Abstract
Chronic total occlusions (CTO) are found commonly in patients with prior coronary artery bypass grafting (CABG). We sought to determine the effect of CABG on collateral robustness in patients with a CTO. Patients with a CTO diagnosed on coronary angiography between July 2010 and December 2019 were included in this study. Patients were classified as either CTO supplied by a functional graft, CTO supplied by collaterals from a non-grafted donor vessel (non-grafted) or a CTO supplied by collaterals from a grafted donor vessel (grafted). The degree of collateral robustness was determined by the Rentrop classification and collateral connection (CC) grade. Demographic, angiographic and clinical outcomes were recorded. A total of 2088 CTO lesions were identified, of which 878 (42.0%) were supplied by a functional graft, 994 (47.6%) CTOs were supplied by a non-grafted donor vessel and 216 (10.3%) CTOs were supplied by a grafted donor vessel. CTOs supplied by a grafted donor vessel had lower rates of robust collaterals (37.0% vs 83.0%, p < 0.0001) with less mature collaterals as determined by the Rentrop grade (p < 0.0001) and CC grade (p < 0.0001) as compared to CTOs supplied by a non-grafted donor vessel. In patients with a previous CABG, a grafted donor vessel results in less robust coronary collaterals with lower Rentrop and CC grade compared to an ungrafted donor vessel. This may be attributable to changes in coronary blood flow and shear stress, and may be a factor in the lower procedural success rates for CTO intervention in patients with prior CABG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A coronary chronic total occlusion (CTO) is defined as the presence of TIMI 0 flow in a epicardial vessel present for 3 months, appreciated angiographically as the presence of late filling of the occluded vessel by collaterals [1]. The true incidence of a CTO has varied widely in the published literature, with up to 90% in patients undergoing angiography with a history of prior coronary artery bypass grafting (CABG) [2], 15–30% in those without prior CABG [3] and 6.6% in those presenting with an acute coronary syndrome [4].
Bypass grafting results in significant alterations in coronary blood flow and consequently vascular shear stress in the native circulation distal to the graft [5]. As collateral recruitment and maturation is exquisitely related to vascular shear stress [6], bypass grafting may affect the collateral circulation. However, what impact the presence of a bypass graft, and consequent alterations in coronary blood flow have on coronary collaterals has not previously been assessed.
We sought to determine the effect of previous CABG on the coronary collateral circulation as determined by invasive angiography. We also sought to determine the effect of graft type and location on collateral recruitment as well as demographic, clinical and angiographic differences in patients with a CTO.
Methods
We reviewed all patients undergoing clinically indicated coronary angiography at a tertiary centre from July 2010 to December 2019. We identified patients who had a reported CTO through a commercially available reporting system on a local server (McKesson, Irving TX, USA). Patients presenting with ST elevation myocardial infarction (STEMI) or those with only ipsilateral collaterals such as bridging collaterals, were excluded. Procedural characteristics, baseline medications, in-hospital course along with left ventricular function, and biochemical results were reviewed using electronic medical records. Mortality and last medical contact was determined through medical record linking systems. Left ventricular function was assessed by transthoracic echocardiography, or if not performed, by ventriculography at the time of angiography.
Patients were divided into one of three groups based on the perfusion to the CTO (Fig. 1): CTO supplied by a functional graft, CTO supplied by collaterals from a non-grafted donor vessel or a CTO supplied by collaterals from a grafted donor vessel. A functional graft was defined as an arterial or venous graft through which there was perfusion to the myocardium subtended by the CTO. In those patients with a CTO supplied by a non-grafted or grafted vessel, the presence and degree of collaterals was graded according to the Rentrop classification [7]. Robust collaterals were defined as Rentrop grade 2 or 3, as has been done previously [8,9,10,11]. The Collateral Connection (CC) grade was also assessed [12] [Table 1]. The donor vessel was defined as the epicardial coronary artery from which collaterals arose. In cases where two vessels provided collaterals, the vessel from which the predominant collaterals arose was defined as the donor vessel. Stenosis in the donor vessel and graft was calculated using quantitative coronary angiography (QCA) (McKesson, Irving Tx, USA). In the setting of a grafted donor, this was assessed between the graft anastomosis and collaterals, thereby determining the degree of stenosis (and hence alteration to blood flow) impacting on the collaterals. Bypass grafts were recorded as left internal mammary artery, right internal mammary artery, saphenous vein graft or radial arterial graft. All coronary angiograms were reviewed by UKA, AE and NM, with a subset of patients having two blinded senior clinicians assessing collateral robustness to ensure a high degree of interobserver correlation. This methodology was chosen as previous studies have shown very low rates of interobserver or intraobserver variability for Rentrop grading [12, 13].
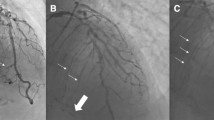
Differing perfusion of a chronic total occlusion of the right coronary artery. A Diagnostic angiography identifying a CTO of the right coronary artery (RCA) (7-point star). B a patent SVG graft (chevron) anastomosed to the distal RCA (4-point star) perfusing the CTO in both anterograde and retrograde manner. C A patent SVG graft (chevron) anastomosed to a diagonal artery (4-point star) perfusing the LAD in a retrograde manner which subsequently perfuses through septal collaterals (thin arrows) the occluded RCA (triangle). D Septal collaterals (thin arrows) from an ungrafted LAD supplying the occluded RCA (triangle)
Indication for angiography was defined as either emergent (unstable angina, non ST elevation myocardial infarction, ventricular arrhythmia or cardiac arrest not fulfilling criteria for STEMI) or non-emergent (stable angina or angina equivalent symptoms). Left ventricular impairment was defined as left ventricular ejection fraction (LVEF) ≤ 50% while valvular heart disease was defined as moderate or severe mitral or aortic valve disease. Medications were defined as regular medications being taken by the patient at the time of angiography. Project approval by the local human ethics committee was obtained.
Continuous variables were presented as means ± standard deviation in those with normally distributed data or medians and interquartile ranges in those with non-normally distributed data. Categorical data was presented as percentages. Comparisons between groups were performed using Pearson’s chi square test for all categorical variables. Continuous variables were firstly assessed by the Shapiro–Wilk test to ascertain normality of distribution, after which a student t-test was used for data that was normally distributed or Mann–Whitney U test for non-normally distributed continuous data. All tests were two-sided, and p < 0.05 was considered statistically significant. Analyses were performed using SPSS (version 24, IBM, New York, New York).
Results
A total of 2088 CTO lesions were included in the analysis with 959 (45.9%) not having had a previous CABG. Of the 1129 lesions with a previous CABG, 870 (77.1%) had a functional graft perfusing the CTO while 216 (19.1%) had a grafted donor vessel and 35 (3.1%) had a CTO supplied by either an ungrafted vessel or vessel with an occluded graft. Consequently, 994 (47.6%) CTOs were supplied by an ungrafted vessel, 216 (10.3%) CTOs supplied by a grafted vessel and 878 (42.0%) supplied by a functional graft (Fig. 2).
Patients with a CTO supplied by a grafted donor were less likely to have a LAD CTO (7.9% vs 23.6% vs 49.8%, p < 0.0001) and a lower left ventricular ejection fraction (45% vs 55% vs 50%, p < 0.0001) compared with those with a CTO supplied by an ungrafted donor or functional graft respectively. Patients with a CTO supplied by a functional graft were older (76.3 yrs vs 75.5 yrs vs 71.8 yrs, p < 0.0001) compared to those with a CTO supplied by a grafted donor or ungrafted donor respectively.
Table 2 shows the demographic and angiographic differences between the CTO supplied by a grafted donor compared with the CTO supplied by an ungrafted donor. Those with a grafted donor were older (74.0 yrs vs 70.5 yrs, p < 0.0001) and more likely to have multiple CTOs (90.8% vs 27.4%, p < 0.0001). Those with a grafted donor vessel had a lower degree of stenosis in the donor vessel, proximal to the collaterals (37.1% vs 50.8%, p < 0.0001). CTOs supplied by a grafted vessel had lower rates of robust collaterals (37.0% vs 83.0%, p < 0.0001) with less mature collaterals as determined by the Rentrop grade (p < 0.0001) and CC grade (p < 0.0001) (Fig. 3).
In CTOs supplied by a grafted donor, neither the graft type nor the site of anastomoses relative to the collaterals were associated with robustness of coronary collaterals. Similarly, there was no difference in the degree of stenosis in the donor vessel graft or donor vessel and robustness of coronary collaterals (Table 3).
Discussion
CTOs supplied by a grafted donor vessel have significantly poorer and less mature coronary collaterals as compared to those CTOs supplied by an ungrafted donor, irrespective of location of graft relative to collaterals, or type of graft. Previous studies have suggested that in patients undergoing CTO PCI, the presence of a prior CABG is associated with lower procedural success rates, higher risk of in-hospital mortality and higher complication rates [14, 15]. Histopathological studies [16] have shown that the presence of a bypass graft to a CTO is associated with more calcification and severe negative remodelling, which can also be appreciated angiographically following successful CTO PCI [17]. Whilst the degree of calcification is associated with lower CTO PCI success [18], poorer coronary collaterals are also associated with lower procedural success [19, 20], attributable not only to the ability to utilise collaterals as a retrograde option for intervention, but also reflecting better distal opacification and hence vessel visualisation. The finding of poorer collaterals may be a factor in the lower success rates of CTO PCI in this cohort.
Extensive calcification occurs in both proximal and distal segments to the CTO, providing a histological explanation for the clinical finding of more rapid progression of atherosclerosis in grafted coronary arteries [21, 22]. It has been postulated that blood stasis and lower shear stress resulting from competitive flow between the native vessel and the bypass graft may be the underlying mechanism of greater calcification [21, 23]. These alterations in flow and shear stress are also associated with poor collateral maturation. Coronary blood flow, particularly in the left coronary system is predominantly (> 60%) diastolic due to the effect of systolic myocardial compressive forces reducing coronary driving pressure and maximally increasing coronary vascular resistance [24]. However in certain situations, such as a non-dominant RCA, there is greater systolic flow owing to the thin walled right ventricle and low systolic intracavitatory pressure, resulting in lower vascular resistance. Similarly, in the setting of a dyskinetic or hypokinetic segment, graft and coronary systolic blood flow can significantly increase, thereby affecting endothelial shear stress and the ability to recruit collaterals [24]. Furthermore, canine studies have suggested that, in the acute setting, diastolic flow through a LIMA anastomosed to the LAD is significantly lower than in the normal setting [25]. Over time however, flow through the LIMA has a large diastolic component, characteristic of native coronary artery flow [26,27,28], with modulation from predominantly systolic flow proximally, to predominantly diastolic in the distal segment to match coronary vascular resistance [29]. SVGs, however, act as passive conduits with diastolic flow throughout their length [29]. Diastolic flow velocity in a LIMA graft is greater and more sustained than in an SVG [30] and as a result wall shear stress is higher [29]. The radial artery as a conduit is more susceptible to spasm [31], which may reflect its relative muscular structure and endothelial and smooth muscle cell response to platelet activation [31]. These dynamic and varied perturbations to flow and shear stress likely impact on the ability to recruit and mature coronary collaterals.
Computational fluid dynamic modelling suggests that wall shear stress is significantly lower in bypass grafts compared with native coronary flow, with resultant downstream reduction in shear stress in the native circulation [32]. In a doppler wire study [33], wall shear stress was greater in the LIMA as compared with the SVG, suggesting acceleration of atherosclerosis in the low flow state of a grafted coronary artery. This unfavourable low flow and decreased shear stress may also result in poorer collateral recruitment [6]. Although we did not detect any difference in robustness of collaterals in patients with an SVG graft compared with a LIMA graft, this requires further assessment with larger numbers. Similarly, the effect of the graft location relative to collaterals did not affect robustness of collaterals. In the setting of an occluded donor vessel, all flow will be in a single direction (either anterograde to normal flow, or retrograde to normal flow in the setting of graft landing distal to collaterals). However, in the setting of native flow in the donor vessel, there may be areas of competitive flow, which is associated with unfavourable wall shear stress, endothelial dysfunction and possible impairment of collateral recruitment [34].
In a previous study [35] of 217 patients, previous CABG was associated with improved collaterals, with a significantly lower rate of non-interventional collaterals compared with those patients who had not undergone prior CABG. Furthermore, they found no difference in patients with an occluded graft compared to those without prior grafts with respect to degree of collaterals. Despite more robust collaterals, the presence of a previous CABG was associated with a significantly lower rate of successful CTO PCI, although no differences in complication rates. Whilst the authors of that study did not quantify how they classified ‘non-interventional collaterals’, in the present study patients with a grafted donor vessel had a lower CC grade along with Rentrop grading compared to those without a grafted donor vessel. These differences may reflect a predilection to utilising an occluded graft itself for intervention to a native vessel CTO, thereby classifying a vessel as having ‘non-interventional collaterals’. Instead, the Rentrop classification which relates to contrast opacification of the occluded vessel removes any inherent bias in consideration of a collateral for intervention.
This is a single centre retrospective study which has limitations with respect to possible underlying bias, however the relatively large numbers allow hypothesis generating analysis of the data. The semi-quantitative method of collateral grading may have been influenced by degree of catheter engagement and duration of cine acquisition. However, robust collaterals are generally seen relatively early with a previous study suggesting that collaterals opacify the epicardial vessel in 20–30 frames [36], which in the setting of a cine acquisition of 15 frames per second, does not require prolonged injections and acquisition compared to standard care. Only anatomical assessment of collaterals was included in the study, and therefore it is possible that collateral perfusion through vessels not seen on angiography is possible. Future studies may benefit from other modalities of collateral flow assessment to determine the effect of bypass grafting on myocardial perfusion to a CTO [37]. However further assessment particularly to assess the impact of alterations in wall shear stress and flow dynamics is required to determine the impact of collateral maturation in patients with previous CABG and an ungrafted CTO. Furthermore angiograms prior to CABG were not reviewed, and it is possible that some collaterals were pre-existing to the CABG. However, as collaterals are dynamic, with rapid regression and recruitment, the influence of flow alterations following grafting are likely to result in alterations in the angiographically appreciated collaterals, and hence more relevant for the current study. Finally, only 18% of patients in the study were females, and while this is consistent with real world data from international registries [38], sex specific effect of collateral robustness may need to be considered in future studies.
Conclusions
In patients with a previous CABG, a donor vessel which is supplied by a bypass graft results in less robust coronary collaterals and less interventional coronary collaterals. This may be a factor in the lower procedural success rates for CTO intervention in patients with prior CABG. Further research into the effect of alterations in coronary flow dynamics on collateral recruitment and maturation in the setting of CABG should be considered.
References
Allahwala UK, Brilakis ES, Byrne J, Davies JE, Ward MR, Weaver JC, Bhindi R (2019) Applicability and interpretation of coronary physiology in the setting of a chronic total occlusion. Circ Cardiovasc Interv 12:e007813. https://doi.org/10.1161/CIRCINTERVENTIONS.119.007813
Jeroudi OM, Alomar ME, Michael TT, El Sabbagh A, Patel VG, Mogabgab O, Fuh E, Sherbet D, Lo N, Roesle M, Rangan BV, Abdullah SM, Hastings JL, Grodin J, Banerjee S, Brilakis ES (2014) Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital. Catheter Cardiovasc Interv 84:637–643. https://doi.org/10.1002/ccd.25264
Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR (2005) Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol 95:1088–1091. https://doi.org/10.1016/j.amjcard.2004.12.065
Allahwala UK, Jolly SS, Dzavik V, Cairns JA, Kedev S, Balasubramanian K, Stankovic G, Moreno R, Valettas N, Bertrand O, Lavi S, Velianou JL, Sheth T, Meeks B, Brilakis ES, Bhindi R (2018) The Presence of a CTO in a non-infarct-related artery during a STEMI treated with contemporary primary PCI is associated with increased rates of early and late cardiovascular morbidity and mortality: the CTO-TOTAL substudy. JACC Cardiovasc Interv 11:709–711. https://doi.org/10.1016/j.jcin.2017.12.005
Ghista DN, Kabinejadian F (2013) Coronary artery bypass grafting hemodynamics and anastomosis design: a biomedical engineering review. Biomed Eng Online 12:129. https://doi.org/10.1186/1475-925X-12-129
Allahwala UK, Khachigian LM, Nour D, Ridiandres A, Billah M, Ward M, Weaver J, Bhindi R (2020) Recruitment and maturation of the coronary collateral circulation: current understanding and perspectives in arteriogenesis. Microvasc Res 132:104058. https://doi.org/10.1016/j.mvr.2020.104058
Rentrop KP, Cohen M, Blanke H, Phillips RA (1985) Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 5:587–592. https://doi.org/10.1016/s0735-1097(85)80380-6
Allahwala UK, Weaver JC, Nelson GI, Nour D, Ray M, Ciofani JL, Ward M, Figtree G, Hansen P, Bhindi R (2020) Effect of recruitment of acute coronary collaterals on in-hospital mortality and on left ventricular function in patients presenting with ST elevation myocardial infarction. Am J Cardiol 125:1455–1460. https://doi.org/10.1016/j.amjcard.2020.02.023
Allahwala UK, Nour D, Alsanjari O, Bhatia K, Nagaraja V, Khatri JJ, Cockburn J, Hildick-Smith D, Sakata Y, Ward M, Weaver JC, Bhindi R (2020) Prognostic implications of the rapid recruitment of coronary collaterals during ST elevation myocardial infarction (STEMI): a meta-analysis of over 14,000 patients. J Thromb Thrombolysis. https://doi.org/10.1007/s11239-020-02282-6
Allahwala U, Nour D, Bhatia K, Ward M, Lo S, Weaver JC, Bhindi R (2020) Prognostic impact of collaterals in patients with a coronary chronic total occlusion (CTO): a meta-analysis of over 3000 patients. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.29348
Allahwala UK, Weaver JC, Bhindi R (2020) Spontaneous coronary collateral recruitment in patients with recurrent ST elevation myocardial infarction (STEMI). Heart Vessels 35:291–296. https://doi.org/10.1007/s00380-019-01493-z
Werner GS, Ferrari M, Heinke S, Kuethe F, Surber R, Richartz BM, Figulla HR (2003) Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation 107:1972–1977. https://doi.org/10.1161/01.CIR.0000061953.72662.3A
Tasolar H, Balli M, Cetin M, Otlu YO, Altun B, Bayramoglu A (2015) Effects of the coronary collateral circulation on the Tp-e interval and Tp-e/QT ratio in patients with stable coronary artery disease. Ann Noninvasive Electrocardiol 20:53–61. https://doi.org/10.1111/anec.12173
Nikolakopoulos I, Choi JW, Khatri JJ, Alaswad K, Doing AH, Dattilo P, Abi Rafeh N, Maalouf A, Abou Jaoudeh F, Tamez H, Shah A, Gkargkoulas F, Lembo NJ, Parikh M, Kirtane AJ, Ali ZA, Vemmou E, Xenogiannis I, Rangan BV, Abdullah S, Banerjee S, Garcia S, Burke MN, Brilakis ES, Karmpaliotis D (2020) Follow-up outcomes after chronic total occlusion percutaneous coronary intervention in patients with and without prior coronary artery bypass graft surgery: insights from the PROGRESS-CTO registry. J Invasive Cardiol 32:315–320
Liu MJ, Chen CF, Gao XF, Liu XH, Xu YZ (2020) In-hospital outcomes of chronic total occlusion percutaneous coronary intervention in patients with and without prior coronary artery bypass graft: a protocol for systematic review and meta analysis. Medicine (Baltimore) 99:e19977. https://doi.org/10.1097/MD.0000000000019977
Sakakura K, Nakano M, Otsuka F, Yahagi K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R (2014) Comparison of pathology of chronic total occlusion with and without coronary artery bypass graft. Eur Heart J 35:1683–1693. https://doi.org/10.1093/eurheartj/eht422
Allahwala UK, Ward MR, Bhindi R (2018) Change in the distal vessel luminal diameter following chronic total occlusion revascularization. Cardiovasc Interv Ther 33:345–349. https://doi.org/10.1007/s12928-017-0491-8
Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Hinohara T, Tanaka H, Mitsudo K, Investigators JCR (2011) Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv 4:213–221. https://doi.org/10.1016/j.jcin.2010.09.024
Allahwala UK, Kott K, Bland A, Ward M, Bhindi R (2020) Predictors and prognostic implications of well-matured coronary collateral circulation in patients with a chronic total occlusion (CTO). Int Heart J 61:223–230. https://doi.org/10.1536/ihj.19-456
Allahwala UK, Nour D, Bhatia K, Ward MR, Lo S, Weaver JC, Bhindi R (2020) Prognostic impact of collaterals in patients with a coronary chronic total occlusion: a meta-analysis of over 3,000 patients. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.29348
Hwang MH, Meadows WR, Palac RT, Piao ZE, Pifarre R, Loeb HS, Gunnar RM (1990) Progression of native coronary artery disease at 10 years: insights from a randomized study of medical versus surgical therapy for angina. J Am Coll Cardiol 16:1066–1070. https://doi.org/10.1016/0735-1097(90)90533-u
Maurer BJ, Oberman A, Holt JH Jr, Kouchoukos NT, Jones WB, Russell RO Jr, Reeves TJ (1974) Changes in grafted and nongrafted coronary arteries following saphenous vein bypass grafting. Circulation 50:293–300. https://doi.org/10.1161/01.cir.50.2.293
Chiu JJ, Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91:327–387. https://doi.org/10.1152/physrev.00047.2009
Olinger GN, Mulder DG, Maloney JV Jr, Buckberg GD (1976) Phasic coronary flow: intraoperative evaluation of flow distribution, myocardial function, and reactive hyperemic response. Ann Thorac Surg 21:397–404. https://doi.org/10.1016/s0003-4975(10)63887-8
Tedoriya T, Kawasuji M, Ueyama K, Sakakibara N, Takemura H, Watanabe Y (1993) Physiologic characteristics of coronary artery bypass grafts. Ann Thorac Surg 56:951–956. https://doi.org/10.1016/0003-4975(93)90362-l
Dobrin P, Canfield T, Moran J, Sullivan H, Pifarre R (1977) Coronary artery bypass. The physiological basis for differences in flow with internal mammary artery and saphenous vein grafts. J Thorac Cardiovasc Surg 74:445–454
Fujiwara T, Kajiya F, Kanazawa S, Matsuoka S, Wada Y, Hiramatsu O, Kagiyama M, Ogasawara Y, Tsujioka K, Katsumura T (1988) Comparison of blood-flow velocity waveforms in different coronary artery bypass grafts. Sequential saphenous vein grafts and internal mammary artery grafts. Circulation 78:1210–1217. https://doi.org/10.1161/01.cir.78.5.1210
de Bono DP, Samani NJ, Spyt TJ, Hartshorne T, Thrush AJ, Evans DH (1992) Transcutaneous ultrasound measurement of blood-flow in internal mammary artery to coronary artery grafts. Lancet 339:379–381. https://doi.org/10.1016/0140-6736(92)90076-f
Bach RG, Kern MJ, Donohue TJ, Aguirre FV, Caracciolo EA (1993) Comparison of phasic blood flow velocity characteristics of arterial and venous coronary artery bypass conduits. Circulation 88:II133-140
Fusejima K, Takahara Y, Sudo Y, Murayama H, Masuda Y, Inagaki Y (1990) Comparison of coronary hemodynamics in patients with internal mammary artery and saphenous vein coronary artery bypass grafts: a noninvasive approach using combined two-dimensional and Doppler echocardiography. J Am Coll Cardiol 15:131–139. https://doi.org/10.1016/0735-1097(90)90188-u
Chardigny C, Jebara VA, Acar C, Descombes JJ, Verbeuren TJ, Carpentier A, Fabiani JN (1993) Vasoreactivity of the radial artery. Comparison with the internal mammary and gastroepiploic arteries with implications for coronary artery surgery. Circulation 88:II115-127
Goubergrits L, Affeld K, Wellnhofer E, ZurbruggR HT (2001) Estimation of wall shear stress in bypass grafts with computational fluid dynamics method. Int J Artif Organs 24:145–151
Shimizu T, Ito S, Kikuchi Y, Misaka M, Hirayama T, Ishimaru S, Yamashina A (2004) Arterial conduit shear stress following bypass grafting for intermediate coronary artery stenosis: a comparative study with saphenous vein grafts. Eur J Cardiothorac Surg 25:578–584. https://doi.org/10.1016/j.ejcts.2003.12.039
Nordgaard H, Swillens A, Nordhaug D, Kirkeby-Garstad I, Van Loo D, Vitale N, Segers P, Haaverstad R, Lovstakken L (2010) Impact of competitive flow on wall shear stress in coronary surgery: computational fluid dynamics of a LIMA-LAD model. Cardiovasc Res 88:512–519. https://doi.org/10.1093/cvr/cvq210
Budassi S, Zivelonghi C, Dens J, Bagnall AJ, Knaapen P, Avran A, Spratt JC, Walsh S, Faurie B, Agostoni P, of all the Ri (2020) Impact of prior coronary artery bypass grafting in patients undergoing chronic total occlusion-percutaneous coronary intervention: procedural and clinical outcomes from the REgistry of crossboss and hybrid procedures in FrAnce, the NetheRlands, BelGium, and UnitEd Kingdom (RECHARGE). Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.28954
Gatzov P, Manginas A, Voudris V, Pavlides G, Genchev GD, Cokkinos DV (2003) Blood flow velocity in donor coronary artery depends on the degree and pattern of collateral vessel development: a study using thrombolysis in myocardial infarction frame count method. Catheter Cardiovasc Interv 60:462–468. https://doi.org/10.1002/ccd.10694
Allahwala UK, Brilakis ES, Kiat H, Ayesa S, Nour D, Ward M, Lo S, Weaver JC, Bhindi R (2020) The indications and utility of adjunctive imaging modalities for chronic total occlusion (CTO) intervention. J Nucl Cardiol. https://doi.org/10.1007/s12350-020-02381-0
Vemmou E, Alaswad K, Khatri JJ, Nikolakopoulos I, Karacsonyi J, Xenogiannis I, Karmpaliotis D, Garcia S, Burke MN, Brilakis ES (2020) Patient radiation dose during chronic total occlusion percutaneous coronary intervention: insights from the PROGRESS-CTO registry. Circ Cardiovasc Interv 13:e009412. https://doi.org/10.1161/CIRCINTERVENTIONS.120.009412
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors confirm no potential conflict of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Allahwala, U.K., Ekmejian, A., Mughal, N. et al. Impact of coronary artery bypass grafting (CABG) on coronary collaterals in patients with a chronic total occlusion (CTO). Int J Cardiovasc Imaging 37, 3373–3380 (2021). https://doi.org/10.1007/s10554-021-02327-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02327-8