Abstract
Mucins are heavily O-glycosylated proteins primarily produced by glandular and ductal epithelial cells, either in membrane-tethered or secretory forms, for providing lubrication and protection from various exogenous and endogenous insults. However, recent studies have linked their aberrant overexpression with infection, inflammation, and cancer that underscores their importance in tissue homeostasis. In this review, we present current status of the existing mouse models that have been developed to gain insights into the functional role(s) of mucins under physiological and pathological conditions. Knockout mouse models for membrane-associated (Muc1 and Muc16) and secretory mucins (Muc2) have helped us to elucidate the role of mucins in providing effective and protective barrier functions against pathological threats, participation in disease progression, and improved our understanding of mucin interaction with biotic and abiotic environmental components. Emphasis is also given to available transgenic mouse models (MUC1 and MUC7), which has been exploited to understand the context-dependent regulation and therapeutic potential of human mucins during inflammation and cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
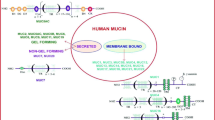
Mucins comprise a complex family of high-molecular-weight, membrane-bound, or secreted O-glycoproteins which are produced by glandular and ductal epithelial cells. Mucins play critical roles in lubrication and protection of mucosa, renewal and differentiation of the epithelia, cell adhesion, and cellular signaling (Fig. 1a) [2–4]. So far, 21 mucins have been recognized in human, out of them 12 are attached to the cell membrane, whereas the others are secreted by the cells [2]. Multiple studies have shown the diverse and tissue-specific expression profile of mucins. Nonetheless, a single tissue can express number of different mucins (Table 1). Qualitative and quantitative alterations in mucins have been correlated with the inflammatory, pre-neoplastic, and neoplastic conditions [5, 7–12]. Studies have shown that some of the membrane-spanning mucins can serve as cell surface receptors and facilitate signal transduction in response to external stimuli that lead to cell proliferation, differentiation, apoptosis, migration, and invasion of cancer cells (Fig. 1b) [2, 13–18]. Despite ongoing research efforts, the structure and function of various mucins and mucin-mediated molecular mechanisms under normal and pathological conditions remain poorly understood. Moreover, the awry molecular and cellular mechanisms which lead to the aberrant expression and upregulation of various mucins under different disease conditions have not been completely comprehended [19].
Illustration of the various physiological outcomes of aberrant mucin expression under normal and pathological conditions. a Under normal physiological condition, mucins provide lubrication and protection to the epithelial surface by providing a physical barrier from a hostile environment. Mucins shield the epithelium against the action of various pathogens ( ), enzymes, gastric, and bile acids [1]. Mucins are involved in the cellular differentiation of epithelial and immune cells. The expression of mucins in bone marrow (BM) progenitors and mature immune cells is involved in hematopoiesis. b Under pathological conditions, mucins are aberrantly expressed and undergo differential posttranslational modifications. The mucous layer sequesters many molecules involved in inflammation, cellular migration, and healing processes [2]. Mucins help transformed cells to avoid immune surveillance by masking epitopes of tumor antigens on the cell surface. Loss of apical–basolateral polarity allows interaction between membrane-bound mucins (MBMs) and growth factor receptors such as receptor tyrosine kinases (
), enzymes, gastric, and bile acids [1]. Mucins are involved in the cellular differentiation of epithelial and immune cells. The expression of mucins in bone marrow (BM) progenitors and mature immune cells is involved in hematopoiesis. b Under pathological conditions, mucins are aberrantly expressed and undergo differential posttranslational modifications. The mucous layer sequesters many molecules involved in inflammation, cellular migration, and healing processes [2]. Mucins help transformed cells to avoid immune surveillance by masking epitopes of tumor antigens on the cell surface. Loss of apical–basolateral polarity allows interaction between membrane-bound mucins (MBMs) and growth factor receptors such as receptor tyrosine kinases ( ), leading to sustained proliferative signaling cascades. Furthermore, the overexpression of mucins promotes cell motility and invasiveness and induces resistance to chemotherapeutic agents. Interestingly, the aberrant expression of secretory mucins (SMs) occasionally facilitates pathogenic infection, though the exact mechanism is still not understood
), leading to sustained proliferative signaling cascades. Furthermore, the overexpression of mucins promotes cell motility and invasiveness and induces resistance to chemotherapeutic agents. Interestingly, the aberrant expression of secretory mucins (SMs) occasionally facilitates pathogenic infection, though the exact mechanism is still not understood
Although cell-based in vitro studies have yielded crucial knowledge about the role of mucins under various pathological conditions, information regarding the physiologically relevant functions of mucins in context of the microenvironment which exists in tissue/tumor and complex interactions among different cell types remains obscure and underexplored. Therefore, the development of suitable animal models will provide an improved understanding of the consequence of dysregulation under normal and pathological conditions. Xenograft models have been helpful in validating the in vitro findings [20–24]; however, these models have several limitations including lack of competent immune system and factual microenvironment. Since recent reports have implicated the importance of mucins in immune system regulation, it is critical to study the role of mucins in the context of a functional immune system [25, 26]. These shortcomings could be overcome by utilizing transgenic (Tg), knockin (KI), and knockout (KO) mouse models, collectively known as genetically engineered mouse (GEM) models. Cre-Lox and Flp-FRT recombination systems are the most commonly used approaches for the development of conditional and inducible KO and KI mouse models [27, 28]. Some of the mucin mouse models have provided crucial information about the importance of mucins including their role under physiological and pathological conditions. In this review article, we intend to provide an overall picture of the currently available mucin mouse models and discuss their utility for understanding mucin function under inflammatory and malignant pathologies.
2 Comparative analysis and synteny of mouse and human mucins
Mice are frequently used as a model system to study the biology of human diseases because of their small size, ease of manipulation, short generation interval, and relatively inexpensive maintenance costs. Before describing the usefulness of murine models to understand the function of human mucins, it is important to emphasize the homology between murine and human mucins. Table 2 summarizes different types of human mucins, their homologues in mice, and their genomic localization. Mucins consist of multiple domains (Fig. 2): sperm protein, enterokinase, and the agrin domain (SEA) involved in protein interactions; epidermal growth factor (EGF)-like domain that can act as a ligand; cysteine-rich dimerization or D domain (including D1, D2, D′, D3 similar to von Willebrand (vWD) domains) for oligomerization; variable number of tandem repeats (VNTR or TRs) rich in serine (Ser), threonine (Thr), proline (Pro) (collectively known as S/T/P) for O-linked glycosylation; the hydrophobic transmembrane (TM) domain for cell surface localization and cytoplasmic tail (CT) to facilitate signal transduction [31, 32]. The domain organization and expression pattern of mucins is largely conserved between human and mouse and is discussed below in detail.
Representation of the prototype structure of mucins along with the characterized and putative roles of their functional domains: MBMs and SMs have a TR domain with variable numbers and lengths of the repeats. They are predominantly get O-glycosylated and separated by unique sequences. They also have few N-glycosylation sites with varying localization with different mucins. Most of the MBMs possess SEA domains with a potential cleavage site (G/SVVV), except MUC4 where GDPH (also present in MUC2 and MUC5 AC secretory mucins) is considered to be a putative site for cleavage. Mucins have varying lengths of CT (MUC4 CT is shortest with 22 amino acids), which are believed to facilitate signal transduction due to the presence of potential phosphorylation sites such as Ser, Thr, and Tyr residues. Other domains present in mucins include EGF-like motifs, nidogen and adhesion-associated NIDO and AMOP, and vWD domains. SMs are rich in cys-rich domains (D1, D2, D3, and D4), which are similar to the D domains of the vWD factor and flank the TR region. These domains are important for disulfide cross-linking to allow oligomerization between the mucin molecules required for gel-forming network
2.1 Membrane-bound mucins
Muc1 was the first murine mucin gene identified and characterized [33]. The human MUC1 gene and its murine ortholog are 87 % identical in the non-TR domains and 74 % in the promoter regions. The VNTR region of human MUC1 consists of 20 amino acid repeats, while that of mouse Muc1 has 20 to 21 amino acids each [34]. The maximum similarity between Muc1 and MUC1 exists in their TM and cytoplasmic domains. The tissue-specific expression pattern of the mouse Muc1 is also very similar to that of its human counterpart (Table 1). Similarities in the sequence and expression pattern of human MUC1 and murine Muc1 are indicative of their similarities in function(s), interacting partners, mode of internalization, subcellular localization, and routing to the plasma membrane during their recycling or after their synthesis.
Muc4, like its human ortholog, is encoded by 25 exons [35]. It consists of at least 20 TRs of 124–126 amino acids each, whereas human MUC4 has 146–500 repeats of 16 amino acid residues. The Ser/Thr region located upstream of TRs in murine Muc4 is significantly different and much smaller in size (63 amino acids) as compared to human sequence (951 amino acids) [35]. Interestingly, 12 potential N-glycosylation sites, which are downstream of TR region, are perfectly conserved in Muc4 and MUC4 and both orthologs exhibit similar expression patterns [35].
Two large exons of mouse Muc16 at the N terminal region have sequence homology to exons 1 and 3 of human MUC16. Murine Muc16 possesses only one SEA domain in its extracellular (EC) region, whereas the number of SEA domains in the EC region of human MUC16 goes up to 60. Muc16 also shares the similar characteristic repeat structure of human MUC16 along with 66 % homology in their C-terminus [36]. The overall expression pattern of Muc16 and MUC16 is similar (Table 1). Both of them are expressed by the ovarian surface epithelial cells, though their cellular localization is different. Human MUC16 is present on the cell surface and soluble fraction due to its shedding from the cell membrane, whereas murine Muc16 has shown to be secreted by MOVCAR ovarian cells [37].
Other membrane-bound murine mucins including Muc13, Muc15, Muc3, Muc20, and Muc21 are either partially characterized or have not been characterized yet. The C-terminus of Muc13 shows 52 % identity to the human MUC13 ortholog. However, the N-terminus of the Muc13 mucin domain shows a significant divergence from the human MUC13, as the murine form has a nearly perfect repeat structure in contrast to the human form which retains many degenerate repeats [38, 39]. The carboxyl terminal of MUC17 was found to be 59.6 % similar to murine Muc3, while there is only 46.4 % amino acid sequence similarity between murine and human MUC3. MUC17 has 52 % similarity with the first EGF domain and 63.5 % similarity with the second EGF domain of Muc3. Altogether, there is greater similarity between Muc3 and MUC17 compared to Muc3 and MUC3, suggesting that MUC17 is the ortholog of Muc3 [29, 30]. Comparison of the amino acid sequences of human and mouse Muc20 showed 48 % overall similarity [40]. Both mucins comprise several hydrophobic domains and three mucin-like repeats of 18 amino acid residues in their N-terminal regions and are expressed predominantly in the kidney.
2.2 Gel-forming mucins
Gel-forming mucins are the main components of mucus and consist of multiple “cysteine-rich” vWF C and vWF D domains in the flanking region of the mucin-like Thr/Ser-rich repeats and C-terminal cystine knot-like domain (CTCK) [41], which allow them to oligomerize by forming intermolecular disulfide bonds. Currently, five gel-forming murine mucins have been recognized: Muc2, Muc5ac, Muc5b, Muc6 and Muc19. Interestingly, four of these genes (Muc2, Muc5ac, Muc5b, and Muc6) are clustered on chromosome 7 F5 [42], a region that exhibits synteny with the human chromosome 11p15 [43]. The order of clustering of secretory mucin genes is Muc6–Muc2–Muc5ac–Muc5b, which is conserved in both human and mouse [43].
Muc2 forms the basic framework for the formation of an intraluminal mucus gel of various gastrointestinal (GI) organs [44]. Apart from their 75 % homology at the N-terminus, mouse and human MUC2 promoter regions also exhibit a strong sequence similarity which might subject them to similar transcriptional regulation [45]. Muc2, like its human counterpart, is predominantly expressed in the colon, to a lesser extent in the small intestine and undetectable in the stomach [46].
The TR of Muc5ac contains a 16-amino acid sequence, whereas the human MUC5AC has only eight amino acid residues per repeat [34, 47]. The TR domain of Muc5ac is followed by a 133-amino acid cysteine-rich non-repetitive region (CRRI), a 63-residue non-repetitive Ser/Thr-rich domain, and a second cysteine-rich region (CRRII) which share around 81 and 76 % similarity, respectively [34]. Despite the lack of sequence similarity between the TR units of the murine and human MUC5AC, their non-repetitive regions are nearly identical.
Alignment of the Muc5b gene with its human ortholog indicated few common features. Overall, there is 43 % identity between the murine Muc5b and human MUC5B which is predominantly contributed by N- and C-terminal regions which are 64 and 62 % similar, respectively [48]. However, the expression pattern of Muc5b does not match the human MUC5B, as the murine form is principally expressed in the laryngeal mucous glands and at a low level in the stomach and duodenum, whereas the MUC5B gene is expressed in many tissues including the airway, gall bladder, and tongue [48].
The mouse Muc19 gene is located on chromosome 15, which is homologous to human MUC19 on chromosome 12 [41]. Like other gel-forming mucins, Muc19 also has vWD, vWC, and CTCK domains [41]. Paired analysis of mouse Muc19 and human MUC19 has shown 27 % homology [49], mainly at the C-terminus and the putative N-terminus of the peptide sequences, whereas the central repetitive regions did not show any homology [41]. Similar to the human MUC19, Muc19 is predominantly expressed in the salivary glands.
The murine Muc6 is composed of 33 exons, and comparative analysis suggested that the human Muc6 and mouse Muc6 lack both the cysteine-rich domains and the cysteine-rich subdomains which are frequently found in the S/T/P-rich regions of other human and mouse secretory mucins (MUC2, 5AC, and 5B). The absence of these cysteine-rich domains and subdomains possibly makes them resistant to proteolytic degradation [50] and could be the reason for their high expression in the stomach in both humans and mice. In addition to the stomach, murine Muc6 also exhibits high expression in the duodenum, whereas it is expressed at low levels in the salivary glands [42].
3 Utilization of mucin mouse models for studying pathological conditions
Although studies of mucins under in vitro culture conditions have helped in elucidating the pathways operative in specific cell types, research in animal models incorporates the intricacies of the organ system in the context of dynamic immunological, hormonal, and physiological milieus. Mucin mouse models can provide improved understanding of the pathobiological implications of individual mucins in a more comprehensive and holistic manner in a physiologically relevant host microenvironment.
3.1 Mucin KO mice and associated pathologies
To date, KO mouse models lacking the expression of Muc1, Muc2, and Muc16 have been generated. Strategies to generate these mice along with their utility to comprehend the role of these mucins in various diseases are discussed in detail in the following sections.
3.1.1 The Muc1 KO mouse model
The Muc1 KO mouse (Muc1−/−) was generated by targeted inactivation of Muc1 gene by inserting a neomycin selection cassette within exon 2 [51]. Interestingly, Muc1−/− mice displayed no alterations in survival rate and fertility and appeared phenotypically normal. In these mice, Muc4 expression increased in lactating mammary gland, salivary gland, lung, stomach, and colon (Fig. 3a), but there was no significant difference in the expression levels of other mucin-like genes, including ASGP-2, CD34, CD43, glycophorin, and Mad-CAM-1 [51]. Muc1−/− mice fed on lithogenic diet for 56 days exhibited significantly reduced secretion and accumulation of mucin (Muc1 and Muc5ac) in the gallbladder as compared to the wild-type (WT) mice, resulting in reduced crystallization of cholesterol and inhibition of gallstone formation (Fig. 3a) [52]. Like human disease, genetic mouse model of cystic fibrosis (CF), driven by CFTR S489X mutation, is characterized by high mucus production and accumulation which results in severe intestinal obstruction and death at weaning. CFTR mice in Muc1 null background exhibited significant reduction in the intestinal mucus accumulation, suggesting that Muc1 predominantly contributes to the mucinous obstruction of the GI tract during CF [53].
Representation of the pathological phenotypes that arise upon the knockout of individual mucins in the mouse models: a Muc1 knockout (KO) mice express higher levels of Muc4 in the salivary gland, stomach, colon, and mammary glands, which could probably act as a compensatory mechanism. Muc1 null mice exhibited reduced breast tumor growth upon polyoma middle T antigen induction as compared to WT mice. The CFTR mouse when crossed with the Muc1−/− mouse showed less severe intestinal obstruction as compared to the control mice. Muc1−/− mice also demonstrated reduced cholesterol absorption, signifying the importance of mucin in cholesterol uptake in the small intestine. Furthermore, Muc1-deficient mice showed reduction in the formation of cholesterol crystals and, consequently, reduced incidence of gallstone formation when fed the lithogenic diet. MUC1−/− mice on a KrasG12D-Cre background demonstrated higher survival and reduced high-grade PanIN lesions as compared to the KC and KCM mice. Muc1−/− mice exhibited increase susceptibility to get infected by H. pylori. In Muc1-deficient mice, increased differentiation of bone marrow progenitor cells (BMPC) to immature cell population of myeloid origin (MDSC) was observed upon GM-CSF and IL-4 stimulation. b Muc2−/− KO mice develop abnormalities predominantly in the lower gastrointestinal track (LGIT). There is an increase in crypt length and a higher proliferation rate at 4 weeks of age, which subsequently progresses to invasive carcinoma in older mice. IL-10 and p21 KO on a Muc2-deficient background further increased the incidence of tumor formation, mortality, and mucosal thickening in LGIT. IL-10−/−, Muc2−/− mice have shown a significant reduction in the expression of other mucins like Muc4 and Muc3 in the intestinal track, predisposing to environmental insult. Abscence of Muc2 also increases the propensity for T. muris infection
The Muc1−/− mouse model has helped in understanding the critical role played by MUC1 in cancer pathobiology. The growth of mammary tumors induced by the polyoma middle T antigen was significantly reduced in Muc1 null mice compared to WT littermates, although histologically, both groups developed poorly differentiated adenocarcinomas. The incidence and the metastasis were not remarkably different in the Muc1−/− and WT mice [51]. In a separate study, Cre-LSL-KrasG12D (KC) mouse expressing constitutively active Kras in pancreas was crossed with the Muc1 KO mouse to generate KC mice with Muc1 knockout (KCKO) mouse which showed higher survival and did not develop high-grade pancreatic intraepithelial neolplastic (PanIN) lesions even at 40 weeks of age, signifying the importance of Muc1 in pancreatic cancer progression (Fig. 3a) [54]. Only 10 % of KCKO mice exhibited metastasis to the lung, liver, and peritoneal sites, which was significantly less than KC and KC mice with human MUC1 overexpression (KCM) (generated by crossing KC mice with MUC1.Tg mice). Presence of MUC1 significantly accelerated the PC progression and metastasis. Thirty percent of the KC mice and 61 % of the KCM mice developed lung metastasis, and 20 % of the KC mice and 33 % of the KCM mice developed liver metastasis, whereas peritoneal metastasis was exhibited by 10 % of the KC mice and 23 % of the KCM mice [54]. Mechanistically, reduced tumorigenicity in KCKO mouse was associated with the absence of CDC25 and decreased phosphorylation of mitogen-activated protein kinase (MAPK) [54]. In addition, MUC1 resulted in the development of chemoresistance due to noticeable increase in multidrug resistance protein-1 expression in KCM as compared with KCKO tumors [55].
MUC1 has also been shown to regulate the development of the immune system. Myeloid-derived suppressor cells (MDSCs) play important role in suppressing the immune system under cancerous conditions via various mechanisms [56]. Recently, Poh TW et al. demonstrated that compared to WT animals, bone marrow (BM) progenitor cells in Muc1−/− mice predominantly differentiated into MDSCs under granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 signaling (Fig. 3a). This was attributed to reduced β-catenin stability due to Muc1 deficiency, as in vitro studies revealed an inverse relationship between β-catenin stability and the expansion of CD11b+Gr1+ MDSCs [26]. Given this new role of Muc1 in the differentiation and proliferation of myeloid progenitors, it will be of interest to utilize the Muc1 mouse models to study hematological malignancies and evaluate efficacy of immunotherapies for various malignancies.
Besides participating in oncogenesis and differentiation of immune cells, Muc1 expression also impacts the outcome of pathogenic infections [57, 58]. In a recent study, Helicobacter pylori (H. pylori)—a pathogen regarded as an etiological factor for a number of pathologies including peptic ulcers, gastric mucosal-associated lymphoid tissue lymphoma, and gastric adenocarcinoma—was found to infect and colonize Muc1−/− mice with a 5-fold higher propensity as compared to the WT mice (Fig. 3a) [58]. This study further demonstrated the ability of Helicobacter strains (SS9 and J99) to bind to purified MUC1, which limits the access of H. pylori to the epithelial surface, and thereby providing protection from infection.
Altogether, studies on Muc1 KO mice have established that inhibition of Muc1 expression can reduce the tumorigenic properties of cancer and gallstone formation. Muc1 targeting can improve the clinical outcome of CF patients, though it can elevate the susceptibility of H. pylori-associated pathological conditions. In contrast, overexpression of MUC1 in mice promotes metastasis and drug resistance.
3.1.2 The Muc2 KO mouse model
Muc2 KO mice (Muc2−/−) were generated by substituting a genomic fragment spanning exons 2 to 4 of Muc2 with a phosphoglycerate kinase-neomycin (PGK-Neo) cassette [59]. Muc2−/− mice showed no significant developmental defects as compared to heterozygous and WT littermates till 12 months of age and showed no compensatory increase in the expression of other intestinal apomucins (e.g., Muc5ac, Muc3, and Muc13). Muc2−/− mice developed GI tumors by 6 months which spontaneously progressed to invasive carcinoma at an older age (Fig. 3b). Around 65 % of the Muc2−/− mice develop tumors by 24 months of age with the incidence of more than 1.5 tumors per mouse [59]. There was increased cellular proliferation, reduced goblet cell number, decreased apoptosis, and increased migration of intestinal epithelial cells. The most distinct difference between the WT and the Muc2−/− mice was the absence of distinguishable goblet cells in the Muc2−/− mice. Tff3 and SI, two important markers to check the functionality of goblet cells and enterocytes, displayed no alteration in their expression levels in small intestine between WT and Muc2−/− mice [59, 60]. At a younger age (6 months), the Muc2 KO mice showed tumor development only in small intestine, whereas in older animals (1 year), tumors were observed in the small intestine (47 %), colon (21 %), and rectum (15.7 %). Similar to Muc2−/− mice, p21−/− (inhibitor of cyclin-independent kinase), Muc2−/− double-KO (DKO) mice also exhibited increased tumor formation in the small intestine, colon, and rectum but at a faster rate compared to p21−/− mice (Fig. 3b) [60]. Intestinal tumors size was 35 % larger in Muc2−/−, p21−/− (P = 0.02) or Muc2−/−, p21+/− (P = 0.03) mice than the tumors in Muc2−/−, p21+/+ mice.
Muc2 deficiency has also been related with the different outcomes during both prenatal and postnatal stages and during the suckling–weaning transition [61]. Before birth, and before weaning, no significant differences in the body weights between Muc2−/− mice and WT mice were observed. However, after weaning, Muc2−/− mice had considerably less body weight compared to WT mice, suggesting growth retardation. Before weaning (P14), the levels of pro-inflammatory cytokines (IL-12 p35, IL-12 p40, and tumor necrosis factor alpha (TNF-α)) and immune cells influx (Foxp3-positive Treg cells and the immune suppressive cytokines TGF-β1 and IL-10) were significantly higher in Muc2−/− mice as compared to WT mice. After weaning (P28), the pro-inflammatory response remained active, but expression of immunosupressive markers such as Foxp3 and TGF-β1 decreased [61]. This suckling–weaning transition exacerbated the development of colitis in Muc2-deficient mice, as reflected by reduced body weights, increase in distal colonic crypt lengthening, and influx of T cells in the mucosa. Altogether, these studies indicate that colitis in Muc2−/− mice is restricted before weaning but worsens in the distal colon after weaning, establishing the protective role of Muc2. Additionally, microarray analysis on 2–4-week-old Muc2−/− mice following colitis development revealed upregulation of genes involved in various immunological processes including the following: antigen processing/presentation, B cell receptor- and T cell receptor-mediated signal transduction, and Jak-STAT signaling (Fig. 3b) [62]. These mice expressed elevated levels of immunoglobulins, pro-inflammatory cytokines, chemokines, and antimicrobial proteins. Moreover, 4-week-old Muc2−/− mice showed significant alteration in the expression of certain cell structure maintenance genes, including upregulation of claudin-10 and downregulation of claudin-1 and claudin-5 [62].
To gain insight into the protective functions of Muc2 and its possible role in intestinal bowel disease (IBD), Muc2 KO (Muc2−/−) and heterozygous (Muc2+/−) mice were utilized from birth to 16 weeks of age before the development of adenomas. The Muc2−/− animals exhibited significantly lower body weight at 5 weeks of age as compared to the WT and heterozygous (Muc2+/−) littermates, indicating a significant growth retardation which has been associated with IBD and attributed to malnutrition [63]. Muc2−/− mice when challenged with colitis-inducing agent, dextran sulfate sodium, showed pathological signs of colitis by 5 weeks of age [64]. The microscopic analysis of the colon indicated mucosal thickening, increased proliferation, and superficial erosions. Muc2−/− mice also exhibited increase infiltration of lymphocytes in the colon as compared to WT mice. Further, a significant increase in the transcriptsof the inflammatory cytokines TNF-α (5-fold) and IL-1β (4-fold) was observed in the colon of Muc2−/− mice as compared to WT mice. This study was further substantiated by the observations in interleukin-10 KO (IL-10−/−) mice, which spontaneously developed colitis by 3 months of age and displayed reduced Muc2 levels [65, 66]. Further, KO of Muc2 on IL-10−/− background, resulted in significant growth retardation, altered goblet cell morphology, crypt hyperplasia, infiltration of lymphocytes, flattening of epithelial cells, and alteration of the lamina propria and developed colitis by 4 weeks of age in the proximal colon (Fig. 3b). These mice also had increased circulating levels of inflammatory cytokines such as IL-12p70, TNF-α, IFN-γ, MIP-1, and IL-6 compared to WT mice as early as 4 weeks, which remained persistently high at 5 weeks of age. Additionally, these animals exhibited apoptotic bodies along the crypts and had a higher mortality rate as compared to the WT or individual gene KO animals [67]. Thus, Muc2 in the intestinal mucus layer appears to be essential for mucosal protection, and abrogation of mucus barrier function is a major cause of colitis development.
Mucositis is one of the most common and severe outcomes of anti-cancer chemotherapy and predisposes patients to life-threatening infections. Mucositis has been associated with anincreased espression of MUC2 [68] and pro-inflammatory cytokines such as TNF-α, which exacerbates intestinal damage, and IL-10 [69, 70], which provides protection to the intestine. Due to the established protective role of Muc2 in colitis as discussed earlier, De Koning et al. utilized Muc2−/− mice to understand the contribution of Muc2 in providing the intestinal epithelial defense against methotrexate (MTX)-induced mucositis [71]. A significant increase in the expression of protective IL-10 in WT mice was observed, whereas Muc2−/− mice exhibited increased expression of damage inducing TNF-α, indicating that thelack of Muc2 expression leads to an altered immune response prior to MTX challenge.
The presence of Muc2 has also been found to impact host–pathogen interactions during infection [72]. Bergstorm et al. infected Muc2−/− mice with Citrobacter rodentium, a murine attaching–effacing (A/E) pathogen related to diarrheagenic A/E E. coli [72]. Upon infection, Muc2−/− mice exhibited a rapid loss of weight and high mortality of up to 90 %. Mucin secretion was significantly higher in WT mice during infection as compared to the uninfected WT controls, signifying the importance of mucin production to clear the mucosal surface from pathogenic bacteria (Fig. 3b). Protective function of Muc2 was also demonstrated in Trichuris muris (T. muris) parasitic infection. WT mice showed 46 % reduction in T. muris worm burden by day 15 and 84 % reduction by day 20 after infection, whereas Muc2-deficient mice did not exhibit decrease in worm burden even at 20 days postinfection [73]. The nematodes also demonstrated noticeable decrease in their energy status in WT mice than that in the susceptible Muc2−/− mice. Reduced mucus permeability has been observed in Muc2−/− mice, suggesting the involvement of Muc2 in the formation of effective physical barrier and network to concentrate and present defense proteins against pathogenic infection to protect gastric epithelia. Proteins required for the maintenance and restitution of GI tract, such as trefoil factor 3, angiogenin, and RELM-β, might be enriched in the mucus layer formed by Muc2 [73]. After infection, Muc5ac was found to be unexpectedly upregulated in the cecal mucus of WT and Muc2−/− mice, implying the importance of mucins in pathogenic infections.
Overall, studies on Muc2 KO mice have established that Muc2 not only functions as a tumor suppressor in colon cancer but it also provides protection against the development of inflammatory conditions, bacterial and parasitic infections.
3.1.3 The Muc16 KO mouse model
Muc16 KO mice (Muc16−/−) were generated by targeted inactivation of the gene by replacing a 6-kb genomic fragment, harboring the majority of exon 3 and a portion of intron 3 with a IRES-T-lacZ reporter cassette, followed by a PGK-NeobpA expression cassette flanked by the loxP sites [74]. The absence of any distinct phenotype in Muc16 homozygous mutant mice suggested that CA125/Muc16 is not essential for the normal development and reproduction. Similarly, overexpression of MUC16 carboxyl-terminus construct, also known as MUC16ΔC354 as it includes 354 amino acids of the carboxy terminus, with chicken β actin (CAG) promoter had no effect on the viability, fertility, and development of mice [75]. Meanwhile, Muc16−/− male mice exhibited high reproductive ability, as their crossing with WT females yielded more progenies as compared to the ones crossed with WT male mice. Muc16−/− mice did not exhibit compensatory increase in other mucins genes; instead, there was a downregulation of Muc1 in the uterus (p = 0.01) and lung of Muc16 −/− mice [74].
Conditional Muc16−/− KO mice could be helpful in validating and defining the role of Muc16 in oncogenesis in the background of p53−/− or constitutively active Kras mice in future studies, particularly in pancreatic, ovarian, and breast cancers, where MUC16 overexpression is frequently observed [76–78].
3.2 Mucin Tg mice and their phenotypes
To date, MUC1, Muc5ac, and MUC7 Tg mice models have been generated, characterized, and studied. Of these, MUC1 Tg mice have been extensively studied for evaluating immunotherapeutic approaches for various cancers.
3.2.1 MUC1 Tg mouse
The MUC1.Tg mouse was generated by successfully inserting the entire MUC1 gene using either 40- or 10.6-kb vector constructs in the mouse genome. The 40-kb construct contained 4.5-kb upstream of the 5′ sequence and 27-kb downstream of the 3′ sequence, whereas the 10.6-kb construct included 1.6-kb upstream of the 5′ sequence and 1.9-kb downstream of the 3′ sequence of the MUC1 gene from the human genome [79]. MUC1.Tg mice expressed human MUC1 in a tissue-specific manner with a profile similar to that seen in humans [79]. Schroeder et al. further confirmed the role of MUC1 in tumorigenesis by generating the number of Tg animals overexpressing the full-length and deletion constructs of human MUC1 in the mouse mammary gland [80]. Interestingly, significantly high interaction between MUC1 and epidermal growth factor receptor (EGFR) was observed at the apical surface of lactating mammary gland due to presence of EGFR all over the alveolar lumen. Therefore, strong downstream activation of MAP kinases was observed upon EGF stimulation in the mouse mammary gland of MUC1.Tg mice as compared to the Muc1−/− and WT mice. Therefore, it is possible that the modulation of EGFR signaling and activation of MAPK are mechanistically important for MUC1-associated mammary gland tumorigenesis [80]. MMTV-MUC1.Tg mice exhibited a 62 % higher incidence of mammary tumor formation as compared to the WT mice [81]. The mammary glands in MMTV-MUC1.Tg mice were histologically atypical and hyperplastic and showed significantly reduced apoptosis and delayed involution. MUC1.Tg mice also showed the formation of pulmonary carcinomas, suggesting the requirement of MUC1 in the development of breast and lung tumors [81]. Increased tumor angiogenesis and increased tumor growth were also observed in a PyMT-MMTV/hMUC1.Tg mouse model [82]. The data demonstrated that both xenograft and double-Tg MMTV-PyMT/hMUC1 mice had larger tumors, along with the increased frequency of tumor formation as compared to the MMTV-PyMT control mice. Higher VEGF expression was also noticed in the tumors of the MMTV-PyMT/hMUC1 mice than the control animals, suggesting the pro-angiogenic role of MUC1 during tumor progression. Additionally, MUC1.Tg mice when crossed with IL-10−/− mice showed an early onset of inflammatory bowel disease (IBD), higher inflammation score, and a higher incidence of colon cancers compared to IL-10−/− mice [83], demonstrating the ability of aberrant MUC1 expression to propagate chronic inflammation and tumor progression.
As mentioned earlier, MUC1.Tg mice (KCM) displayed increased metastasis, higher tumorigenic ability, and higher frequency of high-grade PanIN lesions as compared to the KCKO and KC mice along with significantly reduced survival rate as compared to the KCKO mice [54]. Subsequently, the same mice were used to study the influence of MUC1 on immune response during cancer progression to invasive adenocarcinoma [84]. Anti-MUC1 responses were observed in KCM (denoted as PDA.MUC1) mice in the earlier stages, but this effect is reduced at the later stages due to increment in the population of Tregs and MDSCs in the tumor microenvironment along with high tumor-draining lymph node (TDLNs) than KC mice [84]. This study was further substantiated when MUC1.Tg mice were utilized to examine the immunological tolerance to MUC1 and to test the efficacy of MUC1-targeted immunotherapy [85]. Upon immunization with MUC1 peptides, WT mice implanted with MUC1-expressing tumors produced IgM and IgG antibodies, whereas MUC1.Tg mice had strong IgM and feeble IgG antibody response, suggesting a significantly reduced class switching in B lymphocytes that resulted in failure to reject MUC1-expressing tumors. Mukherjee et al. further established MUC1 tolerance by crossing MUC1.Tg mice with ET mice (expressing the first 127 amino acids of SV40 T antigen under the control of the elastase promoter) to generate MET mice that developed pancreatic tumor [86]. These mice demonstrated acinar cell dysplasia at birth, which was ensued by the development of microadenomas and acinar cell carcinomas. Cytotoxic T cells (CTLs) were identified in all MET mice and were effective in eliminating implanted tumors in vivo, whereas the MUC1.Tg mice with the B16.MUC1 s.c. tumor elicited ineffective CTL response. These CTLs exhibited low affinity due to the presence of MUC1 in mouse thymus which led to the development of central tolerance toward MUC1. In spite of the stimulation of a tumor-specific immune response, MUC1 did not cause the rejection of a spontaneously occurring tumor, possibly due to the presence of elaborate means of escaping MHC class I-restricted immune response [86]. Altogether, these studies suggested that MUC1.Tg mice are a useful model for scrutinizing and comprehending the mechanistic aspect of central and peripheral tolerance to the tumor antigen MUC1.
In order to assess the efficiency of MUC1-specific immunotherapy on intestinal adenomas, Akporiaye et al. developed a bigenic MUC1.Tg/MIN mouse model by crossing Apc/(MIN/+) mice with MUC1.Tg mice [87]. The immunotherapy involved the combination of two MHC class I restricted MUC1 TR peptides and a class II pan helper peptide along with the biological adjuvants CpG-ODN and GM-CSF. This combination did not show change in the number of adenomas, but flattening of adenomas and reduction in the frequency of large-sized adenomas were quite evident. Glycosylation of the mucins is a critical determinant of their immunogenicity [88], and the extent and pattern of glycosylation are altered during tumorigenesis. Most of the early efforts to develop MUC1-based cancer vaccines involved unglycosylated MUC1 TR peptides with variable lengths, conjugated to different carriers and/or co-administered with an adjuvant. Unfortunately, these strategies showed insignificant immune responses against MUC1-expressing cancer cells [89–92], which could possibly be due to the conformational discrepancies that arise due to differential glycosylation of MUC1 or the use of carrier proteins leading to immunosuppression [93]. Unlike MUC1 TR peptide, densely glycosylated MUC1 cannot be processed efficiently by antigen-presenting cells (APCs) [94], thereby compromising its presentation through the MHC class II and class I pathway. A recent study has shown that a covalent linkage between a glycosylated MUC1-derived glycopeptide and a Toll-like receptor (TLR) agonist can stimulate strong humoral and cellular immune responses and is efficacious in reversing tolerance and generating a therapeutic response [88]. The use of tripartite peptide vaccine composed of the TLR2 agonist Pam3CysSK4, a peptide T helper epitope and an aberrantly glycosylated MUC1 peptide had a significant therapeutic effect in MUC1.Tg mice due to the adjuvant mediated nonspecific antitumor responses and MUC1-peptide specific humoral and cellular immune responses. Glycosylation of the MUC1 peptide and its covalent attachment with TLR agonist was found to be critical for inducing CTLs and ADCC-mediating antibodies and had an optimal immunological response [88].
Studies comparing immunotherapy-induced anti-MUC1 responses in WT and Tg mice revealed that MUC1.Tg mice elicit reduced T helper responses, even in the presence of efficiently processed and well-presented MUC1 by APCs. MUC1 is a self antigen that is expressed under normal conditions, which makes MUC1.Tg mice tolerant to immunization against synthetic 100-mer MUC1 peptide-primed dendritic cells (DCs). Therefore, Turner MS et al. performed adoptive transfer of CD4+ T cells from WT mice to the MUC1.Tg mice, followed by the immunization with the same MUC1-loaded DCs [95]. Surprisingly, rather than expected increase in immune response, these mice showed decrease in cellular and humoral response, indicating the presence of immunosuppressive component in the WT CD4+ T cells. The assumption was further supported as the adoptive transfer after T-reg-depleted T helper cells derived from WT mice improved the efficacy of MUC1-specific vaccine and immunity in MUC1.Tg animals [95]. Beatty et al. have also demonstrated delayed progression of IBD to colitis-associated colon cancer (also known as CACC) upon administration of 100-mer TnMUC1 peptide-based immunotherapy in MUC1-positive IBD (generated by crossing IL-10−/− mice with MUC1.Tg mice). This delay in progression was attributed to the induction of adaptive immunity (CTLs specific to MUC1), reduced neutrophil infiltration, and systemic reduction in MDSCs, leading to the depletion of abnormal MUC1-expressing cells in IBD colons [96]. On the other hand, IBD in control mice exhibited elevated population of immunosuppressive MDSC cells in the spleen. These studies in MUC1.Tg mice underscore the role of Tregs and MDSCs in limiting the adaptive immune responses following immunotherapy with self-antigens. Hence, the removal of these immunosuppressive components can possibly improve the efficacy of anti-MUC1 vaccines. Activation of adaptive immunity can modulate local and systemic microenvironment and combat the inability of the MUC1-targeted vaccines to elicit adaptive immune response to kill MUC1+ tumors, which is currently one of the major obstacles in MUC1-based vaccination in cancer patients.
3.2.2 Muc5ac Tg mice
Recently, rat Muc5ac cDNA was cloned with the GFP inserted immediately upstream of the 5′ of the VNTR [97]. This fusion product of 9.1 kb was placed under the control of the rat Clara cell secretory protein (rCCSP) promoter. Murine airways harboring Clara cells were used to overexpress Muc5ac, as these cells contain the complete machinery required for the proper posttranslational modifications and secretion of mucins. The overexpression of rat Muc5ac did not affect fetal development, survival, and growth. No change in endogenous Muc5b expression was noticed, suggesting that in spite of their tandem localization on the same chromosome 7 F5, these mucins are possibly regulated by different mechanisms. Despite nearly 20-fold hypersecretion of rat Muc5ac compared to WT levels, Muc5ac.Tg mice model did not show any sign of obstruction in the small airways during physiological and inflammatory conditions. The Muc5ac.Tg mice exhibited significant decrease in PR8/H1N1 influenza virus susceptibility and neutrophil infiltration. Mechanistically, terminal α2,3-sialic acids present on Muc5ac have the ability to bind to the surface proteins of PR8/H1N1 influenza virus and thus act as a decoy receptors to prevent the viral entry into the respiratory epithelial cells [97]. Furthermore, the reduced neutrophilic response in Muc5ac.Tg mice following infection might restrict tissue damage to the lungs, thus providing additional protective advantage. Altogether, the studies in Muc5ac.Tg mice suggest that Muc5ac overexpression plays a protective role in respiratory infections.
3.2.3 MUC7 Tg mice and its phenotype
MUC7 is a small, soluble, and non-gel forming mucin. It is mainly involved in the clearance of various bacteria and inhibits bacterial colonization in the respiratory tract [98]. MUC7 gene spans approximately 10 kb and comprises three exons and two introns, which encode for a 39 kDa protein having six VNTRs with 23 amino acids each [99]. Bobek et al. have generated a 16-kb construct of MUC7 transgene having all of the three exons along with two introns and 3 kb of both downstream and upstream sequences [100]. MUC7 was found to be expressed in a tissue-specific manner in the sublingual glands of both male and female mice and at low levels in the submandibular glands of females. All other tested tissues did not show detectable MUC7 expression, though a separate study indicated its expression in the pancreas and bladder [101]. This model has been used to elucidate the role of MUC7 following cigarette extract exposure which resulted in the statistically significant increase in MUC7 transcripts in both the trachea and the lungs [102].
4 Conclusions and perspectives
Currently available and established mucin KO mouse models have provided useful information regarding their role in carcinogenesis and inflammatory diseases. Muc1−/− mouse model has provided the information regarding the importance of Muc1 in mammary and pancreatic cancer, which has been further substantiated by using MUC1.Tg mice. Additionally, Muc2−/− mice showed the protective roles of Muc2 in GI segment, as Muc2 silencing abrogated the mucus barrier function of intestine, and thus made mice more susceptible for drug-induced or inflammation-induced damage. Muc2 KO mice also displayed tumor formation within just 6 months. Furthermore, Muc5ac.Tg mice revealed the protective function of Muc5ac against respiratory infection. Altogether, mucins express differentially under different pathological conditions including pathogenic infections, IBD, and cancer, which further emphasizes the need to generate additional mucin-targeted mouse models for their potential diagnostic and therapeutic implications.
Mucin overexpression in human cancers, like MUC4 in pancreatic cancer, appears very early during the pre-neoplastic lesions, suggesting its role as an oncogene, which is further corroborated by the utilization of spontaneous mouse models of several cancers [103]. Hence, mucin KO and/or expression of the mucin transgene in the background of spontaneous cancer models for pancreatic (Kras), colon (APC), lung (Kras), and prostate (PTEN) cancers will allow us to discern their pro- and anti-tumorigenic roles and provide a unique opportunity to test mucin-targeted combination therapies. In fact, such Tg models have been recently developed and utilizedto study more precisely the oncogenic functions of mucins. For example, importance of MUC16 in tumorigenesis was confirmed when mice progenies resulting from the breeding of MUC16 carboxyl-terminus Tg mouse (MUC16ΔC354) mice with p53 heterozygotes showed high susceptibility to form spontaneous tumors. These mice exhibited increase incidence of neoplastic lesion formation (particularly, sarcoma and lymphoma) as compared to the p53+/− heterozygote mice [104]. Additionally, these mouse models can also be helpful to understand the aberrant regulation of mucins in pathological conditions. We can highlight the involvement of extrinsic and intrinsic factors which are responsible for the aberrant expression and overexpression of mucins in different pathological conditions.
Another important aspect of the mucin biology is the compensatory mechanism where the loss of one mucin is compensated by the upregulation of another. These compensatory mechanisms may mask the significance of a given mucin in tissue homeostasis when studied using KO models. As discussed earlier, Muc1−/− mice exhibited a compensatory increase in Muc4 in mammary tissue [51], while Muc16−/− mice demonstrated reduced Muc1 expression in the uterus and lung [74]. Thus, KO of multiple mucins in the same animal model can potentially circumvent such compensatory mechanisms and provide insights into the interplay between their regulatory networks.
It is an established fact that the tumors are sustained by a relatively small population of cells called cancer stem cells (CSC) or cancer-initiating cells [105]. Monoclonality of the primary tumor and metastatic lesions also strengthens this belief. The MUC1 cleavage product (ectodomain) functions as a growth factor receptor on undifferentiated human embryonic stem cells and interacts with NM23 to maintain the stemness [106]. Similarly, our studies have shown that the interaction between MUC4 and HER2 is involved in the maintenance of stem cells in ovarian cancer cell lines and there was a significant increase in the stem cell population after MUC4 overexpression in the SKOV3 cell line [107, 108]. MUC1.Tg and KO mice have shown a drastic difference in the tumor progression and hematopoiesis abnormalities, again reinforcing their critical role in the CSC population [26, 109]. The involvement of mucins in the stemness has not been directly addressed in the mucin mouse models, but the aforementioned studies hint at their plausible involvement in the maintenance of the stem cell population in the tumor. It will be of immediate interest to undertake studies in GEM models to validate the involvement of mucins in the context of CSC origin, stemness, and clinical implications.
There is growing evidence supporting the role of mucins in modulating pathogenic infections [97]. Infection with H. pylori to the gastric mucosa has been positively associated with the aberrant expression of MUC13, and eradication of this pathogen leads to the reduction in various cell surface molecules including MUC13 [110, 111]. In contrast, expression of Muc1 has been shown protective effects for H. pylori infection, as it confines its colonization and resulting pathogen-mediated inflammation [58]. Interestingly, MUC4 overexpression has been observed in cervical cancer and cholangiocarcinoma [112, 113], though its expression has not been related with the associated pathogens (HPV and HCV, respectively). Therefore, the availability of the relevant mouse models will allow the research community to understand the involvement of these mucins in the early stages of pathogen-induced carcinomas.
Mucin hypersecretion is believed to contribute to the morbidity and mortality in airway obstructive diseases such as asthma, COPD, or CF [114, 115] and anti-secretory therapies are often used in managing these patients. However, the aforementioned observations in the GEM models indicating the protective role of mucins in preventing pathogenic infections [58, 73] support the notion that frequently observed increase in mucin secretion might be occurring as a response rather than a cause of these airway obstructions. Thus, the strategies to utilize anti-secretory therapies for defensive and preemptive purposes need to be carefully examined and evaluated for the risk of epithelial infections by viruses and bacteria.
In human mucin genes, most of the polymorphism is TR region encoding DNA sequence, which leads to different numbers of these tandem repeats in mucins. VNTR polymorphism of the MUC1 mucin gene has shown to influence the susceptibility toward H. pylori-induced gastritis [116]. Additionally, a wide cohort study in northern Europe demonstrated a 100 % linkage disequilibrium between the frequently occurring MUC7*5 (in the 5th VNTR) polymorphism and the single-nucleotide polymorphism rs9982010 [117]. It has been associated with a reduced risk of being asthmatic with a better lung function. Similarly, MUC7 polymorphism has shown remarkably high incidence of asthma in African–Americans compared to Whites [118]. A lower predominance of the MUC7*5 allele in asthma patients is suggestive of the protective functions of MUC7 in the respiratory system. Due to the established clinical association of MUC7 with asthma, MUC7 transgenic mouse model could also have high applicability to the study of cancer-associated inflammation, as recent studies have associated MUC7 expression in the pathogenesis of various cancers [119, 120]. Development of mucin mouse models can also highlight the importance of mucin polymorphism in clinical settings, which is currently at its infancy.
Lastly, studies have long demonstrated that mucin domains (vWD, SEA, EGF-like) and their glycosylation state dictate their function. Thus, it will be important to evaluate the contribution of individual domains in mucin biology by utilizing domain-deleted KO or knockin mucin mouse models. Developing the next-generation mucin mouse model should also entail combination with genes that allow for lineage tracing and real-time imaging for a better appreciation of the involvement of mucins in the etiologies and progression of pathologies.
Abbreviations
- MUC1:
-
Human mucin
- Muc1:
-
Mouse mucin
- KO:
-
Knockout
- KI:
-
Knockin
- Tg:
-
Transgenic
- KC:
-
Cre-LSL-KrasG12D
- KCM:
-
KC mice with human MUC1 overexpression
- KCKO:
-
KC mice with Muc1 knockout
- CF:
-
Cystic fibrosis
- SEA:
-
Sperm protein, enterokinase, and agrin domain
- EGF-L:
-
Epidermal growth factor (EGF)-like domain
- D domain:
-
Dimerization domain
- TR:
-
Variable number of tandem repeats
- Ser:
-
Serine
- Thr:
-
Threonine
- Pro:
-
Proline
- TM:
-
Transmembrane
- CT:
-
Cytoplasmic tail
- CTCK:
-
Cystine knot-like domain
- rCCSP:
-
Rat Clara cell secretory protein
- vWD:
-
Von Willebrand
- T-reg:
-
Regulatory T cells
- WT:
-
Wild type
- CTL:
-
Cytotoxic T lymphocytes
- APCs:
-
Antigen-presenting cells
- CSCs:
-
Cancer stem cells
References
Montagne, L., Piel, C., & Lalles, J. P. (2004). Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutrition Reviews, 62, 105–114.
Kufe, D. W. (2009). Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer, 9, 874–885.
Rachagani, S., Torres, M. P., Moniaux, N., & Batra, S. K. (2009). Current status of mucins in the diagnosis and therapy of cancer. Biofactors, 35, 509–527.
Kaur, S., Kumar, S., Momi, N., Sasson, A. R., & Batra, S. K. (2013). Mucins in pancreatic cancer and its microenvironment. Nature Reviews Gastroenterol Hepatology, 10, 607–620.
Andrianifahanana, M., Moniaux, N., & Batra, S. K. (2006). Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1765, 189–222.
Itoh, Y., Kamata-Sakurai, M., Denda-Nagai, K., Nagai, S., Tsuiji, M., Ishii-Schrade, K., et al. (2008). Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology, 18, 74–83.
Buisine, M. P., Desreumaux, P., Leteurtre, E., Copin, M. C., Colombel, J. F., Porchet, N., et al. (2001). Mucin gene expression in intestinal epithelial cells in Crohn’s disease. Gut, 49, 544–551.
Corfield, A. P., Myerscough, N., Longman, R., Sylvester, P., Arul, S., & Pignatelli, M. (2000). Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut, 47, 589–594.
Singh, A. P., Chauhan, S. C., Bafna, S., Johansson, S. L., Smith, L. M., Moniaux, N., et al. (2006). Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate, 66, 421–429.
Mukhopadhyay, P., Lakshmanan, I., Ponnusamy, M. P., Chakraborty, S., Jain, M., Pai, P., et al. (2013). MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One, 8, e54455.
Senapati, S., Chaturvedi, P., Sharma, P., Venkatraman, G., Meza, J. L., El-Rifai, W., et al. (2008). Deregulation of MUC4 in gastric adenocarcinoma: potential pathobiological implication in poorly differentiated non-signet ring cell type gastric cancer. British Journal of Cancer, 99, 949–956.
Chauhan, S. C., Singh, A. P., Ruiz, F., Johansson, S. L., Jain, M., Smith, L. M., et al. (2006). Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Modern Pathology, 19, 1386–1394.
Chaturvedi, P., Singh, A. P., Moniaux, N., Senapati, S., Chakraborty, S., Meza, J. L., et al. (2007). MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Molecular Cancer Research, 5, 309–320.
Chaturvedi, P., Singh, A. P., Chakraborty, S., Chauhan, S. C., Bafna, S., Meza, J. L., et al. (2008). MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Research, 68, 2065–2070.
Ponnusamy, M. P., Singh, A. P., Jain, M., Chakraborty, S., Moniaux, N., & Batra, S. K. (2008). MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. British Journal of Cancer, 99, 520–526.
Singh, P. K., & Hollingsworth, M. A. (2006). Cell surface-associated mucins in signal transduction. Trends in Cell Biology, 16, 467–476.
Ahmad, R., Raina, D., Trivedi, V., Ren, J., Rajabi, H., Kharbanda, S., et al. (2007). MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nature Cell Biology, 9, 1419–1427.
Rachagani, S., Macha, M. A., Ponnusamy, M. P., Haridas, D., Kaur, S., Jain, M., et al. (2012). MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis, 33, 1953–1964.
Kumar, S., Das, S., Rachagani, S., Kaur, S., Joshi, S., Johansson, S. L., et al. (2014). NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene. doi:10.1038/onc.2014.409.
Li, Y., Liu, D., Chen, D., Kharbanda, S., & Kufe, D. (2003). Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene, 22, 6107–6110.
Moniaux, N., Chaturvedi, P., Varshney, G. C., Meza, J. L., Rodriguez-Sierra, J. F., Aubert, J. P., et al. (2007). Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. British Journal of Cancer, 97, 345–357.
Singh, A. P., Moniaux, N., Chauhan, S. C., Meza, J. L., & Batra, S. K. (2004). Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Research, 64, 622–630.
Tsutsumida, H., Swanson, B. J., Singh, P. K., Caffrey, T. C., Kitajima, S., Goto, M., et al. (2006). RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clinical Cancer Research, 12, 2976–2987.
Qiu, W., & Su, G. H. (2013). Challenges and advances in mouse modeling for human pancreatic tumorigenesis and metastasis. Cancer Metastasis Review, 32, 83–107.
Li, Q., Ren, J., & Kufe, D. (2004). Interaction of human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochemical and Biophysical Research Communications, 315, 471–476.
Poh, T. W., Bradley, J. M., Mukherjee, P., & Gendler, S. J. (2009). Lack of Muc1-regulated beta-catenin stability results in aberrant expansion of CD11b+Gr1+ myeloid-derived suppressor cells from the bone marrow. Cancer Research, 69, 3554–3562.
Gu, H., Marth, J. D., Orban, P. C., Mossmann, H., & Rajewsky, K. (1994). Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science, 265, 103–106.
Long, D. P., Zhao, A. C., Chen, X. J., Zhang, Y., Lu, W. J., Guo, Q., et al. (2012). FLP recombinase-mediated site-specific recombination in silkworm, Bombyx mori. PLoS One. doi:10.1371/journal.pone.0040150.
Shekels, L. L., & Ho, S. B. (2003). Characterization of the mouse Muc3 membrane bound intestinal mucin 5′ coding and promoter regions: regulation by inflammatory cytokines. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression, 1627, 90–100.
Gum, J. R., Jr., Crawley, S. C., Hicks, J. W., Szymkowski, D. E., & Kim, Y. S. (2002). MUC17, a novel membrane-tethered mucin. Biochemical and Biophysical Research Communications, 291, 466–475.
Senapati, S., Das, S., & Batra, S. K. (2010). Mucin-interacting proteins: from function to therapeutics. Trends in Biochemical Sciences, 35, 236–245.
Joshi, S., Kumar, S., Choudhury, A., Ponnusamy, M. P., & Batra, S. K. (2014). Altered mucins (MUC) trafficking in benign and malignant conditions. Oncotarget, 5, 7272–7284.
Spicer, A. P., Parry, G., Patton, S., & Gendler, S. J. (1991). Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. Journal of Biological Chemistry, 266, 15099–15109.
Shekels, L. L., Lyftogt, C., Kieliszewski, M., Filie, J. D., Kozak, C. A., & Ho, S. B. (1995). Mouse gastric mucin: cloning and chromosomal localization. Biochemical Journal, 311(Pt. 3), 775–785.
Desseyn, J. L., Clavereau, I., & Laine, A. (2002). Cloning, chromosomal localization and characterization of the murine mucin gene orthologous to human MUC4. European Journal of Biochemistry, 269, 3150–3159.
Maeda, T., Inoue, M., Koshiba, S., Yabuki, T., Aoki, M., Nunokawa, E., et al. (2004). Solution structure of the SEA domain from the murine homologue of ovarian cancer antigen CA125 (MUC16). Journal of Biological Chemistry, 279, 13174–13182.
Goodell, C. A., Belisle, J. A., Gubbels, J. A., Migneault, M., Rancourt, C., Connor, J., et al. (2009). Characterization of the tumor marker muc16 (ca125) expressed by murine ovarian tumor cell lines and identification of a panel of cross-reactive monoclonal antibodies. Journal of Ovarian Research. doi:10.1186/1757-2215-2-8.
Dougherty, G. J., Kay, R. J., & Humphries, R. K. (1989). Molecular cloning of 114/A10, a cell surface antigen containing highly conserved repeated elements, which is expressed by murine hemopoietic progenitor cells and interleukin-3-dependent cell lines. Journal of Biological Chemistry, 264, 6509–6514.
Williams, S. J., Wreschner, D. H., Tran, M., Eyre, H. J., Sutherland, G. R., & McGuckin, M. A. (2001). Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. Journal of Biological Chemistry, 276, 18327–18336.
Higuchi, T., Orita, T., Nakanishi, S., Katsuya, K., Watanabe, H., Yamasaki, Y., et al. (2004). Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. Journal of Biological Chemistry, 279, 1968–1979.
Chen, Y., Zhao, Y. H., Kalaslavadi, T. B., Hamati, E., Nehrke, K., Le, A. D., et al. (2004). Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. American Journal of Respiratory Cell and Molecular Biology, 30, 155–165.
Desseyn, J. L., & Laine, A. (2003). Characterization of mouse muc6 and evidence of conservation of the gel-forming mucin gene cluster between human and mouse. Genomics, 81, 433–436.
Pigny, P., Guyonnet-Duperat, V., Hill, A. S., Pratt, W. S., Galiegue Zouitina, S., D’Hooge, M. C., et al. (1996). Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics, 38, 340–352.
Johansson, M. E., Larsson, J. M., & Hansson, G. C. (2011). The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl 1), 4659–4665.
Aslam, F., Palumbo, L., Augenlicht, L. H., & Velcich, A. (2001). The Sp family of transcription factors in the regulation of the human and mouse MUC2 gene promoters. Cancer Research, 61, 570–576.
Van Klinken, B. J., Einerhand, A. W., Duits, L. A., Makkink, M. K., Tytgat, K. M., Renes, I. B., et al. (1999). Gastrointestinal expression and partial cDNA cloning of murine Muc2. Americal Journal of Physiology, 276, G115–G124.
Inatomi, T., Tisdale, A. S., Zhan, Q., Spurr-Michaud, S., & Gipson, I. K. (1997). Cloning of rat Muc5AC mucin gene: comparison of its structure and tissue distribution to that of human and mouse homologues. Biochemical and Biophysical Research Communications, 236, 789–797.
Escande, F., Porchet, N., Aubert, J. P., & Buisine, M. P. (2002). The mouse Muc5b mucin gene: cDNA and genomic structures, chromosomal localization and expression. Biochemical Journal, 363, 589–598.
Culp, D. J., Latchney, L. R., Fallon, M. A., Denny, P. A., Denny, P. C., Couwenhoven, R. I., et al. (2004). The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiological Genomics, 19, 303–318.
Toribara, N. W., Ho, S. B., Gum, E., Gum, J. R., Jr., Lau, P., & Kim, Y. S. (1997). The carboxyl-terminal sequence of the human secretory mucin, MUC6. Analysis of the primary amino acid sequence. Journal of Biological Chemistry, 272, 16398–16403.
Spicer, A. P., Rowse, G. J., Lidner, T. K., & Gendler, S. J. (1995). Delayed mammary tumor progression in Muc-1 null mice. Journal of Biological Chemistry, 270, 30093–30101.
Wang, H. H., Afdhal, N. H., Gendler, S. J., & Wang, D. Q. (2004). Targeted disruption of the murine mucin gene 1 decreases susceptibility to cholesterol gallstone formation. Journal of Lipid Research, 45, 438–447.
Parmley, R. R., & Gendler, S. J. (1998). Cystic fibrosis mice lacking Muc1 have reduced amounts of intestinal mucus. Journal of Clinical Investigation, 102, 1798–1806.
Besmer, D. M., Curry, J. M., Roy, L. D., Tinder, T. L., Sahraei, M., Schettini, J., et al. (2011). Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Research, 71, 4432–4442.
Nath, S., Daneshvar, K., Roy, L. D., Grover, P., Kidiyoor, A., Mosley, L., et al. (2013). MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. doi:10.1038/oncsis.2013.16.
Nagaraj, S., Collazo, M., Corzo, C. A., Youn, J. I., Ortiz, M., Quiceno, D., et al. (2009). Regulatory myeloid suppressor cells in health and disease. Cancer Research, 69, 7503–7506.
Linden, S. K., Sheng, Y. H., Every, A. L., Miles, K. M., Skoog, E. C., Florin, T. H., et al. (2009). MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathogens. doi:10.1371/journal.ppat.1000617.
McGuckin, M. A., Every, A. L., Skene, C. D., Linden, S. K., Chionh, Y. T., Swierczak, A., et al. (2007). Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology, 133, 1210–1218.
Velcich, A., Yang, W., Heyer, J., Fragale, A., Nicholas, C., Viani, S., et al. (2002). Colorectal cancer in mice genetically deficient in the mucin Muc2. Science, 295, 1726–1729.
Yang, W., Velcich, A., Lozonschi, I., Liang, J., Nicholas, C., Zhuang, M., et al. (2005). Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2-/- mice. American Journal of Pathology, 166, 1239–1246.
Burger-van, P. N., van der Sluis, M., Bouma, J., Korteland-van Male, A. M., Lu, P., Van, S. I., et al. (2011). Colitis development during the suckling-weaning transition in mucin Muc2-deficient mice. American Journal of Physiology-Gastrointestinal and Liver Physiology, 301, G667–G678.
Lu, P., Burger-van, P. N., van der Sluis, M., Witte-Bouma, J., Kerckaert, J. P., van Goudoever, J. B., et al. (2011). Colonic gene expression patterns of mucin Muc2 knockout mice reveal various phases in colitis development. Inflammatory Bowel Diseases, 17, 2047–2057.
Moeeni, V., & Day, A. S. (2011). Impact of Inflammatory bowel disease upon growth in children and adolescents. International Scholarly Research Notices: Pediatrics, 2011, 365712.
Van der Sluis, M., De Koning, B. A., De Bruijn, A. C., Velcich, A., Meijerink, J. P., Van Goudoever, J. B., et al. (2006). Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology, 131, 117–129.
Berg, D. J., Davidson, N., Kuhn, R., Muller, W., Menon, S., Holland, G., et al. (1996). Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. Journal of Clinical Investigation, 98, 1010–1020.
Schwerbrock, N. M., Makkink, M. K., van der Sluis, M., Buller, H. A., Einerhand, A. W., Sartor, R. B., et al. (2004). Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflammatory Bowel Diseases, 10, 811–823.
van der Sluis, M., Bouma, J., Vincent, A., Velcich, A., Carraway, K. L., Buller, H. A., et al. (2008). Combined defects in epithelial and immunoregulatory factors exacerbate the pathogenesis of inflammation: mucin 2-interleukin 10-deficient mice. Laboratory Investigation, 88, 634–642.
Verburg, M., Renes, I. B., Meijer, H. P., Taminiau, J. A., Buller, H. A., Einerhand, A. W., et al. (2000). Selective sparing of goblet cells and paneth cells in the intestine of methotrexate-treated rats. American Journal of Physiology-Gastrointestinal and Liver Physiology, 279, G1037–G1047.
Guy-Grand, D., DiSanto, J. P., Henchoz, P., Malassis-Seris, M., & Vassalli, P. (1998). Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. European Journal of Immunology, 28, 730–744.
Sellon, R. K., Tonkonogy, S., Schultz, M., Dieleman, L. A., Grenther, W., Balish, E., et al. (1998). Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and Immunity, 66, 5224–5231.
de Koning, B. A., Sluis, M., Lindenbergh-Kortleve, D. J., Velcich, A., Pieters, R., Buller, H. A., et al. (2007). Methotrexate-induced mucositis in mucin 2-deficient mice. Journal of Cellular Physiology, 210, 144–152.
Bergstrom, K. S., Kissoon-Singh, V., Gibson, D. L., Ma, C., Montero, M., Sham, H. P., et al. (2010). Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathogens. doi:10.1371/journal.ppat.1000902.
Hasnain, S. Z., Wang, H., Ghia, J. E., Haq, N., Deng, Y., Velcich, A., et al. (2010). Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology, 138, 1763–1771.
Cheon, D. J., Wang, Y., Deng, J. M., Lu, Z., Xiao, L., Chen, C. M., et al. (2009). CA125/MUC16 is dispensable for mouse development and reproduction. PLoS One. doi:10.1371/journal.pone.0004675.
Tian,H., Spriggs, DR (2011). Conditional transgenic mice for the carboxyl-terminus of MUC16. Cancer Research, 71(8 Suppl).doi:10.1158/1538-7445.AM2011-4332.
Shimizu, A., Hirono, S., Tani, M., Kawai, M., Okada, K., Miyazawa, M., et al. (2012). Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Science, 103, 739–746.
Wang, Y., Cheon, D. J., Lu, Z., Cunningham, S. L., Chen, C. M., Luo, R. Z., et al. (2008). MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse. Differentiation, 76, 1081–1092.
Lakshmanan, I., Ponnusamy, M. P., Das, S., Chakraborty, S., Haridas, D., Mukhopadhyay, P., et al. (2012). MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene, 31, 805–817.
Peat, N., Gendler, S. J., Lalani, N., Duhig, T., & Taylor-Papadimitriou, J. (1992). Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Research, 52, 1954–1960.
Schroeder, J. A., Thompson, M. C., Gardner, M. M., & Gendler, S. J. (2001). Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. Journal of Biological Chemistry, 276, 13057–13064.
Schroeder, J. A., Masri, A. A., Adriance, M. C., Tessier, J. C., Kotlarczyk, K. L., Thompson, M. C., et al. (2004). MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene, 23, 5739–5747.
Woo, J. K., Choi, Y., Oh, S. H., Jeong, J. H., Choi, D. H., Seo, H. S., et al. (2012). Mucin 1 enhances the tumor angiogenic response by activation of the AKT signaling pathway. Oncogene, 31, 2187–2198.
Beatty, P. L., Plevy, S. E., Sepulveda, A. R., & Finn, O. J. (2007). Cutting edge: transgenic expression of human MUC1 in IL-10-/- mice accelerates inflammatory bowel disease and progression to colon cancer. Journal of Immunology, 179, 735–739.
Tinder, T. L., Subramani, D. B., Basu, G. D., Bradley, J. M., Schettini, J., Million, A., et al. (2008). MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. Journal of Immunology, 181, 3116–3125.
Rowse, G. J., Tempero, R. M., VanLith, M. L., Hollingsworth, M. A., & Gendler, S. J. (1998). Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Research, 58, 315–321.
Mukherjee, P., Ginardi, A. R., Madsen, C. S., Sterner, C. J., Adriance, M. C., Tevethia, M. J., et al. (2000). Mice with spontaneous pancreatic cancer naturally develop MUC-1-specific CTLs that eradicate tumors when adoptively transferred. Journal of Immunology, 165, 3451–3460.
Akporiaye, E. T., Bradley-Dunlop, D., Gendler, S. J., Mukherjee, P., Madsen, C. S., Hahn, T., et al. (2007). Characterization of the MUC1.Tg/MIN transgenic mouse as a model for studying antigen-specific immunotherapy of adenomas. Vaccine, 25, 6965–6974.
Lakshminarayanan, V., Thompson, P., Wolfert, M. A., Buskas, T., Bradley, J. M., Pathangey, L. B., et al. (2012). Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proceedings of the National Academy of Sciences of the United States of America, 109, 261–266.
Goydos, J. S., Elder, E., Whiteside, T. L., Finn, O. J., & Lotze, M. T. (1996). A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. Journal of Surgical Research, 63, 298–304.
Karanikas, V., Hwang, L. A., Pearson, J., Ong, C. S., Apostolopoulos, V., Vaughan, H., et al. (1997). Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. Journal of Clinical Investigation, 100, 2783–2792.
Soares, M. M., Mehta, V., & Finn, O. J. (2001). Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. Journal of Immunology, 166, 6555–6563.
Von Mensdorff-Pouilly, S., Petrakou, E., Kenemans, P., van Uffelen, K., Verstraeten, A. A., Snijdewint, F. G., et al. (2000). Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. International Journal of Cancer, 86, 702–712.
Dziadek, S., Griesinger, C., Kunz, H., & Reinscheid, U. M. (2006). Synthesis and structural model of an alpha (2,6)-sialyl-t glycosylated MUC1 eicosapeptide under physiological conditions. Chemistry, 12, 4981–4993.
Ninkovic, T., & Hanisch, F. G. (2007). O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. Journal of Immunology, 179, 2380–2388.
Turner, M. S., Cohen, P. A., & Finn, O. J. (2007). Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells. Journal of Immunology, 178, 2787–2793.
Beatty, P. L., Narayanan, S., Gariepy, J., Ranganathan, S., & Finn, O. J. (2010). Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prevention Research (Philadelphia), 3, 438–446.
Ehre, C., Worthington, E. N., Liesman, R. M., Grubb, B. R., Barbier, D., O’Neal, W. K., et al. (2012). Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proceedings of the National Academy of Sciences of the United States of America, 109, 16528–16533.
Li, S., Intini, G., & Bobek, L. A. (2006). Modulation of MUC7 mucin expression by exogenous factors in airway cells in vitro and in vivo. American Journal of Respiratory Cell and Molecular Biology, 35, 95–102.
Bobek, L. A., Tsai, H., Biesbrock, A. R., & Levine, M. J. (1993). Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). Journal of Biological Chemistry, 268, 20563–20569.
Bobek, L. A., Li, H., Rojstaczer, N., Jones, C., Gross, K. W., & Levine, M. J. (1998). Tissue-specific expression of human salivary mucin gene, MUC7, in transgenic mice. Transgenic Research, 7, 195–204.
Biesbrock, A. R., Bobek, L. A., & Levine, M. J. (1997). MUC7 gene expression and genetic polymorphism. Glycoconjugate Journal, 14, 415–422.
Fan, H., & Bobek, L. A. (2010). Regulation of human MUC7 mucin gene expression by cigarette smoke extract or cigarette smoke and Pseudomonas aeruginosa lipopolysaccharide in human airway epithelial cells and in MUC7 transgenic mice. Open Respiratory Medicine Journal, 4, 63–70.
Rachagani, S., Torres, M. P., Kumar, S., Haridas, D., Baine, M., Macha, M. A., et al. (2012). Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy. Journal of Hematology & Oncology, 5, 68.
Tian, H., Zhou, Q., Lasonos, A., Spriggs DR. (2013). Overexpression of the carboxyl-terminus of MUC16 can accelerate the occurrence of phenotypes in p53 heterozygous mouse model. Cancer Research, 73(8 Suppl). doi:10.1158/1538-7445.AM2013-326.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer. Nature, 414(6859), 105–111.
Hikita, S. T., Kosik, K. S., Clegg, D. O., & Bamdad, C. (2008). MUC1* mediates the growth of human pluripotent stem cells. PLoS One. doi:10.1371/journal.pone.0003312.
Ponnusamy, M. P., & Batra, S. K. (2008). Ovarian cancer: emerging concept on cancer stem cells. Journal of Ovarian Research. doi:10.1186/1757-2215-1-4.
Ponnusamy, M. P., Seshacharyulu, P., Vaz, A., Dey, P., & Batra, S. K. (2011). MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells. Journal of Ovarian Research. doi:10.1186/1757-2215-4-7.
Sugiura, D., Denda Nagai, K., Takashima, M., Murakami, R., Nagai, S., Takeda, K., et al. (2012). Local effects of regulatory T cells in MUC1 transgenic mice potentiate growth of MUC1 expressing tumor cells in vivo.8 PLoS One. doi:10.1371/journal.pone.0044770.
Tsai, C. J., Herrera-Goepfert, R., Tibshirani, R. J., Yang, S., Mohar, A., Guarner, J., et al. (2006). Changes of gene expression in gastric preneoplasia following Helicobacter pylori eradication therapy. Cancer Epidemiology, Biomarkers & Prevention, 15, 272–280.
Shimamura, T., Ito, H., Shibahara, J., Watanabe, A., Hippo, Y., Taniguchi, H., et al. (2005). Overexpression of MUC13 is associated with intestinal-type gastric cancer. Cancer Science, 96, 265–273.
Munro, E. G., Jain, M., Oliva, E., Kamal, N., Lele, S. M., Lynch, M. P., et al. (2009). Upregulation of MUC4 in cervical squamous cell carcinoma: pathologic significance. International Journal of Gynecological Pathology, 28, 127–133.
Shibahara, H., Tamada, S., Higashi, M., Goto, M., Batra, S. K., Hollingsworth, M. A., et al. (2004). MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology, 39, 220–229.
Chung, M. H., Choi, J. Y., Lee, W. S., Kim, H. N., & Yoon, J. H. (2002). Compositional difference in middle ear effusion: mucous versus serous. Laryngoscope, 112, 152–155.
Yuta, A., Ali, M., Sabol, M., Gaumond, E., & Baraniuk, J. N. (1997). Mucoglycoprotein hypersecretion in allergic rhinitis and cystic fibrosis. American Journal of Physiology, 273, L1203–L1207.
Vinall, L. E., King, M., Novelli, M., Green, C. A., Daniels, G., Hilkens, J., et al. (2002). Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology, 123, 41–49.
Kirkbride, H. J., Bolscher, J. G., Nazmi, K., Vinall, L. E., Nash, M. W., Moss, F. M., et al. (2001). Genetic polymorphism of MUC7: allele frequencies and association with asthma. European Journal of Human Genetics, 9, 347–354.
Watson, A. M., Ngor, W. M., Gordish-Dressman, H., Freishtat, R. J., & Rose, M. C. (2009). MUC7 polymorphisms are associated with a decreased risk of a diagnosis of asthma in an African American population. Journal of Investigative Medicine, 57, 882–886.
Carrara, S., Cangi, M. G., Arcidiacono, P. G., Perri, F., Petrone, M. C., Mezzi, G., et al. (2011). Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. American Journal of Gastroenterology, 106, 1359–1363.
Retz, M., Lehmann, J., Roder, C., Plotz, B., Harder, J., Eggers, J., et al. (1998). Differential mucin MUC7 gene expression in invasive bladder carcinoma in contrast to uniform MUC1 and MUC2 gene expression in both normal urothelium and bladder carcinoma. Cancer Research, 58, 5662–5666.
Acknowledgments
The authors on this work are supported, in part, by grants from the National Institutes of Health (TMEN U54 CA163120, EDRN UO1 CA111294, RO1 CA183459, SPORE P50 CA127297, RO1 CA78590, P20 GM103480, R21 CA155175, R21 CA156037, and RO3 CA167342).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, S., Kumar, S., Bafna, S. et al. Genetically engineered mucin mouse models for inflammation and cancer. Cancer Metastasis Rev 34, 593–609 (2015). https://doi.org/10.1007/s10555-015-9549-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-015-9549-1