Abstract
This study aims to evaluate cardiac function in cases of intrahepatic cholestasis of pregnancy (ICP) and compare results with those from healthy controls using the fetal left ventricular modified myocardial performance index (LMPI) and E-wave/A-wave peak velocities (E/A ratio). Moreover, the association between LMPI values, total bile acid (TBA) levels, fetal Doppler measurements, and adverse neonatal outcomes was evaluated. A prospective cross-sectional study of 120 pregnant women was conducted, with 60 having ICP and the other 60 serving as controls. Doppler ultrasound and two-dimensional gray-scale fetal echocardiography were used to calculate the LMPI values and E/A ratios, respectively. The association between LMPI values and TBA levels, fetal Doppler measurements, and adverse neonatal outcomes was evaluated. Fetal LMPI values were significantly higher in the ICP group than in the control group (0.54 ± 0.54 vs. 0.44 ± 0.03; p < 0.001), but the E/A ratio was similar in both groups (0.69 ± 0.10 vs. 0.66 ± 0.14; p = 0.203). TBA levels were positively and significantly correlated with LMPI values (r = 0.546, p < 0.01); however, no significant correlation was found between umbilical arterial pulsatility index values and LMPI values (r = 0.071, p > 0.01). LMPI values were not associated with adverse neonatal outcomes in ICP cases. Fetal cardiac function (LMPI) is associated with increased bile acid levels in ICP. However, because it was not associated with adverse neonatal outcomes in ICP cases, the clinical significance of this finding is unclear. Further studies are required to evaluate the implications of increased LMPI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-related liver disease that causes maternal pruritus and elevated liver enzymes and bilirubin levels. It is characterized by high serum total bile acids (TBAs) [1, 2]. It affects 0.4–15% of pregnancies and varies according to geography and ethnicity [1, 3]. Although the underlying etiology includes hormonal, environmental, and genetic influences, clinical findings are associated with increased serum TBA levels [4]. The importance of the disease derives from its association with adverse perinatal outcomes. It causes adverse perinatal outcomes, such as spontaneous preterm birth, fetal distress, meconium-stained amniotic fluid, and stillbirth. These outcomes are associated with increased maternal serum TBA levels [5, 6]. Although the mechanism of the increase in bile acid levels and adverse perinatal outcomes is not fully understood, it was emphasized that high bile acids might cause stillbirth via cardiac rhythm abnormalities or placental vascular vasospasm and spontaneous preterm delivery via oxytocin receptors [2, 5].
The myocardial performance index (MPI) is a Doppler index that measures ventricular myocardial systolic and diastolic performance. It is not influenced by heart rate and ventricular geometry. First, Tei et al. [7, 8] defined and proposed a global cardiac function predictor. MPI is calculated by dividing the sum of isovolumetric relaxation time (IRT) and isovolumetric contraction time (ICT) by ejection time (ET). This index is increasingly being used as a monitoring tool to identify abnormalities in fetal cardiac function in complicated pregnancies, such as those involving mothers with diabetes or preeclampsia or fetuses with growth restrictions [9, 10]. However, due to wide variations in normal reference values, which are assumed to be caused by the absence of distinct landmarks in Doppler waveforms, many authors have suggested various adjustments [11, 12]. Hernandez-Andrade et al. introduced the modified myocardial performance index (mod-MPI), an alternative technique with improved interobserver reproducibility. The three time periods used for MPI are revealed using clicks of the mitral valve (MV) and aortic valve (AV) opening and closing [13]. Mod-MPI is simple to obtain and replicate. Therefore, studies have been conducted in recent years to determine fetal cardiac dysfunction in complicated pregnancies and predict perinatal outcomes [14,15,16,17].
Previous studies have shown that mod-MPI rises as an indicator of cardiac dysfunction in diseases associated with increased placental vascular resistance, such as preeclampsia and fetal growth retardation [14, 15, 17]. Furthermore, recent studies have found that mod-MPI increases ICP and may predict adverse perinatal outcomes. It has been proposed that the cardiotoxic effect of increased bile acids may cause fetal cardiac dysfunction in ICP [18,19,20]. Bile acids accumulate in the cardiac conduction system and may lead to arrhythmias. Additionally, it can cause cardiac hypertrophy, cardiomyocyte apoptosis, and abnormal heart hemodynamics [21]. Therefore, we aimed to evaluate fetal cardiac function using mod-MPI measurements in ICP cases and the association of mod-MPI values and TBA levels, fetal Doppler measurements, and adverse neonatal outcomes.
Materials and methods
This prospective case–control study was conducted on pregnant women at the Perinatology Department of a tertiary center in Turkey between January 1, 2020, and December 31, 2021. The study protocol was approved by the Local Institutional Ethics Committee (approval number: 2020/14-45), and informed consent was obtained from all participants.
The study includes pregnant women with ICP and who gave birth in our hospital between the pertinent dates. Based on the presence of pruritus and increased TBA levels (≥ 10 μmol/L), ICP is diagnosed [22]. According to TBA levels, ICP severity was classified as mild between 10 and 40 μmol/L and severe above 40 μmol/L. Ursodeoxycholic acid (10–15 mg/kg/day) was administered to all pregnant women with ICP. In ICP cases, delivery was decided after 37 weeks of gestation if no complications might affect fetal well-being or cause maternal morbidity. By computerized randomization, pregnant women who delivered in the same period and had no chronic disease were selected as controls for each patient. The exclusion criteria for the ICP and control groups were in vitro fertilization, multiple pregnancies, congenital fetal malformations, fetal growth retardation (sonographic estimated fetal weight less than 10th percentile), and maternal comorbidities (hypertensive disorders, gestational and pregestational diabetes mellitus, and liver disease).
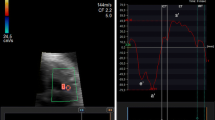
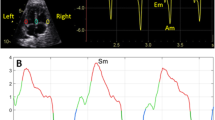
Ultrasound assessment was performed without fetal movements using a Samsung Ultrasound System HS70A (Samsung Medison Company, Republic of Korea) equipped with an abdominal 4–8-MHz curvilinear transducer. All sonographic examinations and fetal and left ventricular modified MPI (LMPI) measurements were performed at the initial referral of the cases. Measurements were taken by two experts (I.O. and T.D.). Each observer calculated LMPI values twice in the same fetus. The measurements were performed offline from video recordings by a second observer who was unaware of the results of the first examination to test the interobserver variability. Fetal biometry and umbilical artery (UA) Doppler were measured, and biophysical scoring evaluated fetal well-being. For the calculation of LMPI, a cross-sectional image of the fetal thorax was obtained at the four-chamber view level with the apical projection of the heart with the Doppler sample opened to 3–4 mm and placed in the internal leaflet of the MV. Due to the proximity of this position to the AV, clicks of both valves corresponding to opening and closing were recorded. The insolation angle was kept at 0–30. The wall motion filter was calibrated at 300 Hz. Doppler sweep velocity was calibrated at 5 cm/s. The time cursor was positioned at the beginning of each Doppler click to create three time periods. ICT, ET, and IRT were the measurements used to determine the times from the beginning of MV closure and the opening of the AV (Fig. 1). The (ICT + IRT)/ET equation was used to determine the LMPI. The LMPI measurement demonstrated high intraobserver [ICC 0.89 (0.75; 0.98)] and interobserver [ICC 0.81 (0.61; 0.98)] agreement levels. The E wave is caused by premature ventricular filling, whereas the A wave is caused by active atrial filling. By plotting the peak velocities of these two waves, the E/A was calculated. The E/A ratio measurement demonstrated high intraobserver [ICC 0.92 (0.79; 0.98)] and interobserver [ICC 0.84 (0.65; 0.98)] agreement levels.
Maternal characteristics, laboratory findings, ultrasonographic measurements, and perinatal outcomes were compared in both groups. A composite adverse neonatal outcome was defined as the presence of at least one of the following findings: respiratory distress syndrome (RDS), hyperbilirubinemia, sepsis, infection of unknown origin, and neonatal intensive care unit (NICU) admission. The correlation between TBA levels and UA doppler and LMPI measurements in ICP cases was examined. Furthermore, the association between adverse neonatal outcomes and LMPI values in ICP cases was evaluated.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences version 26.0 (IBM Corporation, Armonk, NY). Normality distribution was determined using the Shapiro–Wilk test and histogram graphs. The Student’s t test was used to compare paired groups for normally distributed data. For non-normally distributed data, the Mann–Whitney U test was used to compare paired groups, and the Kruskal–Wallis H test was used to compare triple groups. The homogeneity of the variances was checked in the significance sub-analysis of triple groups to determine which groups differed. Because the variances were homogeneous, the Scheffe test, one of the post hoc multiple comparison tests, was used. For categorical variables between groups, the chi-square test was used. The predictive performance of mod-MPI for adverse neonatal outcomes was evaluated using receiver operating characteristic (ROC) curves. Spearman's correlation test was used to evaluate the correlation between groups. The interobserver and intraobserver difference was calculated using the intraclass correlation coefficient. p < 0.05 was considered significant for results.
Results
The study included 120 pregnant women, 60 with ICP and 60 controls. The maternal characteristics, laboratory findings, and perinatal outcomes of both groups are shown in Table 1. The mean maternal age was significantly higher in the ICP group (29 ± 6 vs. 25 ± 5, p < 0.001). Gestational weeks were similar in both groups when measured using ultrasound (31 ± 4 vs. 30 ± 5, p = 0.693). The control group had a significantly higher mean week of delivery (36 ± 1 vs. 38 ± 2, p < 0.001). Mean birth weight was significantly higher in the control group (p = 0.002). Non-reassuring fetal heart rate tracing and primary cesarean delivery rates were significantly higher in ICP cases (p = 0.008 and p = 0.005, respectively). The ICP group had significantly higher adverse perinatal outcomes, such as meconium-stained amniotic fluid, non-reassuring fetal heart rate tracing, and composite adverse neonatal outcomes.
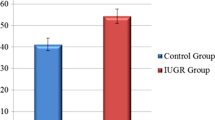
Ultrasonographic measurements of both groups are presented in Table 2. Fetal LMPI values in the ICP group were significantly higher than in the controls (0.54 ± 0.54 vs. 0.44 ± 0.03, p < 0.001). ICT and IRT values were significantly higher in the ICP group (p < 0.001 for both), and ET value was significantly higher in the control group (p = 0.011). There was no statistically significant difference in E/A values between the groups (0.69 ± 0.10 vs. 0.66 ± 0.14, p = 0.203). Umbilical arterial pulsatility index (UA PI) values were significantly higher in the ICP group (0.95 ± 0.29 vs. 0.82 ± 0.16, p = 0.004).
Ultrasonographic measurements of mild ICP, severe ICP, and control groups are presented in Table 3. The LMPI values of the mild ICP and severe ICP groups were significantly higher than the control group, and the LMPI values of the severe ICP group were significantly higher than the mild ICP group (p < 0.001 for all). E/A values did not significantly differ between the groups (p = 0.355). Additionally, the correlation between LMPI values and serum TBA levels and UA doppler measurements in ICP cases was examined (Figs. 2, 3). There was a positive and significant correlation between TBA levels and LMPI values (r = 0.546, p < 0.01). However, no significant correlation was found between UA PI and LMPI values (r = 0.071, p > 0.01).
LMPI values and E/A ratio in ICP cases with and without adverse perinatal outcomes are presented in Table 4. There was no significant difference in LMPI values and E/A ratios in ICP cases with and without adverse perinatal outcomes (p = 0.163 and p = 0.507, respectively). The ROC curve for LMPI was evaluated to predict adverse neonatal outcomes and had no significant effect (AUC 0.638; 95% CI 0.424–0.852, p = 0.120; Fig. 4).
Discussion
This study evaluated fetal LMPI measurements in ICP and their relationship with adverse perinatal outcomes. Our findings revealed that ICP fetuses had significantly higher LMPI values, indicating cardiac involvement, than controls. LMPI values were significantly related to ICP severity and TBA levels. However, ROC analysis did not reveal significance in predicting adverse neonatal outcomes. The ICP cases had significantly higher UA PI Doppler measurements, which affect fetal afterload, but there was no significant correlation between UA PI Doppler measurements and LMPI values. The E/A ratios, which indicate diastolic cardiac dysfunction, did not differ significantly between the two groups.
The MPI indicates the systolic and diastolic functions of the fetal heart [23]. The right side of the heart is dominant during the intrauterine period, and evaluation of the right side of the fetal heart may be more useful in predicting cardiac function [24]. However, to evaluate right ventricular MPI, a biplane image with two different waveforms, one for the pulmonary, and one for the tricuspid valve, is required. The left cardiac ventricle has anatomical advantages for MPI assessment. The valvular proximity of the AVs and MVs allows the evaluation of the E/A ratio with ICT, ET, and IRT in a single Doppler image. Previous studies have shown no significant difference in right ventricular and left ventricular MPI values when evaluating cardiac function in the same fetus without fetal heart rate; they are consistent with each other [25, 26]. There may be inconsistencies between observers in MPI measurements. Mod-MPI is an alternative technique with increased interobserver reproducibility developed to avoid these inconsistencies and has been widely used in recent studies to evaluate fetal cardiac function [13].
Many fetal and pregnancy-related conditions affect fetal heart function significantly. Mod-MPI measures both systolic and diastolic heart functions [23]. An increase in fetal cardiac afterload is one condition affecting cardiac function. Hypervolemia and placental vascular resistance increase fetal cardiac afterload. In previous studies, mod-MPI has been shown to increase in association with myocardial dysfunction in cases of increased afterload, such as intrauterine fetal transfusion [27] and recipient fetuses in twin-to-twin transfusion syndrome [28]. Furthermore, pregnancy-related diseases such as preeclampsia and intrauterine growth retardation are associated with increased placental vascular resistance. Studies have shown increased mod-MPI values in these diseases due to fetal cardiac dysfunction [14, 15, 17]. This study excluded diseases associated with increased placental resistance, such as intrauterine growth retardation and hypertensive diseases of pregnancy. However, our findings revealed that UA PI Doppler measurements were significantly higher in the ICP group. Although previous studies have shown that fetal UA doppler measurements in ICP are ineffective at predicting fetal complications, it has been proposed that high bile acid levels may lead to adverse consequences by causing placental vasoconstriction in the pathogenesis of ICP [2, 5, 29]. Therefore, we examined the correlation between UA PI Doppler measurements and LMPI values, but there was no significant correlation.
Although the etiopathogenesis of ICP is unknown, the association between clinical findings and pregnancy outcomes with increased TBA levels has been proven [22, 30]. Additionally, animal studies have shown that high bile acid levels in ICP have a toxic effect on the fetal heart, resulting in cardiac contraction disorders, fatal arrhythmias, and sudden fetal death [31, 32]. Recent studies have found that cardiac dysfunction in ICP significantly increases echocardiographic mod-MPI measurements. Henry et al. [19] examined both left and right MPI values of the fetal heart in ICP cases and showed that LMPI values were significantly higher in ICP in correlation with TBA levels, but right ventricular MPI values did not significantly increase. Sanhal et al. [20] also found that mod-MPI values of the left fetal ventricle were significantly higher and E/A ratios were significantly lower in ICP. Similarly, Ozel et al. [18] found that LMPI values were significantly higher in ICP cases. Our findings confirmed previous studies and LMPI values were significantly higher in ICP cases and were positively correlated with TBA levels. However, our study found that E/A ratios, indicators of diastolic dysfunction, were not significantly affected in ICP cases. The E/A ratio is associated with cardiac damage and indicates diastolic dysfunction [15, 33]. However, because fetal LMPI and E/A measurements of the cases were performed at the first visit, no relevant results regarding changes in long-term fetal cardiac function could be obtained in our study.
Studies have shown that evaluating fetal well-being, such as non-stress testing or fetal Doppler measurement, is ineffective in predicting adverse perinatal outcomes in ICP [34, 35]. However, Sanhal et al. [20] demonstrated that measuring LMPI can be useful in predicting adverse perinatal outcomes in ICP, and they found a cut-off level of 0.48 at 81.8% sensitivity and 67.6% specificity for predicting adverse perinatal outcomes (non-reassuring fetal heart rate tracing, umbilical cord pH < 7.15, presence of meconium in amnion, and NICU admission) for LMPI. Similarly, Ozel et al. [18] defined the LMPI cut-off level as 0.41 for the same adverse perinatal outcomes. In this study, we performed ROC curve analysis for LMPI to predict adverse neonatal outcomes (RDS, hyperbilirubinemia, sepsis, infection of unknown origin, and NICU admission), but the results did not show significance (AUC 0.638; 95% CI 0.424–0.852, p = 0.120). These differences between studies are due to different selection criteria. We believe that large-scale studies in which all adverse perinatal outcomes are evaluated separately in predicting perinatal outcomes of LMPI measurements are required.
Limitations
Placental diseases (e.g., preeclampsia) associated with increased ventricular afterload were excluded from this study. However, although there was no significant correlation between LMPI and UA PI values, UA PI values were significantly higher in ICP cases. Because the UA PI value may be associated with increased placental vascular resistance, cardiac loading conditions might affect LMPI changes in ICP cases. Furthermore, the number of cases included in the study was limited due to the prospective design of the study and the fact that it was conducted in a single center.
Conclusions
Our findings indicate that fetal cardiac function (LMPI) is affected in ICP and is related to elevated bile acid levels. However, the clinical significance of this finding is unknown because it was not associated with adverse neonatal outcomes in ICP cases. Further research is needed to evaluate the implications of increased LMPI.
References
Geenes V, Chappell LC, Seed PT et al (2014) Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatol Baltim Md 59:1482–1491. https://doi.org/10.1002/hep.26617
Williamson C, Geenes V (2014) Intrahepatic cholestasis of pregnancy. Obstet Gynecol 124:120–133. https://doi.org/10.1097/AOG.0000000000000346
Wikström Shemer E, Marschall HU, Ludvigsson JF et al (2013) Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG Int J Obstet Gynaecol 120:717–723. https://doi.org/10.1111/1471-0528.12174
Dixon PH, Williamson C (2016) The pathophysiology of intrahepatic cholestasis of pregnancy. Clin Res Hepatol Gastroenterol 40:141–153. https://doi.org/10.1016/j.clinre.2015.12.008
Brouwers L, Koster MPH, Page-Christiaens GCML et al (2015) Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol 212(100):e1-7. https://doi.org/10.1016/j.ajog.2014.07.026
Madazli R, Yuksel MA, Oncul M et al (2015) Pregnancy outcomes and prognostic factors in patients with intrahepatic cholestasis of pregnancy. J Obstet Gynaecol J Inst Obstet Gynaecol 35:358–361. https://doi.org/10.3109/01443615.2014.968102
Tei C (1995) New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol 26:135–136
Tei C, Ling LH, Hodge DO et al (1995) New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function–a study in normals and dilated cardiomyopathy. J Cardiol 26:357–366
Severi FM, Rizzo G, Bocchi C et al (2000) Intrauterine growth retardation and fetal cardiac function. Fetal Diagn Ther 15:8–19. https://doi.org/10.1159/000020969
Wong SF, Chan FY, Cincotta RB et al (2003) Cardiac function in fetuses of poorly-controlled pre-gestational diabetic pregnancies–a pilot study. Gynecol Obstet Invest 56:113–116. https://doi.org/10.1159/000073191
Eidem BW, Edwards JM, Cetta F (2001) Quantitative assessment of fetal ventricular function: establishing normal values of the myocardial performance index in the fetus. Echocardiogr Mt Kisco N 18:9–13. https://doi.org/10.1046/j.1540-8175.2001.t01-1-00009.x
Tsutsumi T, Ishii M, Eto G et al (1999) Serial evaluation for myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int 41:722–727. https://doi.org/10.1046/j.1442-200x.1999.01155.x
Hernandez-Andrade E, Figueroa-Diesel H, Kottman C et al (2007) Gestational-age-adjusted reference values for the modified myocardial performance index for evaluation of fetal left cardiac function. Ultrasound Obstet Gynecol 29:321–325. https://doi.org/10.1002/uog.3947
Bhorat IE, Bagratee JS, Pillay M et al (2015) Determination of the myocardial performance index in deteriorating grades of intrauterine growth restriction and its link to adverse outcomes. Prenat Diagn 35:266–273. https://doi.org/10.1002/pd.4537
Api O, Emeksiz MB, Api M et al (2009) Modified myocardial performance index for evaluation of fetal cardiac function in pre-eclampsia. Ultrasound Obstet Gynecol 33:51–57. https://doi.org/10.1002/uog.6272
Sanhal CY, Daglar HK, Kara O et al (2017) Assessment of fetal myocardial performance index in women with pregestational and gestational diabetes mellitus. J Obstet Gynaecol Res 43:65–72. https://doi.org/10.1111/jog.13174
Alici Davutoglu E, Ozel A, Oztunc F et al (2020) Modified myocardial performance index and its prognostic significance for adverse perinatal outcome in early and late onset fetal growth restriction. J Matern-Fetal Neonatal Med 33:277–282. https://doi.org/10.1080/14767058.2018.1489534
Ozel A, Alici Davutoglu E, Eric Ozdemir M et al (2020) Assessment of fetal left ventricular modified myocardial performance index and its prognostic significance for adverse perinatal outcome in intrahepatic cholestasis of pregnancy. J Matern-Fetal Neonatal Med 33:2000–2005. https://doi.org/10.1080/14767058.2018.1535588
Henry A, Welsh AW (2015) Monitoring intrahepatic cholestasis of pregnancy using the fetal myocardial performance index: a cohort study. Ultrasound Obstet Gynecol 46:571–578. https://doi.org/10.1002/uog.14769
Sanhal CY, Kara O, Yucel A (2017) Can fetal left ventricular modified myocardial performance index predict adverse perinatal outcomes in intrahepatic cholestasis of pregnancy? J Matern-Fetal Neonatal Med 30:911–916. https://doi.org/10.1080/14767058.2016.1190824
Vasavan T, Ferraro E, Ibrahim E et al (2018) Heart and bile acids: clinical consequences of altered bile acid metabolism. Biochim Biophys Acta BBA 1864:1345–1355. https://doi.org/10.1016/j.bbadis.2017.12.039
Herrera CA, Manuck TA, Stoddard GJ et al (2018) Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern-Fetal Neonatal Med 31:1913–1920. https://doi.org/10.1080/14767058.2017.1332036
Mahajan A, Henry A, Meriki N et al (2015) The (pulsed-wave) Doppler fetal myocardial performance index: technical challenges, clinical applications and future research. Fetal Diagn Ther 38:1–13. https://doi.org/10.1159/000363181
Mielke G, Benda N (2001) Cardiac output and central distribution of blood flow in the human fetus. Circulation 103:1662–1668. https://doi.org/10.1161/01.cir.103.12.1662
Bui YK, Kipps AK, Brook MM et al (2013) Tissue Doppler is more sensitive and reproducible than spectral pulsed-wave Doppler for fetal right ventricle myocardial performance index determination in normal and diabetic pregnancies. J Am Soc Echocardiogr 26:507–514. https://doi.org/10.1016/j.echo.2013.02.006
Ghawi H, Gendi S, Mallula K et al (2013) Fetal left and right ventricle myocardial performance index: defining normal values for the second and third trimesters–single tertiary center experience. Pediatr Cardiol 34:1808–1815. https://doi.org/10.1007/s00246-013-0709-1
de Assunção RA, Liao AW, de Brizot ML et al (2016) Changes in fetal myocardial performance index following intravascular transfusion: preliminary report. J Matern-Fetal Neonatal Med 29:2697–2702. https://doi.org/10.3109/14767058.2015.1101757
Ichizuka K, Matsuoka R, Hasegawa J et al (2005) The Tei index for evaluation of fetal myocardial performance in sick fetuses. Early Hum Dev 81:273–279. https://doi.org/10.1016/j.earlhumdev.2004.07.003
Mullally BA, Hansen WF (2002) Intrahepatic cholestasis of pregnancy: review of the literature. Obstet Gynecol Surv 57:47–52. https://doi.org/10.1097/00006254-200201000-00023
Fan HM, Mitchell AL, Williamson C (2021) ENDOCRINOLOGY IN PREGNANCY: metabolic impact of bile acids in gestation. Eur J Endocrinol 184:R69–R83. https://doi.org/10.1530/EJE-20-1101
Gorelik J, Shevchuk A, de Swiet M et al (2004) Comparison of the arrhythmogenic effects of tauro- and glycoconjugates of cholic acid in an in vitro study of rat cardiomyocytes. BJOG Int J Obstet Gynaecol 111:867–870. https://doi.org/10.1111/j.1471-0528.2004.00166.x
Williamson C, Gorelik J, Eaton BM et al (1979) The bile acid taurocholate impairs rat cardiomyocyte function: a proposed mechanism for intra-uterine fetal death in obstetric cholestasis. Clin Sci Lond Engl 2001(100):363–369
Narin N, Cetin N, Kiliç H et al (1999) Diagnostic value of troponin T in neonates of mild pre-eclamptic mothers. Biol Neonate 75:137–142. https://doi.org/10.1159/000014089
Kawakita T, Parikh LI, Ramsey PS et al (2015) Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol 213(570):e1-8. https://doi.org/10.1016/j.ajog.2015.06.021
Lee RH, Incerpi MH, Miller DA et al (2009) Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol 113:528–531. https://doi.org/10.1097/AOG.0b013e31818db1c9
Acknowledgements
None.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
Authors state no conflict of interest.
Ethical approval
Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the authors. The study protocol was approved by the Local Institutional Ethics Committee (approval number: 2020/ 14-45).
Informed consent
Informed consent was obtained from all individuals included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omeroglu, I., Golbasi, H., Bayraktar, B. et al. Modified myocardial performance index for evaluation of fetal heart function and perinatal outcomes in intrahepatic pregnancy cholestasis. Int J Cardiovasc Imaging 39, 907–914 (2023). https://doi.org/10.1007/s10554-022-02789-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02789-4