Abstract
Myocardial performance index (MPI), or Tei index, has become a commonly used parameter for the noninvasive, Doppler-derived assessment of global systolic and diastolic performance of the heart in both adults and children. Normal values have been established in adults and children; however, limited data exist in fetal hearts. The aim of this study was to further elucidate normal values of fetal left (LV) and right ventricle (RV) MPI values in second- and third-trimester fetuses and compare these values with other previously published data. This was a retrospective study to measure MPI in healthy fetuses. After Institutional Review Board approval, 2000 fetal echocardiography studies (FES) were acquired during a period of 4 years. Demographic parameters examined included gestational age (GA), maternal age (MA), and indication for fetal echocardiography. Fetuses with congenital heart disease, arrhythmias, or significant noncardiac fetal anomalies were excluded. The following echocardiography parameters were collected: LV ejection time (LVET), mitral valve close-to-open time (MVCO), RVET, tricuspid valve CO (TVCO), and fetal heart rate. For simplicity, LV and RV MPI values were calculated as follows: LV MPI = MVCO − LVET/LVET and RV MPI = TVCO − RVET/RVET. Four hundred twenty FES met the study criteria. LV MPI was evaluated in 230 and 190 FES in the second and third trimester, respectively. Of the 420 FES, 250 (150 in the second trimester and 100 in the third trimester) had all of the measurements required for RV MPI calculation. MA ranged between 16 and 49 years. Indications for FES included diabetes mellitus (N = 140; 33 %), suspected fetal anomalies on routine obstetrical ultrasound (N = 80; 20 %), autoimmune disorder (N = 60; 14 %), family history of CHD (N = 76; 18 %), medication exposure (N = 22; 5 %), increase nuchal thickness (N = 13; 3 %), and other indications (N = 29; 6 %). Averaged LV and RV MPI values were 0.464 ± 0.08 and 0.466 ± 0.09, respectively. Further analysis based on gestational period showed slightly greater LV and RV MPI values during the third compared with the second trimester, i.e., 0.48 and 0.49, respectively, with no statistically significant difference. There was no significant association of LV and RV MPI with heart rate. To our knowledge, this is the first study to establish normal values of fetal MPI based on a large fetal population from a single tertiary center. LV and RV MPI values were independent of GA and fetal heart rate. MPI is a useful parameter for the assessment of global cardiac function in the fetus and demonstrates good reproducibility with narrow interobserver and intraobserver variability. Its usefulness should be studied in fetal hearts with complex congenital anomalies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The last few decades in fetal cardiology have witnessed many advances in the field of perinatal cardiology. Collaborative efforts are evolving between the fetal maternal medicine specialist and the pediatric cardiologist. The importance of assessing fetal cardiac function and the timing for intervention continues to grow. Noninvasive and efficient imaging modalities to evaluate cardiac function have become the focus for both specialties. Significant progress has been achieved during the last three decades to facilitate determination of fetal cardiac anatomy and function. Fetal cardiac anatomy was initially documented by Dolkart and Reimers, who assessed 52 fetuses during the first and second trimesters. This study of normal cardiac anatomy suggested that there may be significant potential for the diagnosis of many fetal cardiac anomalies [7].
Many parameters to assess fetal cardiac function, such as cardiac size and wall thickness, have been studied and validated [20, 24]. Basic systolic function is assessed through measurement of ventricular shortening fraction using either two-dimensional images or M-mode interrogation. Ventricular ejection fraction (EF) to assess systolic function can also be obtained using Simpson technique [25]. However, the reproducibility of these different measurements is poor because of continuous fetal motion [26]. Diastolic dysfunction during a disease state, compared with healthy myocardium, was assessed by way of pulsed Doppler of ventricular inflow, inferior vena cava, ductus venosus, and umbilical vein flow. Changes in patterns and velocities obtained at these different sites were helpful in recognizing cardiac dysfunction that may lead to heart failure [5, 6]. Successful documentation of both transmitral and transtricuspid flow velocity waveforms was achieved in six fetuses only [33], and it was considered the initial attempt to measure the flow in early 1990’s.
MPI, or the Tei index, was initially described by Tei in 1995 as a Doppler-derived, noninvasive method to assess global systolic and diastolic performance of the heart in both adults and children [29, 30]. By incorporating only time intervals, the index is less dependent on anatomy or precise imaging. Furthermore, the MPI is independent of both heart rate and ventricular geometry [21], which renders other methods less attractive. Whether MPI is load-dependent or -independent is still debatable. Some studies have suggested that it is load-independent [13, 21], whereas others, mostly animal studies, have suggested that MPI is strongly related to preload and afterload [5, 13]. The MPI value is defined as the sum of isovolumic contraction time and isovolumic relaxation time divided by ejection time (ET), which requires measurement of the time interval between the end and onset of mitral or tricuspid inflow and ET of the left (LV) or right ventricle (RV) outflow.
This index does not rely on the size, shape, or orientation of the heart; therefore, it can be applied easily in the measurement of ventricular function. Because MPI can be easily acquired, reproduced, and strongly correlated with EF, its use to assess the global function of the heart has become more widely applied.
Normal values have been established in adults and children [18]; however, for fetuses, normal values have been reported in few studies with limited patient populations [9, 10, 12] (Table 1). In 2001, Eidem et al. published their experience with 125 normal fetuses: LV MPI values were 0.36 ± 0.06, and RV MPI values were 0.35 ± 0.05 [9]. Two years later, Friedman et al. evaluated 74 healthy fetuses and calculated the fetal LV MPI value to be 0.53 ± 0.13 [10].
In 2006, Chen et al. published a larger sample study in which 225 normal single fetuses were evaluated. The investigators found that MPI decreased linearly with advanced GA for both the LV and RV but was independent of heart rate [4]. In 2007, Szymkiewicz-Dangel published data for fetuses in the first trimester (11–13.6 weeks GA). LV MPI values ranged from 0.28 to 0.59 (mean 0.41 ± 0.08), and RV MPI values ranged from 0.23 to 0.56 (mean 0.37 ± 0.11) [28]. The most recent study was published by Hamela-Olkowska et al. The group evaluated 140 normal fetuses between 18 to 40 weeks’ gestation. Mean LV MPI was 0.47 ± 0.07, and RV MPI was 0.48 ± 0.1 [12].
Materials and Methods
This is a retrospective study. Two thousand fetal echocardiographic studies (FES) performed between January 2008 and January 2012 were evaluated. The study was approved by the Institutional Review Board. Data was obtained from electronically stored echocardiographic studies of each patient by way of software Xcelera storing system (Xcelera R2.2L1 SP2 2009, Philips Medical Systems Nederland B.V., Veenpluis, The Netherlands). Four hundred twenty FES met the inclusion criteria. Demographic parameters (Table 2) included GA, MA, and single or multiple gestations.
Study Population
MPI values were measured in normal fetuses’ hearts. Pregnant women had been referred to our service for a fetal echocardiography and/or consultation. Maternal indications for fetal echocardiography referals included maternal metabolic disorder, such as diabetes mellitus (N = 140; 33 %); maternal exposure to teratogens, e.g., valproic acid and lithium (N = 22; 5 %); family history of congenital heart disease (CHD) or cardiomyopathy (N = 76; 18 %); and maternal autoimmune disorder (N = 60; 14 %). These indications met the American Society of Echocardiography requirements. Fetal indications included obstetric ultrasound suspicious of (but found to be normal) cardiac fetal disease (N = 80; 20 %); increased nuchal translucency (N = 13; 3 %); and risk for fetal cardiovascular compromise, such as twin–twin transfusion and others (N = 29; 6 %).
Inclusion criteria were all infants with structurally normal heart scans and normal sinus rhythm. Exclusion criteria, and reasons for exclusion from the study, included congenital heart disease; diseases affecting systolic and/or diastolic function, e.g., diaphragmatic hernia; intrauterine growth restriction (IUGR); premature rupture of membrane (PROM); arrhythmia; and FES with incomplete Doppler data and/or poor Doppler data acquisition.
Four hundred twenty FES met the study criteria. LV MPI was evaluated in 230 and 190 studies for the second and third trimester, respectively. Of these, 250 (150 in the second trimester and 100 in the third trimester) had all of the measurements required for RV MPI calculation. MA age ranged between 16 and 49 years.
Two-Dimensional Echocardiography
Data Acquisition
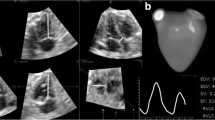
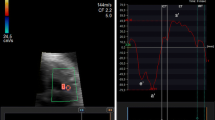
All images were recorded using a C5-1 curved array transducer and iE33 imaging system (Philips Healthcare, Philips Ultrasound, Bothell, WA). Doppler time intervals were measured using an off-line system. The velocities of the mitral and tricuspid inflows were recorded using the apical four-chamber view with the pulsed Doppler sample volume positioned at the tips of the mitral and, subsequently, the tricuspid leaflets during diastole. Velocity patterns of LV and RV outflows were recorded with pulsed Doppler positioned just below the aortic or the pulmonary valves. The following echocardiographic parameters were collected: LVET, mitral valve close-to-open time (MVCO) (Fig. 1a, b), RVET, TVCO (Fig. 1a, c, d), and fetal heart rate (Fig. 1). MPI was calculated according to the following formulae:
Data Collection
Three successive measurements of all the parameters were obtained and averaged. The fetal heart rate was also measured. MPI was calculated as a mean of these successive measurements.
Statistical Analysis
SPSS version 19.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. All numeric data are expressed as mean ± SD) The echocardiographic indices of fetuses were compared with those of the family history or suspected congenital cardiac anomalies and those of the other groups using unpaired Student t tests. Linear regression analysis was used to evaluate the relationship of the MPI to GA and heart rate. Statistical significance was defined as P < 0.05.
Results
Patient Characteristics
Four hundred twenty FES met the study criteria. LV MPI was evaluated in 230 and 190 studies for the second and third trimester, respectively. Of the 420 FES, 250 (150 in the second trimester and 100 in the third trimester) had all of the measurements required for RV MPI calculation (Table 3).
Echocardiographic Parameters and MPI Values
Data analysis was divided into two groups based on GA. LV and RV MPI values for both the second and third trimesters together were 0.464 ± 0.08 and 0.466 ± 0.09, respectively. Further subgroup analysis of LV and RV MPI based on indication for fetal echocardiography revealed no statistical difference in LVMPI between the diabetic group and other groups (P = 0.5). Because there was no statistically significant difference between the diabetic group and the other groups, diabetic group data were included in the main data pool. Although there was a statistical difference between LV MPI values between the second and third trimesters (P = 0.03), correlation coefficient analysis revealed no correlation between LV or RV MPI and GA (R = 0.1 and R = 0.21). Also, there was no correlation between LV and RV MPI and heart rate (R = 0.002 and R = 0.09) (Tables 3, 4; Figs. 2, 3).
Interobserver and Intraobserver Variability
Interaobserver variabilty was 6 % for the calculation of LV MPI and 5 % for that of RV MPI. Intraobserver variability was 4 % and 5 % for LV and RV MPI, respectively.
Discussions
The fetal heart may be influenced by primary cardiovascular and systemic lesions. Our knowledge to assess fetal heart function and predict potential progression to dysfunction continues to advance. Fetal echocardiography will continue to play a crucial role in the management of a large spectrum of primary cardiac and noncardiac fetal pathologies. MPI has been reported in the adult literature in the assessment of myocardial performance in a variety of clinical conditions, including myocardial infarction and dilated cardiomyopathy [22]. In these studies, it was also used as a predictor of clinical outcome [2, 3, 35]. In addition, MPI has been used in the pediatric population to evaluate myocardial function, e.g., in cases of single ventricle, left-to-right shunt, and pulmonary hypertension [1, 8, 36]. Recently the use of MPI has broadened in clinical practice. To detect abnormalities, normal ranges must be well established.
Previously published reference values for MPI values varied from 0.22 to 0.53 [10, 34]; these studies were based on small to medium sample sizes, 50–225 for LV MPI and even fewer for RV MPI, i.e., 27–225 [4, 9, 12, 21, 28, 30, 32]. Both Chen et al. [4] and Tsutsumi et al. [30] found a statistically significant relationship between MPI and advanced GA, whereas other investigators did not. Analyzing a larger sample size allowed us to precisely study this relationship. Using linear regression correlation analysis, there were no correlation between LV and RV MPI and GA or heart rate. Furthermore, with separate analysis of LV and RV MPI in each trimester (second and third), no statistically significant difference was detected; however, MPI was found to be slightly greater for both the LV and RV during the third compared with the second trimester.
MPI was developed as an assessment index of the global function of the RV and the LV. Because it measures both systolic and diastolic functions, it is difficult to determine the mechanism of dysfunction. MPI may be used as a screening and prognostic tool rather than a diagnostic one.
In 2008, Russell et al. [24] noted that MPI values did not change substantially from early to late gestation despite dramatic changes in fetal heart dimensions as well as placental hemodynamic changes. This was in accordance with our results, in which no significant difference was noted in MPI values through the first and second trimesters of pregnancy.
In fetal life, systolic ventricular function, theoretically, should be hyperdynamic in early gestation and less so in late gestation. Diastolic ventricular function, in contrast, should move toward postnatal values as GA advances and approaches term. Diastolic function continues to improve in postnatal life until normalization a few months after birth. Thus, MPI values likely will not change in normal fetuses as GA advances. This may explain our results showing more or less similar MPI values in all trimesters.
Interobserver and intraobserver variability were addressed in this study, and both were similar to other studies. Eidem et al. reported reproducible MPI values [9]. Other investigators modified the measurement of MPI parameters. Raboisson et al. [23] used the Doppler echocardiogram “click” of the opening of the aortic valve as a landmark to delineate the limits of the ejection period. Hernandez et al. [14] further developed the idea of adding the mitral valve closing click to clearly define the three periods and called it the “modified MPI” (Mod-MPI). Their interobserver and intraobserver agreements were greatly improved using the Mod-MPI. Our results, including mean MPI values, are in accordance with those reported by Raboisson et al. MPI strongly correlated with EF and showed less interobserver and intraobserver variability as noted by Van Meighem et al. [32].
The clinical applications for MPI in early detection and counseling were investigated. Early detection of decreased ventricular function was found in fetuses with twin–twin transfusion syndrome, fetuses of diabetic mothers, and other cases [11, 16]. In 2005, Inamura et al. [17] found that the Tei index was highest in fetuses with either pulmonary insufficiency or atresia compared with those having normal forward flow. They also noted that the LV Tei index was significantly greater in fetuses with fetal or neonatal death than in neonatal survivors [17]. More recently, Hernandez-Andrade et al. [15] evaluated MPI as an independent predictor of perinatal death in preterm IUGR fetuses with accuracy similar to that of ductus venosus (DV) flow. He concluded that a combination of DV flow, in addition to MPI, may better stratify the estimated probability of death [17]. In 2009, Szwast et al. [27] studied fetuses with hypoplastic left heart syndrome (HLHS) and concluded that patients with HLHS have inherent limitations in cardiac performance even before birth.
Some investigators have emphasized the importance of strict glycemic control during pregnancy to decrease the risk of many fetal and neonatal cardiac complications Wong et al. [34] assessed fetal MPI values in diabetic mothers with poor glycemic control and noted that MPI was decreased in late gestation [21]. More recently, Turan et al. [31] demonstrated significant differences in first-trimester diastolic myocardial function compared with nondiabetic controls. MPI was inversely related to maternal glycosylated hemoglobin. Wong et al. [34] noted that gestational glucose intolerance was not associated with ventricular hypertrophy and diastolic dysfunction but that MPI decreased in late gestation. In our study, there was no statistically significant difference in LV MPI values between fetuses of diabetic mothers and those of other groups, probably related to strict diabetic control. Diabetic group data were normal and thus were included in the main analysis.
One of the important clinical applications of MPI is to study the effect of IUGR on cardiac performance. In 2010, Comas et al. [6] determined that both systolic and diastolic dysfunction accompanied IUGR. In 2010, Letti et al. [19] evaluated the effect of PROM on cardiac performance. They concluded that cardiac dysfunction is present in the setting of preterm PROM. Therefore, we excluded all IUGR and PROM fetuses from our study.
Our study has several limitations. First, it was a retrospective study. Incomplete data in many studies led to the exclusion of a significant number of normal FES. Second, multiple personnel were involved in obtaining the echocardiographic parameters, which set the stage for a wide range of variation in obtained data. Finally, although evaluation of structurally normal fetal hearts was the main goal in the study, fetal hearts with other factors that may influence MPI like maternal diabetes, exposure to potential teratogens, and maternal autoantibodies when structurally normal, were also included. Although these studies appeared normal echocardiographically, with no difference in mean MPI obtained for these fetuses, subtle effects of such maternal disorders on MPI values may not have been detected. Future prospective studies of each subgroup will be valuable to study such effects.
Conclusion
This is the largest study published to date to better define normal values of fetal MPI based on a large fetal population from a single tertiary center. LV and RV MPI values appear to be independent of GA and fetal heart rate. There was good reproducibility with small interobserver and intraobserver variability in the same center. MPI can be used as a screening/follow-up tool for global cardiac function in normal and complex fetal hearts. MPI should be considered as adjuvant in the initial diagnosis in fetuses with complex heart diseases and their subsequent follow-up scans. Prospective studies are needed to further delineate these relations.
References
Baysal T, Oran B, Doğan M, Cimen D, Karaaslan S (2005) The myocardial performance index in children with isolated left-to-right shunt lesions. Anadolu Kardiyol Derg 5(2):108–111
Blanchard D, Malouf P, Gurudevan S, Auger W, Madani N, De Maria A et al (2009) Utility of right ventricular Tei index in the non invasive evaluation of chronic thromboembolic pulmoanry thromboendartrectomy. JACC Cardiovasc Imaging 2(2):143–149
Carluccio E, Biagolo P, Alunni G, Murrone A, Zuchi C et al (2012) Improvement of myocardial performance index closely reflects intrinsic improvement of cardiac function: assessment in revascularized hibernating myocardium. Echocardiography 29(3):298–306
Chen Q, Sun XF, Liu HJ (2006) Assessment of myocardial performance in fetuses by using Tei index. Zhonghua Fu Chan Ke Za Zhi 41(6):387–390
Cheung MM, Smallhorn JF, Redington AN, Vogel M (2004) The effects of changes in loading conditions and modulation of inotropic state on the myocardial performance index: comparison with conductance catheter measurements. Eur Heart J 25(24):2238–2242
Comas M, Crispi F, Cruz-Martinez R, Martinez JM, Figueras F, Gratacós E (2010) Usefulness of myocardial tissue Doppler versus conventional echocardiography in the evaluation of cardiac dysfunction in early-onset intrauterine growth restriction. Am J Obstet Gynecol 203(1):45.e1–45.e7
Dolkart LA, Reimers FT (1991) Transvaginal fetal echocardiography in early pregnancy: normative data. Am J Obstet Gynecol 165(3):688–691
Dyer KL, Pauliks LB, Das B, Shandas R, Ivy D, Shaffer EM et al (2006) Use of myocardial performance index in pediatric patients with idiopathic pulmonary arterial hypertension. J Am Soc Echocardiogr 19(1):21–27
Eidem BW, Edwards JM, Cetta F (2001) Quantitative assessment of fetal ventricular function: establishing normal values of the myocardial performance index in the fetus. Echocardiography 18(1):9–13
Friedman D, Buyon J, Kim M, Glickstein JS (2003) Fetal cardiac function assessed by Doppler myocardial performance index (Tei Index). Ultrasound Obstet Gynecol 21(1):33–36
Gudmundsson S, Huhta JC, Wood DC et al (1991) Venous Doppler ultrasonography in the fetus with nonimmune hydrops. Am J Obstet Gynecol 164:33–37
Hamela-Olkowska A, Szymkiewicz-Dangel J (2011) Quantitative assessment of the right and the left ventricular function using pulsed Doppler myocardial performance index in normal fetuses at 18 to 40 weeks of gestation. Ginekol Pol 82(2):108–113
Haney MF, A’Roch R, Johansson G, Poelaert J, Biber B (2007) Beat-to-beat change in “myocardial performance index” related to load. Acta Anaesthesiol Scand 51(5):545–552
Hernandez-Andrade E, López-Tenorio J, Figueroa-Diesel H, Sanin-Blair J, Carreras E, Cabero L et al (2005) A modified myocardial performance (Tei) index based on the use of valve clicks improves reproducibility of fetal left cardiac function assessment. Ultrasound Obstet Gynecol 26(3):227–232
Hernandez-Andrade E, Crispi F, Benavides-Serralde JA, Plasencia W, Diesel HF, Eixarch E et al (2009) Contribution of the myocardial performance index and aortic isthmus blood flow index to predicting mortality in preterm growth-restricted fetuses. Ultrasound Obstet Gynecol 34(4):430–436
Ichizuka K, Matsuoka R, Hasegawa J, Farina A, Okai T (2005) The Tei index for evaluation of fetal myocardial performance in sick fetuses. Early Hum Dev 81(3):273–279
Inamura N, Taketazu M, Smallhorn JF, Hornberger LK (2005) Left ventricular myocardial performance in the fetus with severe tricuspid valve disease and tricuspid insufficiency. Am J Perinatol 22(2):91–97
Jurko A Jr, Jurko A, Minarik M (2011) Doppler-derived myocardial performance index in healthy children. Bratisl Lek Listy 112(2):77–79
Letti Müller AL, Barrios Pde M, Kliemann LM, Valério EG, Gasnier R, Magalhães JA (2010) Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet Gynecol 36(1):26–31
Mäkikallio K, Jouppila P, Räsänen J (2005) Human fetal cardiac function during the first trimester of pregnancy. Heart 91(3):334–338
Møller JE, Poulsen SH, Egstrup K (1999) Effect of preload alternations on a new Doppler echocardiographic index of combined systolic and diastolic performance. J Am Soc Echocardiogr 12(12):1065–1072
Pedra SR, Smallhorn JF, Ryan G, Chitayat D, Taylor GP, Khan R, Abdolell M, Hornberger LK (2002) Fetal cardiomyopathies: etiologies, hemodynamic findings and clinical outcome. Circulation 106:585–591
Raboisson MJ, Bourdages M, Fouron JC (2003) Measuring left ventricular myocardial performance index in fetuses. Am J Cardiol 91:919–921
Russell NE, McAuliffe FM (2008) First-trimester fetal cardiac function. J Ultrasound Med 27(3):379–383
Schmidt KG, Silverman NH, Hoffman JI (1995) Determination of ventricular volumes in human fetal hearts by two-dimensional echocardiography. Am J Cardiol 76:1313–1316
Simpson JM, Cook A (2002) Repeatability of echocardiographic measurements in the human fetus. Ultrasound Obstet Gynecol 20:332–339
Szwast A, Tian Z, McCann M, Donaghue D, Rychik J (2009) Right ventricular performance in the fetus with hypoplastic left heart syndrome. Ann Thorac Surg 87(4):1214–1219
Szymkiewicz-Dangel J, Hamela-Olkowska A, Własienko P, Jalinik K, Czajkowski K (2007) The possibility of evaluation of the myocardial performance index in fetuses at 11,0 to 13,6 week of gestation. Ginekol Pol 78(3):218–222
Tei C (1995) New noninvasive index for combined systolic and diastolic ventricular function. J Cardiol 26:135–136
Tsutsumi T, Ishii M, Eto G et al (1999) Serial evaluation for myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int 41:722–727
Turan S, Turan OM, Miller J, Harman C, Reece EA, Baschat AA (2011) Decreased fetal cardiac performance in the first trimester correlates with hyperglycemia in pregestational maternal diabetes. Ultrasound Obstet Gynecol 38(3):231–325
Van Mieghem T, Gucciardo L, Lewi P, Lewi L, Van Schoubroeck D, Devlieger R et al (2009) Validation of the fetal myocardial performance index in the second and third trimesters of gestation. Ultrasound Obstet Gynecol 33(1):58–63
Wladimiroff JW, Huisman TW, Stewart PA (1991) Fetal cardiac flow velocities in the late 1st trimester of pregnancy: a transvaginal Doppler study. J Am Coll Cardiol 17(6):1357–1359
Wong ML, Wong WH, Cheung YF (2007) Fetal myocardial performance in pregnancies complicated by gestational impaired glucose tolerance. Ultrasound Obstet Gynecol 29(4):395–400
Zerguini N, Leger P, Aubert S, Ray R, Ouattara A et al (2008) Tei index to assess perioperative left ventricular systolic function in patients undergoing mitral valve repair. Br J Anaesth 101(4):479–485
Zhang YQ, Sun K, Zhu SL, Wu LP, Chen GZ, Zhang ZF et al (2008) Doppler myocardial performance index in assessment of ventricular function in children with single ventricles. World J Pediatr 4(2):109–113
Acknowledgments
The authors gratefully acknowledge their team of sonographers—John Bokowski, Margaret Durack, Michelle Valmonte—for their contributions in performing the majority of fetal echocardiographic studies.
Conflict of interest
The authors have no conflict of interest related to this study
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghawi, H., Gendi, S., Mallula, K. et al. Fetal Left and Right Ventricle Myocardial Performance Index: Defining Normal Values for the Second and Third Trimesters—Single Tertiary Center Experience. Pediatr Cardiol 34, 1808–1815 (2013). https://doi.org/10.1007/s00246-013-0709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-013-0709-1