Abstract
Acute coronary collateralisation of an infarct-related arterial (IRA) territory may be identified during angiography for ST elevation myocardial infarction (STEMI). Whether the presence or absence of these collaterals affects outcomes remains uncertain. A search of EMBASE, MEDLINE and Cochrane Library, using the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines was conducted to identify studies which reported on the association between coronary collaterals and in-hospital and longer term mortality, left ventricular ejection fraction (LVEF), risk of repeat acute myocardial infarction (AMI) and repeat revascularisation. Patients with Rentrop grade 0 or 1 were defined as poor collaterals whilst those with Rentrop grade two or three were defined as those with robust collaterals. Studies were eligible if they included patients ≥ 18 years of age who had immediate coronary angiography for STEMI. Included studies were observational which recorded the degree of collateral blood flow to the IRA. Two investigators reviewed all citations using a predefined protocol with final consensus for all studies, the data from which was then independently entered to ensure fidelity of results. Inverse variance random effects model for the meta-analysis along with risk of bias assessment was performed. 20 studies with a total of 14,608 patients were identified and included in the analysis. Patients with robust collaterals had lower mortality (OR 0.55, 95% CI 0.48–0.64), both in-hospital (OR 0.47, 95% CI 0.35–0.63) and longer term (OR 0.58, 95% CI 0.46–0.75). Patients with robust collaterals also had a higher mean LVEF (SMD 0.23, 95% CI 0.10–0.37). There was no difference in the rates of AMI or repeat revascularisation between patients with robust or poor collaterals. The presence of robust collaterals during STEMI is associated with reduced in-hospital and longer term mortality and improved left ventricular function. These findings have implications for prognostication and identifying patients who require close monitoring following STEMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
The presence of robust collaterals, visualised during ST elevation myocardial infarction (STEMI), perfusing the territory supplied by the occluded vessel, reduce risk of all-cause mortality.
-
The presence of robust collaterals visualised during a STEMI reduce both short and long term mortality.
-
The presence of robust collaterals visualised during a STEMI is associated with higher LVEF.
-
The degree of collaterals does not predict risk of AMI or revascularisation.
-
The molecular and cell signalling pathways by which collaterals are recruited should be further investigated.
Introduction
During angiography for percutaneous coronary intervention (PCI) in the setting of ST elevation myocardial infarction (STEMI), the finding of coronary collaterals, perfusing the territory distal to the occluded, infarct related artery (IRA), is frequently identified. Whilst the presence of a bystander chronic total occlusion (CTO), with collaterals perfusing the pre-existing CTO is a well described predictor of poorer outcomes [1], the rapid recruitment of collaterals to perfuse the acutely occluded IRA is a separate and distinct entity. Whether the presence of these collaterals is associated with improved prognosis remains uncertain. We performed a systematic review and meta-analysis to examine the impact of rapidly recruited coronary collaterals on risk of mortality, left ventricular function, recurrent acute myocardial infarction (AMI) and repeat revascularisation, in patients presenting with STEMI.
Methods
Search strategy
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines to formulate and conduct the search strategy [2]. We performed a computerised systematic search through MEDLINE, EMBASE, and the Cochrane Library databases. The search was conducted in MEDLINE from 1946–January 10th 2020, EMBASE from 1974–January 10th 2020 and Cochrane Library from 2003–January 10th 2020 and then repeated until March 15th 2020 to identify newer published data. All relevant subject headings as well as free text terms relating to coronary collaterals and STEMI were used. The keywords were searched as text words as well as exploded medical subject headings when feasible. The search strategy for MEDLINE was as follows; (1) Collateral Circulation/(12,137), (2) collateral*.mp. (45,969), (3) Rentrop.mp. (303), (4) CCS.mp (5746), (5) Collateral Connection Score.mp. (3099), (6) 1 or 2 or 3 or 4 or 5 (51,682), (7) ST Elevation Myocardial Infarction/ (3099), (8) STEMI.mp. (10,410), (9) 7 or 8, (10) 6 and 9 (120). The search strategy for EMBASE was (1) Collateral circulation/ (11,049), (2) collateral artery/(409), (3) coronary artery collateral circulation/(1756), (4) collateral*.mp. (56,811), (5) Rentrop.mp. (569), (6) CCS.mp. (9630), (7) Collateral Connection Score.mp. (5), (8) 1 or 2 or 3 or 4 or 5 or 6 or 7 (66,357), (9) ST segment elevation myocardial infarction/ (36,557), (10) STEMI.mp. (27,144), (11) 9 or 10 (41,450), (12) 8 and 11 (434). The search strategy is shown in Appendix 1. We further screened references from the retrieved and included studies as well as prior meta-analyses for any relevant studies which were not retrieved through the initial search.
Data source and searching
Articles were eligible for inclusion if they included patients ≥ 18 years of age who had immediate coronary angiography for STEMI, within 12 h of onset of chest pain or if evidence of ongoing ischaemia. Eligible study designs were ones which recorded the degree of collateral blood flow to the IRA prior to intervention. To standardise the degree of collaterals, we included studies, which quantified the collaterals based upon the Rentrop classification [3], where grade 0 = no filling of any collateral channel; Grade 1 = filling of the side branches of the infarct related artery; Grade 2 = Partial filling of the epicardial vessel of the infarct related artery’ Grade 3 = complete filling of the epicardial vessel. For the analysis, we grouped patients with Rentrop grade zero or one as poor collateral recruiters, whilst those with Rentrop grade two or three were classified as robust collateral recruiters, a dichotomy which was done in the majority of previously published studies, as this corresponds with the degree of perfusion of the occluded vessel.
Two investigators (U.A., D.N.) reviewed all citations identified through the literature search using a predefined protocol. Articles that clearly did not meet inclusion criteria were excluded at the title and/or abstract level. The remaining articles were selected for full text review. When limited information was available from the abstract, full text was always obtained. If further details were sought, the corresponding authors of the study were contacted to obtain further information. Disagreements regarding the selection and quality assessment of articles were resolved through discussion, and full consensus was achieved at each stage of review. Both investigators (U.A., D.N.) also independently entered results and outcomes into separate predetermined tables to ensure fidelity of results. Ethics approval was not deemed necessary given this was a meta-analysis of previously published data.
The outcomes of interest were all-cause mortality, left ventricular function, risk of recurrent AMI and need for repeat revascularisation. We further classified time course of mortality as in-hospital and short term, defined as ≤ 6 months follow up, or longer term mortality, defined as ≥ 12 months follow up. The Newcastle‐Ottawa Scale (NOS) [4] was used to assess the quality of observational studies and risk of bias. The overall quality of the study was determined semi-quantitatively using the Agency for Healthcare Research and Quality (AHRQ) standards [5], whereby studies were graded of good quality, fair quality or poor quality, based upon scoring of the NOS.
Data analysis
For dichotomous outcomes, odds ratio was chosen to present data, whilst for continuous variables, the standardised mean difference (SMD) was used to present data. For continuous variables, the mean and standard deviation was recorded. If studies only presented medians and interquartile ranges, the data was transformed into mean with standard deviations using previously validated methods [6]. The program Review Manager (version 5.3) was used to conduct an inverse variance random effects model for the meta-analysis. The Cochrane Q‐statistic (I2) was used to assess the consistency among studies, with I2 < 25% considered low, I2 > 50% moderate, and I2 > 75% high heterogeneity [7]. Publication bias was estimated visually by funnel plots, where publication bias was considered unlikely if the plot resembles an inverted funnel, and was further tested by using the weighted regression test of Egger [7].
Results
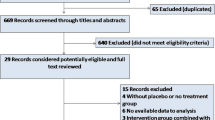
After removing duplicate studies, a total of 554 studies were screened of which, a final 20 studies with 14,608 patients were included in the analysis (Fig. 1). Twelve of the studies [8,9,10,11,12,13,14,15,16,17,18,19] were prospective observational studies, 6 [20,21,22,23,24,25] were retrospective observational, and 2 [26, 27] were retrospective analysis of randomised control trials of patients presenting with STEMI. One study included only patients ≥ 65 years [21], two included only patients with an anterior STEMI, whereby the left anterior descending artery was the IRA [14, 24], one included only patients with inferior STEMI [19] and one included only patients with cardiogenic shock complicating STEMI [18]. Included studies reported on rapidly recruited collaterals, perfusing the territory supplied by the IRA, distinct to preformed collaterals perfusing an existing CTO. All but two studies reported the degree of collateralisation using the Rentrop or equivalent scoring tool. In the study by Alsanjari et al. [8], collaterals were graded as “collaterals” or “no collaterals”. Given the fact that patients with visible collaterals would almost certainly be Rentrop grade two or three, we included this group as robust collateral recruiters whilst those without collaterals were defined as poor collateral recruiters. In the study by Wang et al. [24], patients were grouped as those with no collaterals, defined as Rentrop grade zero, and those with collaterals, which were defined as Rentrop grade 1–3, of which 77% were grade one. For the purposes of this analysis, we characterised patients with visible collaterals as having robust collaterals. Two studies [8, 11] reported outcomes at 6 months, and longer term, with time specific data included in the analysis models.
Included studies were published between 1998 to 2020 with follow up periods between in-hospital alone to 5 years. The number of patients included in each study was between 96 to 3,340 with a mean age of 62.3. The percentage of patients with robust collaterals ranged between 10% and 41.3% with an average of 25.0% across all the studies. The thrombolysis in myocardial infarction (TIMI) flow of 0 in the infarct related artery at the time of angiography, whereby there is complete, persisting occlusion of the IRA, and is a strong predictor of collateral recruitment [20], ranged between 67.3% and 100%. The results of each study included in the analysis are summarised in Table 1. The NOS score for each study is presented in Supplementary Table 1, with 12 studies of a good quality, two fair quality and 6 of a poor quality.
Mortality
Patients with robust collateral recruitment had a lower risk of mortality compared to those with poor collateral recruitment (OR 0.55, 95% CI 0.48–0.64) throughout all included studies, with a very low degree of heterogeneity between studies (I2 = 17%, p = 0.24) (Fig. 2). Asymmetrical appearance of the funnel plot suggested that smaller studies, which do not show a significant mortality difference, were unpublished. Publication bias was supported by Eggers regression analysis (p < 0.01).
In the 11 studies assessing in-hospital and short term mortality, there was a lower rate of mortality in those patients with robust collaterals compared to those with poor collaterals, (OR 0.47, 95%CI 0.35–0.63) (Fig. 3). There was a low degree of heterogeneity between these studies (I2 = 23%, p = 0.22). Publication bias was suggested by both an asymmetrical appearance of the funnel plot, whereby smaller studies which do not show a significant mortality difference were unpublished, and Eggers regression analysis (p < 0.01).
In the 11 studies assessing longer term mortality, patients with robust collateral recruitment had a lower risk of mortality compared to patients with poor collateral recruitment (OR 0.58, 95% CI 0.46–0.75) (Fig. 4). In these studies, there was a very low degree of heterogeneity (I2 = 22%, p = 0.23), whilst there was no evidence of publication bias, with a symmetrical appearance of the funnel plot and Eggers regression analysis (p = 0.13).
As the study of Alsanjari et al. [8] and Hara et al. [11] accounted for 65.3% of the weighted analysis for mortality, sensitivity analysis was performed excluding these 2 heavily weighted studies. After their exclusion, patients with robust collateral recruitment had a lower risk of mortality compared to those with poor collateral recruitment, (OR 0.40, 95% CI 0.31–0.52, p < 0.0001) with no evidence of heterogeneity (I2 = 0%, p = 0.58) and no evidence of publication bias, with a symmetrical appearance of the funnel plot and by Eggers regression analysis (p = 0.35). Similarly, patients with robust collaterals had a lower risk of in-hospital and short term mortality compared to those with poor collaterals (OR 0.36, 95% CI 0.26–051,p < 0.0001) with no evidence of heterogeneity (I2 = 0%, p = 0.94) or evidence of publication bias with a symmetrical appearance of the funnel plot and by Eggers regression analysis (p = 0.47). Patients with robust collaterals had a lower risk of longer term mortality compared to those with poor collaterals (OR 0.47, 95% CI 0.28–0.78, p < 0.01) with low degree of heterogeneity (I2 = 27%, p = 0.20) and no evidence of publication bias, with a symmetrical appearance of the funnel plot and by Eggers regression (p = 0.34) (Supplementary Table 2).
Left ventricular function
Four studies reported on rates of left ventricular impairment, which was defined as < 50% in three studies [8, 13, 20] and not defined in another [18].There was no difference in rates of left ventricular impairment between those with robust collateral recruitment compared to those with poor collateral recruitment (OR 0.69, 95% CI 0.29–1.66) There was, however, a high degree of heterogeneity between studies (I2 = 93%, p < 0.0001) with no evidence of publication bias, with a symmetrical appearance of the funnel plot and by Eggers regression (p = 0.12) (Fig. 5). A total of 13 studies reported mean left ventricular ejection fractions (LVEF), which was performed in-hospital in 11 studies [10, 15, 16, 19, 20, 22, 23, 25,26,27,28], and 6 months in two studies [9, 17]. Patients with robust collaterals had a significantly higher standard mean LVEF compared to those with poor collaterals (standard mean difference (SMD) 0.23, 95% CI 0.10–0.37). There was however a high degree of heterogeneity between studies (I2 = 81%, p < 0.0001) with no evidence of publication bias, with a symmetrical appearance of the funnel plot and by Eggers regression (p = 0.41) (Fig. 6).
AMI and repeat revascularisation
Ten studies analysed the effect of collateral recruitment on risk of recurrent AMI. There was no difference between patients with robust collaterals or poor collaterals on risk of AMI (OR 0.82, 95% CI 0.50–1.34). There was very low heterogeneity between studies (I2 = 0%, p = 0.94) with no evidence of publication bias, with a symmetrical appearance of the funnel plot and by Eggers regression (p = 0.06) (Fig. 7).
Seven studies analysed the effect of collateral recruitment on risk of repeat revascularisation. There was no difference between patients with robust collaterals or poor collaterals on risk of repeat revascularisation (OR 0.99, 95% CI 0.73–1.34). There was very low heterogeneity between studies (I2 = 0%, p = 0.57) with no evidence of publication bias, with a symmetrical appearance of the funnel plot and by Eggers regression (p = 0.15) (Fig. 8).
Discussion
In this meta-analysis of 20 studies with more than 14,500 patients, we found that in those presenting with STEMI, the angiographic appearance of robust collaterals is associated with lower mortality, both in the short term and longer term, with an associated higher LVEF. More robust coronary collaterals, with partial, or complete retrograde perfusion of the occluded epicardial artery, allow oxygenated blood to perfuse the myocardial territory subtended by the IRA. It is perhaps unsurprising that increased perfusion would result in improved LVEF. Whilst the rates of left ventricular impairment did not meet statistical significance, three of the four studies [8, 13, 18] did not report LVEF. In the studies which did report mean ejection fractions, this was significantly higher in patients with robust collaterals compared to those with poor collaterals. It is possible that a greater proportion of those patients with poor collaterals had more severe left ventricular impairment, which may explain these apparent conflicting findings. Whilst the absolute difference in LVEF was small, there was a sustained benefit derived from all studies suggesting the protective effects of collaterals is maintained.
Perhaps driven by the protective effect on left ventricular function, mortality, both in-hospital and short term, as well as longer term was lower in those with robust collaterals. Despite contemporary advancements in management of STEMI, as well as improved post infarct management, mortality rates at 1 year remain at 4.3–4.5% [29]. It is possible that the degree of intrinsic collateral recruitment may explain this remaining mortality penalty, despite optimal management and timely revascularisation. With respect to short-term mortality outcomes, there was high agreement amongst the studies. Furthermore, two studies [8, 11] reported both short and longer term outcomes. The study by Hara et al. [11] showed a trend toward reduced in-hospital mortality in those with robust collaterals (OR 0.80 95% CI 0.57–1.12), whilst at long term follow up of 5 years, there was a clear survival advantage (OR 0.68 95% CI 0.52–0.88). This delay may be explained by the protective effects of collaterals on left ventricular function, with subsequent longer term survival advantage [30]. Alsanjari et al. [8] showed a significant in-hospital mortality benefit which was sustained out to 5 years, suggesting the acute benefit of myocardial salvage persists over time. Even after excluding the studies by Alsanjari et al. [8] and Hara et al. [11] which accounted for a large percentage of weight for the analysis, the presence of robust collaterals remained predictive of total, short-term and long term mortality without evidence of heterogeneity or publication bias, suggesting a sustained and ubiquitous protective effect of collaterals on mortality.
The study by Kim et al. [13], was the only study which suggested a trend toward higher mortality with those with robust collaterals, although this did not reach significance. This may be an issue with respect to appropriate power, as the study was designed as an MRI based study, and found beneficial effects of robust collaterals with respect to smaller infarct size and smaller area at risk. Alternatively, the phenomenon of “collateral decay [31]” which has been described in the setting of a CTO, whereby robust collaterals seen at the time of an acute infarct are no longer evident on repeat angiography may conceivably apply in the STEMI setting to explain these apparent incongruous findings.
There were no differences in risk of recurrent acute myocardial infarction or repeat revascularisation in patients with robust or poor coronary collaterals. Whilst the ability to recruit collaterals is stable in subsequent STEMI presentations [32], it appears that spontaneous collateral recruitment may be protective following onset of acute myocardial infarction rather than influencing risk of subsequent vascular events. Therefore, the innate ability to recruit collaterals appears to be protective once an infarct has commenced rather than preventative, as it does not preclude acute plaque rupture and thromboembolic occlusion.
The incidence of angiographically evident robust coronary collaterals was on average 25% of patients presenting with STEMI. Whilst the mechanisms by which some patients are able to recruit collaterals is uncertain, a combination of patient specific factors (younger age), anatomical considerations (right coronary artery as the culprit vessel) as well as history of angina and presence of more severe bystander coronary disease, mediated through multiple growth factors and cytokines have been previously postulated [20]. Given the protective effects of collateral recruitment, further research is required to ascertain whether adjuvant pharmacological or mechanical treatment may facilitate in their recruitment, to improve outcomes.
The findings of the study are similar to a previous meta-analysis on the impact of collaterals in STEMI [28], although our meta-analysis includes six more studies with 4000 more patients Furthermore, the previous analysis included one study which appraised the effect of collaterals to a concomitant CTO during STEMI, a quite distinct situation to rapidly recruited collaterals to the IRA during STEMI, which may introduce some degree of bias in the results. This confirmation of results, further emphasizes the need to identify biological and mechanistic basis for collateral recruitment.
Limitations
Despite the relative consistent findings between studies of our analysis, there are important limitations which need to be considered. Firstly, all included studies were observational studies, including two which were based on the initial angiographic findings in randomised control trials, prior to intervention, and presented unadjusted outcomes, which may introduce bias into the selection of data although the very nature of spontaneously recruited collaterals suggests that only observational studies are possible. The baseline demographics were relatively similar; however the IRA was variable and in some studies, excluded specific IRA (51,923). Furthermore patient’s background, in particular prior history of angina and or coronary artery disease, particularly disease in the contralateral donor vessel and prior history of angina were not considered and as such may have influenced the degree of collateral recruitment and hence outcomes. Although these collaterals were supplying the territory subtended by an acutely occluded vessel in the setting of a STEMI, it is possible that they were preformed rather than acutely recruited. However, given that the majority of the culprit lesions in patients with STEMI have a mild to moderate degree of stenosis prior to occlusion [33, 34], this is unlikely to be the case. Given included studies ranged from 1998 to 2020, it is conceivable that patient characteristics and management strategy changes may have influenced outcomes, which is an inherent limitation of pooled results. However, whilst studies have shown that patients with STEMI are younger and more likely to have traditional cardiovascular risk factors as compared to 20 years ago, there has been no change in mortality in patients who underwent reperfusion therapy [35]. Given the protective effects of collaterals is likely in the short term prior to revascularisation, it is unlikely however that there is significant bias from including these studies. Another anatomical consideration was that included studies looked at the presence of retrograde filling via contralateral collaterals, and the impact of the presence of anterograde, bridging collaterals was not taken into consideration. Whilst the majority of observed collaterals are from the contralateral vessel, nevertheless this may impact on coronary perfusion and may independently affect prognosis. Finally, it is possible, that there may have been insufficient statistical power to detect associations for some outcomes.
Conclusions
The ability to recruit robust coronary collaterals during a STEMI is associated with a lower in-hospital, short term and longer term mortality as compared to those patients who cannot recruit sufficient collaterals. Similarly, robust collateral recruitment is associated with improved left ventricular function following STEMI, which may be the mechanism by which this survival advantage is achieved. These findings have implications in identifying patients who may benefit from closer monitoring and prognostication in the post infarct setting.
References
Allahwala UK, Jolly SS, Dzavik V, Cairns JA, Kedev S, Balasubramanian K, Stankovic G, Moreno R, Valettas N, Bertrand O, Lavi S, Velianou JL, Sheth T, Meeks B, Brilakis ES, Bhindi R (2018) The presence of a CTO in a non-infarct-related artery during a STEMI treated with contemporary primary PCI is associated with increased rates of early and late cardiovascular morbidity and mortality: the CTO-TOTAL substudy. JACCCardiovascInterv 11(7):709–711. https://doi.org/10.1016/j.jcin.2017.12.005
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Rentrop KP, Cohen M, Blanke H, Phillips RA (1985) Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am CollCardiol 5(3):587–592. https://doi.org/10.1016/s0735-1097(85)80380-6
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2019) The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 22 Dec 2019
Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, Treadwell JR (2008) Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions.In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Methods for Effective Health Care. Rockville (MD)
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Alsanjari O, Chouari T, Williams T, Myat A, Sambu N, Blows L, Cockburn J, de Belder A, Hildick-Smith D (2019) Angiographically visible coronary artery collateral circulation improves prognosis in patients presenting with acute ST segment-elevation myocardial infarction. Catheter CardiovascInterv. https://doi.org/10.1002/ccd.28532
Antoniucci D, Valenti R, Moschi G, Migliorini A, Trapani M, Santoro GM, Bolognese L, Cerisano G, Buonamici P, Dovellini EV (2002) Relation between preintervention angiographic evidence of coronary collateral circulation and clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am J Cardiol 89(2):121–125. https://doi.org/10.1016/s0002-9149(01)02186-5
Desch S, de Waha S, Eitel I, Koch A, Gutberlet M, Schuler G, Thiele H (2010) Effect of coronary collaterals on long-term prognosis in patients undergoing primary angioplasty for acute ST-elevation myocardial infarction. Am J Cardiol 106(5):605–611. https://doi.org/10.1016/j.amjcard.2010.04.010
Hara M, Sakata Y, Nakatani D, Suna S, Nishino M, Sato H, Kitamura T, Nanto S, Hori M, Komuro I, Investigators O (2016) Impact of coronary collaterals on in-hospital and 5-year mortality after ST-elevation myocardial infarction in the contemporary percutaneous coronary intervention era: a prospective observational study. BMJ Open 6(7):e011105. https://doi.org/10.1136/bmjopen-2016-011105
Kajiya T, Yamashita M, Otsuji H, Toyonaga K, Lee S (2014) Assessment of coronary collateral artery by CT angiography in patients with ST-elevation acute myocardial infarction. Int J Cardiol 176(3):1359–1361. https://doi.org/10.1016/j.ijcard.2014.07.281
Kim EK, Choi JH, Song YB, Hahn JY, Chang SA, Park SJ, Lee SC, Choi SH, Choe YH, Park SW, Gwon HC (2016) A protective role of early collateral blood flow in patients with ST-segment elevation myocardial infarction. Am Heart J 171(1):56–63. https://doi.org/10.1016/j.ahj.2015.10.016
Perez-Castellano N, Garcia EJ, Abeytua M, Soriano J, Serrano JA, Elizaga J, Botas J, Lopez-Sendon JL, Delcan JL (1998) Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J Am CollCardiol 31(3):512–518. https://doi.org/10.1016/s0735-1097(97)00521-4
Rechcinski T, Jasinska A, Peruga JZ, Forys J, Krzeminska-Pakula M, Bednarkiewicz Z, Trzos E, Kasprzak JD (2013) Presence of coronary collaterals in ST-elevation myocardial infarction patients does not affect long-term outcome. Pol Arch Med Wewn 123(1–2):29–37. https://doi.org/10.20452/pamw.1587
Sen O, Allahverdiyev S, Topuz M, Baykan AO, Oz F, Koc M (2017) Clinical significance and determinants of prompt recruitment collaterals during primary percutaneous coronary intervention. KardiolPol 75(8):763–769. https://doi.org/10.5603/KP.a2017.0078
Shen Y, Wu F, Pan C, Zhu T, Zhang Q, Zhang R, Ding F, Lu L, Hu J, Yang Z, Shen W, Wu Z (2014) Clinical relevance of angiographic coronary collaterals during primary coronary intervention for acute ST-elevation myocardial infarction. Chin Med J (Engl) 127(1):66–71
Valim LR, Lopez ACA, Bienert RdC, Ribeiro HB, Campos CA, Esper RB, Zalc S, Perin MA, Lemos PA, Ribeiro EE, Ramires JAF (2011) Acute myocardial infarction complicated by cardiogenic shock: effect of collateral circulation in primary coronary intervention results–data from the incor registry. Rev Bras de CardiolInvasiva 19(2):166–171
Yaylak B, Altintas B, Ede H, Baysal E, Akyuz S, Bilge O, Sevuk U, Erdogan G, Ciftci H (2015) Impact of coronary collateral circulation on in-hospital death in patients with inferior ST elevation myocardial infarction. Cardiol Res Pract 2015:242686. https://doi.org/10.1155/2015/242686
Allahwala UK, Weaver JC, Nelson GI, Nour D, Ray M, Ciofani JL, Ward M, Figtree G, Hansen P, Bhindi R (2020) Effect of recruitment of acute coronary collaterals on in-hospital mortality and on left ventricular function in patients presenting with ST elevation myocardial infarction. Am J Cardiol 125(10):1455–1460. https://doi.org/10.1016/j.amjcard.2020.02.023
Chu AA, Li W, Zhu YQ, Meng XX, Liu GY (2019) Effect of coronary collateral circulation on the prognosis of elderly patients with acute ST-segment elevation myocardial infarction treated with underwent primary percutaneous coronary intervention. Medicine (Baltimore) 98(31):e16502. https://doi.org/10.1097/MD.0000000000016502
Elsman P, van’t Hof AW, de Boer MJ, Hoorntje JC, Suryapranata H, Dambrink JH, Zijlstra F, Zwolle Myocardial Infarction Study G (2004) Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J 25(10):854 858. https://doi.org/10.1016/j.ehj.2004.03.005
Hernandez-Perez FJ, Goirigolzarri-Artaza J, Restrepo-Cordoba MA, Garcia-Touchard A, Oteo-Dominguez JF, Silva-Melchor L, Fernandez-Diaz JA, Dominguez-Puente JR, Alonso-Pulpon L, Goicolea-Ruigomez J (2017) Impact of coronary collaterals on long-term prognosis in patients treated with primary angioplasty. Rev EspCardiol (Engl Ed) 70(3):178–185. https://doi.org/10.1016/j.rec.2016.09.023
Wang B, Han YL, Li Y, Jing QM, Wang SL, Ma YY, Wang G, Luan B, Wang XZ (2011) Coronary collateral circulation: effects on outcomes of acute anterior myocardial infarction after primary percutaneous coronary intervention. J GeriatrCardiol 8(2):93–98. https://doi.org/10.3724/SP.J.1263.2011.00093
Ying S, Feng W, Chunzang P, Tianqi Z, Qi Z, Ruiyan Z, Fenghua D, Lin L, Jian H, Zhenkun Y, Weifeng S, Zonggui W (2014) Clinical relevance of angiographic coronary collaterals during primary coronary intervention for acute ST-elevation myocardial infarction. Chin Med J 127(1):66–71
Freund A, Stiermaier T, de Waha S, Eitel I, Schock S, Lurz P, Thiele H, Desch S (2020) Coronary collaterals in patients with ST-elevation myocardial infarction presenting late after symptom onset. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01625-w
Sorajja P, Gersh BJ, Mehran R, Lansky AJ, Krucoff MW, Webb J, Cox DA, Brodie BR, Stone GW (2007) Impact of collateral flow on myocardial reperfusion and infarct size in patients undergoing primary angioplasty for acute myocardial infarction. Am Heart J 154(2):379–384. https://doi.org/10.1016/j.ahj.2007.04.034
Cui K, Lyu S, Song X, Yuan F, Xu F, Zhang M, Zhang M, Wang W, Zhang D, Tian J (2018) Effect of coronary collaterals on prognosis in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction: a meta-analysis. Angiology 69(9):803–811. https://doi.org/10.1177/0003319718768399
Jolly SS, Cairns JA, Yusuf S, Rokoss MJ, Gao P, Meeks B, Kedev S, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemela K, Bernat I, Cantor WJ, Cheema AN, Steg PG, Welsh RC, Sheth T, Bertrand OF, Avezum A, Bhindi R, Natarajan MK, Horak D, Leung RC, Kassam S, Rao SV, El-Omar M, Mehta SR, Velianou JL, Pancholy S, Dzavik V, Investigators T (2016) Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 387(10014):127–135. https://doi.org/10.1016/S0140-6736(15)00448-1
Ng VG, Lansky AJ, Meller S, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie B, Shah R, Mehran R, Stone GW (2014) The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur Heart J Acute Cardiovasc Care 3(1):67–77. https://doi.org/10.1177/2048872613507149
van Dongen IM, Elias J, Gert van Houwelingen K, Agostini P, Claessen BE, Hoebers LP, Ouweneel D, van der Schaaf RJ, Tijssen JG, Henriques JP (2017) TCT-387 collateral quality decay several days after primary PCI a novel observation from the EXPLORE trial. J Am CollCardiol. https://doi.org/10.1016/j.jacc.2017.09.483
Allahwala UK, Weaver JC, Bhindi R (2020) Spontaneous coronary collateral recruitment in patients with recurrent ST elevation myocardial infarction (STEMI). Heart Vessels 35(3):291–296. https://doi.org/10.1007/s00380-019-01493-z
Higuma T, Soeda T, Yamada M, Yokota T, Yokoyama H, Nishizaki F, Xing L, Yamamoto E, Bryniarski K, Dai J, Lee H, Okumura K, Jang IK (2016) Coronary plaque characteristics associated with reduced TIMI (thrombolysis in myocardial infarction) flow grade in patients with ST-segment-elevation myocardial infarction: a combined optical coherence tomography and intravascular ultrasound study. Circ CardiovascInterv. https://doi.org/10.1161/CIRCINTERVENTIONS.116.003913
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92(3):657–671. https://doi.org/10.1161/01.cir.92.3.657
Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, Lemesle G, Motreff P, Popovic B, Khalife K, Labeque JN, Perret T, Le Ray C, Orion L, Jouve B, Blanchard D, Peycher P, Silvain J, Steg PG, Goldstein P, Gueret P, Belle L, Aissaoui N, Ferrieres J, Schiele F, Danchin N, Usik U, investigators F-M (2017) Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French registry of acute ST-elevation or non-ST-elevation myocardial infarction) 1995 to 2015. Circulation 136(20):1908–1919. https://doi.org/10.1161/CIRCULATIONAHA.117.030798
Funding
UA has received an unconditional research grant from Heart Research Australia; RB has received an unconditional future Leader Fellowship from the Heart Foundation of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors have relevant conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Allahwala, U.K., Nour, D., Alsanjari, O. et al. Prognostic implications of the rapid recruitment of coronary collaterals during ST elevation myocardial infarction (STEMI): a meta-analysis of over 14,000 patients. J Thromb Thrombolysis 51, 1005–1016 (2021). https://doi.org/10.1007/s11239-020-02282-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02282-6