Toblli JE, Cao G, Casas G, Mazza ON. In vivo and in vitro effects of nebivolol on penile structures in hypertensive rats. Am J Hypertens. 2006;19:1226–32.
Angulo J, Wright HM, Cuevas P, González-Corrochano R, Fernández A, Cuevas B, et al. Nebivolol dilates human penile arteries and reverses erectile dysfunction in diabetic rats through enhancement of nitric oxide signaling. J Sex Med. 2010;7:2681–97.
Grégoire JP, Moisan J, Guibert R, Ciampi A, Milot A, Gaudet M, et al. Determinants of discontinuation of new courses of antihypertensive medications. J Clin Epidemiol. 2002;55:728–35.
Silvestri A. Report of erectile dysfunction after therapy with beta-blockers is related to patient knowledge of side effects and is reversed by placebo. Eur Heart J. 2003;24:1928–32.
Cocco G. Erectile dysfunction after therapy with metoprolol: the Hawthorne effect. Cardiology. 2009;112:174–7.
Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. β-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. J Am Med Assoc. 2002;288:351–7.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–248.
McLaughlin T, Harnett J, Burhani S, Scott B. Evaluation of erectile dysfunction therapy in patients previously nonadherent to long-term medications: a retrospective analysis of prescription claims. Am J Ther. 2005;12:605–11.
Düsing R. Sexual dysfunction in male patients with hypertension: influence of antihypertensive drugs. Drugs. 2005;65:773–86.
Author information
Authors and Affiliations
Contributions
IF, NP, ID, IM and GG contributed to the conception or design of the work. IF, NP and EA contributed to the acquisition, analysis or interpretation of the data for the work. IF and NP drafted the manuscript. ID, IM, EA and GG critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
No humans were involved in this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farmakis, I.T., Pyrgidis, N., Doundoulakis, I. et al. Effects of Major Antihypertensive Drug Classes on Erectile Function: a Network Meta-analysis. Cardiovasc Drugs Ther 36, 903–914 (2022). https://doi.org/10.1007/s10557-021-07197-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07197-9