Abstract
3D direct planimetry is increasingly used in clinical practice as a rapid way to measure the mitral valve area (MVA) in patients with rheumatic mitral stenosis (MS) who underwent three-dimensional transesophageal echocardiography (3D-TEE). However, data on its accuracy and reliability are scarce. This study aimed to compare the MVA measurements obtained by 3D direct planimetry to the conventional technique multiplanar reconstruction (MPR) in MS patients using 3D-TEE. We retrospectively included 49 patients with rheumatic MS undergoing clinically-indicated 3D-TEE in the study. We determined the 3D direct planimetry measurements of MVA from the left atria aspect (MVALA) and the left ventricle aspect (MVALV), and compared those with the MPR method (MVAMPR). We also assessed the major and minor diameters of the mitral valve orifice using MPR and 3D direct planimetry. We found an excellent agreement between the MVA measurements obtained by the MPR method and 3D direct planimetry (MVALA and MVALV) [intraclass correlation coefficients (ICC) = 0.951 and 0.950, respectively]. However, the MVAMPR measurements were significantly larger than the MVALA and MVALV (p < 0.001; mean difference: 0.12 ± 0.15 cm2 and 0.11 ± 0.16 cm2, respectively).The inter-observer and intra-observer variability ICC were 0.875 and 0.856 for MVAMPR, 0.982 and 0.984 for MVALA, and 0.988 and 0.986 for MVALV, respectively. The major diameter measured by MPR (1.90 ± 0.42 cm) was significantly larger than that obtained by 3D direct planimetry (1.72 ± 0.35 cm for the LA aspect, p < 0.001; 1.73 ± 0.36 cm for the LV aspect, p < 0.001). The minor diameter measured by MPR (0.96 ± 0.25 cm) did not differ from that derived by 3D direct planimetry (0.94 ± 0.25 cm for the LA aspect, p = 0.07; 0.95 ± 0.27 cm for the LV aspect, p = 0.32). 3D direct planimetry provides highly reproducible measurements of MVA and yields data in excellent agreement with those obtained by the MPR method. The discrepancy between the two techniques may be due to differences in major diameter measurements of the mitral valve orifice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatic mitral stenosis (MS) remains the most common heart valve disease in developing countries with high morbidity and mortality [1, 2]. Clinical management of these patients relies on the accurate measurement of the mitral valve area (MVA) [3, 4]. Echocardiography is the first-line diagnostic imaging modality to measure the MVA [5]. Three-dimensional transesophageal echocardiography (3D-TEE) using the multiplanar reconstruction (MPR) method provides better alignment of the image plane at the mitral tips [5,6,7]. This renders a more accurate and reproducible planimetric measurement with better interobserver and intraobserver agreement than 2D transthoracic echocardiography [8,9,10], and is, therefore, deemed the gold standard by some investigators [10,11,12]. However, the analysis must be done off-line. 3D direct planimetry, a technology developed in recent years, permits direct measurements within the 3D dataset, without the need to export the dataset into special software for offline analysis [13]. 3D-TEE provides a unique en-face view of the mitral valve orifice, and direct planimetry of the MVA could, therefore, be performed in a method similar to 2D echocardiography [14]. Recently, 3D direct planimetry has more frequently been used in clinical practice as a fast way to measure the MVA [15,16,17]. However, research assessing the effectiveness of the 3D direct planimetry method and its comparison to the MPR method is lacking [18].
Thus, the purpose of this study was to compare the MVA measurements obtained by 3D direct planimetry and MPR in patients with rheumatic MS who underwent 3D-TEE and to explore the possible cause of discrepancies between the two 3D techniques.
We also determined the reliability of the visual assessment of the 3D direct planimetry method, since one of the critical elements of that technique is that the mitral valve orifice is parallel to the screen.
Methods
Study patient population
We retrospectively evaluated 52 patients with MS who underwent clinically-indicated 3D-TEE between January 2017 and December 2019. We excluded three patients with poor 3D-TEE image quality, resulting in 49 patients in our analyses. Clinical and demographic data were obtained from the patients’ medical records. The study protocol was reviewed and approved by the Local Ethics Committee and informed consent requirements were waived due to the study’s retrospective nature.
3D transesophageal echocardiography
3D-TEE was performed after local anesthesia using a commercially available matrix-array echocardiographic machine (EPIQ 7C, Philips) with an X7-2t transducer. 3D-TEE images [19] were acquired from patients in sinus rhythm using the zoom mode over four consecutive heartbeats while the patient held their breath. For patients in atrial fibrillation or with suboptimal multibeat construction, the 3D-TEE images were obtained using the zoom mode (one beat) focused on the mitral valve, with the highest possible volume rate. Both 2D gain and compression settings were set in the midrange (about 50 U) before acquiring the 3D datasets as recommended in the EAE/ASE guideline [19], to avoid dropouts or over-gain.
Measurement of MVA by MPR
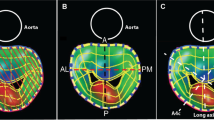
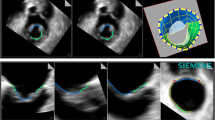
MPR measurements of the MVA (MVAMPR) were performed offline using the Philips QLAB version 10.5 software. First, the maximum early diastolic opening of the mitral valve orifice was determined. Then, multiplanar reconstruction was used to locate two orthogonal planes to cross the tips of the mitral valve, after which a third cropping plane at the level of mitral valve orifice was seen (Fig. 1) and MVAMPR was measured by planimetry (Fig. 2). Additionally, we measured two orthogonal diameters, the major diameter (MajorMPR) and the minor diameter (MinorMPR) of the mitral valve orifice (Fig. 2).
Measurement of MVA by 3D direct planimetry
3D direct planimetry of MVA was determined through an en-face view of the mitral valve in a method similar to 2D echocardiography of the left atrial aspect (MVALA) and left ventricular aspect (MVALV) on the echocardiographic machine. First, the maximum early diastolic opening of the mitral valve orifice was chosen which is the same frame as used for the MPR method. Then, we performed manual rotation of the 3D image to ascertain that the mitral valve orifice was parallel to the screen when the MVA was highest (Fig. 3). The planimetry MVA measurement was obtained from the magnified view (Fig. 4). Additionally, two orthogonal diameters, the major and the minor diameter of the mitral valve orifice, were measured from the left atrial aspect (MajorLA, MinorLA) and left ventricular aspect (MajorLV, MinorLV) (Fig. 4).
In order to test the reliability of the 3D direct planimetry measurement, we used the face-cropping function built into the machine, which can cut 3D images in a plane parallel to the screen. After the 3D direct measurement method was completed, the face-cropping function was used on the same 3D image to adjust the orientation of the 3D image for layer-by-layer cutting, so that the cutting plane could cut both commissures simultaneously, as well as the A2 and P2 segments. In that situation, the mitral valve orifice is considered to be parallel to the screen, resulting in a true-sized orifice that can be measured by 3D direct planimetry (MVALA FACE and MVALV FACE) (Fig. 5).
A mitral valve orifice parallel to the screen as judged by the face-cropping method. a 3D zoom image of a mitral valve orifice from the left ventricular perspective. *A2, P2 scallops and commissures. b Cutting plane cut A2 and P2 scallops simultaneously. c Cutting plane cut both commissures simultaneously
Inter- and intra-observer variability
To determine the inter-observer variability for MVA measurements, images of 15 randomly selected patients were analyzed at different times by two independent, blinded observers. One of these observers analyzed the same images twice on a different day at 1-month intervals, without reference to the previous measurements, to determine the intra-observer variability.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 25.0. All data were normally distributed as determined by Shapiro–Wilk test, except the 3D frame rate. Continuous variables were expressed as mean ± standard deviation or median (range) where appropriate. Categorical variables were expressed as an absolute number (percentage). MVA values determined by 3D MPR and 3D direct planimetry were compared using the paired Student’s t-test. Statistical analysis of the association of variables was performed using Pearson correlation coefficients. Intermethod agreement was evaluated using the Bland–Altman method and intraclass correlation coefficients (ICC). Inter-observer and intra-observer reproducibility were evaluated using the Bland–Altman method and ICC. All tests were performed two-sided, and a p-value < 0.05 was considered significant.
Results
Population
The study population comprised of 49 patients with MS, with a mean age of 50.7 ± 12.0 years, 63.3% were female, and 38.8% were in atrial fibrillation. Forty-five (91.8%) patients had an LV ejection fraction of ≥ 50%. The baseline characteristics of all patients are presented in Table 1.
MVA measurements using 3D TEE methods
The MVA, as determined by the MPR method, was 1.36 ± 0.56 cm2, while the MVA, as determined by 3D direct planimetry, was 1.25 ± 0.54 cm2 for MVALA and 1.25 ± 0.55 cm2 for MVALV. We found an excellent agreement between MVAMPR and MVALA with an ICC value of 0.951 (p < 0.001). The agreement between MVAMPR and MVALV was also excellent, with an ICC value of 0.950 (p < 0.001). However, MVAMPR measurements were significantly larger than the MVALA (mean difference: 0.12 ± 0.15 cm2; p < 0.001) and MVALV measurements (mean difference: 0.11 ± 0.16 cm2; p < 0.001).
Furthermore, the MVAMPR correlated well with the MVALA (r = 0.971, p < 0.001) and the MVALV (r = 0.968, p < 0.001).
We performed a Bland–Altman analysis and found an excellent agreement between the MPR and 3D direct planimetry (Fig. 6). The MVALA did not differ from MVALV (mean difference: − 0.004 ± 0.050cm2, p = 0.57).
The MajorMPR (1.90 ± 0.42 cm) of the mitral valve orifice was significantly larger than the MajorLA (1.72 ± 0.35 cm; mean difference: 0.19 ± 0.22 cm; p < 0.001) and MajorLV (1.73 ± 0.36 cm; mean difference: 0.18 ± 0.23 cm; p < 0.001). However, the MajorMPR correlated well with the MajorLA (r = 0.843, p < 0.001) and the MajorLV (r = 0.842, p < 0.001).
On the other hand, we did not find a significant difference between the MinorMPR (0.96 ± 0.25 cm) and the MinorLA (0.94 ± 0.25 cm; mean difference: 0.019 ± 0.071 cm, p = 0.065) or the MinorLV (0.95 ± 0.27 cm; mean difference: 0.013 ± 0.095 cm, p = 0.32). The Bland–Altman analysis of these data is presented in Fig. 7.
Bland–Altman graphs for mitral valve orifice diameters. a Bland–Altman graph for major diameter as determined by MPR and 3D direct planimetry from the left atrial aspect. b Bland–Altman graph for major diameter as assessed by MPR and 3D direct planimetry from the left ventricular aspect. c Bland–Altman graph for minor diameter as determined by MPR and 3D direct planimetry from the left atrial aspect. d Bland–Altman graph for minor diameter measured by MPR and 3D direct planimetry from the left ventricular aspect
The MVALA measurements we obtained were significantly lower than the MVALA FACE (mean difference: − 0.017 ± 0.042 cm2, p = 0.007). The MVALV measurements were also significantly lower than the MVALV FACE measurements (mean difference: − 0.029 ± 0.058 cm2, p = 0.001).
The time required to perform the analysis of each 3D dataset was different for the 3D direct planimetry and MPR methods. The 3D direct planimetry was done faster and took 0.5 min, while the MPR method took 2 min.
Inter-observer and intra-observer variability
The inter- and intra-observer agreement for MVAMPR values were excellent, with ICC values of 0.875 and 0.856, respectively. The absolute difference between assessments was 0.059 ± 0.209 cm2 and 0.018 ± 0.219 cm2, respectively.
The inter- and intra-observer agreement for MVALA were also excellent, with ICC values of 0.982 and 0.984, with an absolute difference between assessments of 0.028 ± 0.067 cm2 and 0.006 ± 0.070 cm2, respectively.
Finally, also for MVALV the inter- and intra-observer agreement were excellent, with ICC values of 0.988 and 0.986, and an absolute difference of 0.019 ± 0.061 cm2 and 0.003 ± 0.068 cm2, respectively.
Discussion
The present study compared MVA measurements obtained by multiplanar reconstruction to those obtained by the 3D direct planimetry method in patients with rheumatic MS. We found that the agreement between the methods was excellent, but MPR yielded larger MVA compared to the 3D direct planimetry, which has better reproducibility. The discrepancies in MVA measurements between the two techniques may be due to differences in the major diameter measurements of the orifice.
The assessment of MVA by echocardiography has been validated in various settings. The guideline recommends planimetry methods as reference standards because they do not involve any hypothesis regarding flow conditions, cardiac chamber compliance, or associated valvular lesions [20].
MPR and 3D direct planimetry are both methods that determine the MVA by delineating the mitral valve orifice, and could theoretically both produce a reliable measurement. However, there are limited data available on the comparison of the two 3D methods.
In the present study, we observed an excellent agreement between data obtained by the MPR and the 3D direct planimetry methods with excellent correlation and ICC. However, MVAMPR measurements were higher than MVALA and MVALV measurements using the 3D direct planimetry method, with a mean difference of 0.12 and 0.11 cm2, respectively. To our knowledge, few other investigations compared the two 3D methods. Eibel et al. [13] demonstrated that the direct delineation in the en-face 3D-TEE mode leads to a non-significant underestimation of MVA compared to MPR measurements in a cohort of patients who predominately had a normal MVA. Sadeghian et al. [18] found that MVAMPR overestimated the MVA compared to MVA obtained by direct planimetry on the 3D zoom mode in rheumatic MS patients, which is in agreement with our study. The mean difference found in their study was approximately 0.9 cm2.
The reasons for the discrepancies between the MPR and 3D direct planimetry methods based on the same 3D image remain to be elucidated. Measurements of the major and minor diameter of the mitral valve orifice may shed some light on this. The MPR method yielded a larger major diameter than the 3D direct planimetry method, while the minor diameter measurements did not differ between the two 3D methods. Possible explanations for these differences may lie in the 3D geometry of the mitral valve. The anterior leaflet has a rounded free edge, which comprises one-third of the annular circumference. The posterior leaflet, on the other hand, is long and narrow and lines the remainder of the circumference. The leaflet shape, together with the differential depth between the tips and commissures, makes the mitral valve orifice non-planar. This non-planar structure is not optimally represented by a single 2D plane, even when the valve is stenotic [21]. When cropping the stenotic mitral valve orifice using the “face-cropping” setting, we found that most of the orifices had a curved surface. Therefore, the orifice created using the MPR method may not be accurate. In essence, the 3D direct planimetry is also a 2D method which measures the projected area of the actual 3D orifice on the screen and does not need to reconstruct the orifice. This may be why the 3D direct planimetry has better inter- and intra-observer agreement than the MPR method. It should be noted that planimetry methods, including 2D planimetry, MPR and 3D dirct planimetry, do not take into account the differential depth between the tips and commissures of the mitral valve. Mitral valve navigation software may be feasible and accurate when measuring the curved surface area of the mitral valve orifice [21].
The limitation of 3D direct planimetry is that the 3D images are displayed on a 2D screen. This can cause an underestimation of the MVA measurement when the mitral valve orifice is not parallel to the screen. To test the reliability of the 3D direct planimetry method, we compared the eyeball method to the face-cropping method, which acts as the z-axis correction function in MPR. The measurements using the eyeball method underestimated MVA compared to the face-cropping method. This was as expected since it is impossible to align the orifice precisely parallel to the screen. However, the difference we found between the two methods is too small to be of clinical significance.
Due to its ease of use and accuracy, 3D direct planimetry can serve as a reasonable option for the assessment of MVA, especially in clinical scenarios where rapid diagnostics are desirable [22] or when the border of the orifice cannot be identified by the MPR method. In addition, one can choose to measure from the left atrium or the left ventricle, with no statistical difference between the two approaches. Care must be taken to ensure that the orifice is aligned parallel to the screen when applying the 3D direct planimetry method. When this is uncertain, face-cropping can be useful for precise positioning. Because 3D direct planimetry method is susceptible to gain artifacts, the 2D gain settings should be optimized before acquiring the 3D datasets. Both gain and compression settings should be set in the midrange (about 50 U) as per the EAE/ASE guideline [19], to avoid dropouts or over-gain. Furthermore, during 3D direct planimetry one should try to avoid adjusting the gain settings.
Several limitations of this study should be mentioned. First, the lack of an absolute gold standard for MVA measurements [23, 24] precluded us from concluding the relative accuracy of 3D direct planimetry compared with MPR. Secondly, we did not include MVA measurements by 2D transthoracic echocardiography in the analysis as the primary aim of this study was to compare two 3D-TEE methods. Thirdly, this study was conducted as a retrospective study and is subjected to the inherent limitations of such a study design.
Conclusions
3D direct planimetry provides rapid and highly reproducible measurements of MVA, yielding data in excellent correlation and agreement with data obtained by the MPR method. The MPR-derived MVA measurements are systematically larger than those obtained by 3D direct planimetry, which may be due to differences in major diameter measurements of the mitral valve orifice.
Data availability
The data that support the findings of this study are available on request. The data are not publicly available due to the containing information that could compromise the privacy of research participants.
References
Marijon E, Ou P, Celermajer DS et al (2007) Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med 357:470–476. https://doi.org/10.1056/NEJMoa065085
Carapetis JR, Beaton A, Cunningham MW et al (2016) Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2:15084. https://doi.org/10.1038/nrdp.2015.84
Baumgartner H, Falk V, Bax JJ et al (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791. https://doi.org/10.1093/eurheartj/ehx391
Nishimura RA, Otto CM, Bonow RO et al (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:e57–e185. https://doi.org/10.1016/j.jacc.2014.02.536
Wunderlich NC, Beigel R, Siegel RJ (2013) Management of mitral stenosis using 2D and 3D echo-Doppler imaging. JACC Cardiovasc Imaging 6:1191–1205. https://doi.org/10.1016/j.jcmg.2013.07.008
Kupferwasser I, Mohr-Kahaly S, Menzel T et al (1996) Quantification of mitral valve stenosis by three-dimensional transesophageal echocardiography. Int J Card Imaging 12:241–247. https://doi.org/10.1007/BF01797737
Langerveld J, Valocik G, Plokker HWT et al (2003) Additional value of three-dimensional transesophageal echocardiography for patients with mitral valve stenosis undergoing balloon valvuloplasty. J Am Soc Echocardiogr 16:841–849. https://doi.org/10.1067/S0894-7317(03)00402-4
Schlosshan D, Aggarwal G, Mathur G et al (2011) Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc Imaging 4:580–588. https://doi.org/10.1016/j.jcmg.2010.12.009
Dreyfus J, Brochet E, Lepage L et al (2011) Real-time 3D transoesophageal measurement of the mitral valve area in patients with mitral stenosis. Eur J Echocardiogr 12:750–755. https://doi.org/10.1093/ejechocard/jer118
Min SY, Song JM, Kim YJ et al (2013) Discrepancy between mitral valve areas measured by two-dimensional planimetry and three-dimensional transoesophageal echocardiography in patients with mitral stenosis. Heart 99:253–258. https://doi.org/10.1136/heartjnl-2012-302742
Argulian E, Seetharam K (2018) Echocardiographic 3D-guided 2D planimetry in quantifying left-sided valvular heart Disease. Echocardiography 35:695–706. https://doi.org/10.1111/echo.13828
Mannaerts HFJ, Kamp O, Visser CA (2004) Should mitral valve area assessment in patients with mitral stenosis be based on anatomical or on functional evaluation? A plea for 3D echocardiography as the new clinical standard. Eur Heart J 25:2073–2074. https://doi.org/10.1016/j.ehj.2004.10.001
Eibel S, Turton E, Mukherjee C et al (2017) Feasibility of measurements of valve dimensions in en-face-3D transesophageal echocardiography. Int J Cardiovasc Imaging 33:1503–1511. https://doi.org/10.1007/s10554-017-1141-z
Saric M, Lang RM, Kronzon I (2016) Quantification of mitral stenosis. In: Lang RM (ed) ASE’s comprehensive echocardiography, 2nd edn. Elsevier, Philadelphia, pp 460–464
Solomon SD, Wu J, Gillam L (2018) Echocardiography. In: Zipes DP (ed) Braunwald’s heart disease, 11th edn. Elsevier, Philadelphia, pp 173–251
Armstrong WF, Ryan T (2019) Feigenbaum’s echocardography, 8th edn. Wolters Kluwer, Philadelphia, pp 282–323
Fard MS, Rezaeian N, Pourafkari L et al (2019) Level of agreement in three-dimensional planimetric measurement of mitral valve area between transthoracic and transesophageal echocardiography. Echocardiography 36:1501–1508. https://doi.org/10.1111/echo.14431
Sadeghian H, Rezvanfard M, Jalali A (2019) Measurement of mitral valve area in patients with mitral stenosis by 3D echocardiography: a comparison between direct planimetry on 3D zoom and 3D quantification. Echocardiography 36:1509–1514. https://doi.org/10.1111/echo.14397
Lang RM, Badano LP, Tsang W et al (2012) EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 13:1–46. https://doi.org/10.1093/ehjci/jer316
Baumgartner H, Hung J, Bermejo J et al (2009) Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 22:1–23. https://doi.org/10.1016/j.echo.2008.11.029
Mahmoud Elsayed HM, Hassan M, Nagy M et al (2017) A novel method to measure mitral valve area in patients with rheumatic mitral stenosis using three-dimensional transesophageal echocardiography: feasibility and validation. Echocardiography 35:368–374. https://doi.org/10.1111/echo.13786
Biaggi P, Felix C, Gruner C et al (2013) Assessment of mitral valve area during percutaneous mitral valve repair using the MitraClip system: comparison of different echocardiographic methods. Circ Cardiovasc Imaging 6:1032–1040. https://doi.org/10.1161/CIRCIMAGING.113.000620
Faletra F, Pezzano A, Fusco R et al (1996) Measurement of mitral valve area in mitral stenosis: four echocardiographic methods compared with direct measurement of anatomic orifices. J Am Coll Cardiol 28:1190–1197. https://doi.org/10.1016/S0735-1097(96)00326-9
Pérez de Isla L, Casanova C, Almería C et al (2007) Which method should be the reference method to evaluate the severity of rheumatic mitral stenosis? Gorlin’s method versus 3D-echo. Eur J Echocardiogr 8:470–473. https://doi.org/10.1016/j.euje.2006.08.008
Funding
We received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no significant competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
Ethical approval and consent to participate
We further confirm that any aspect of the data collection covered in this manuscript that has involved our patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Consent for publication
We guarantee that the contribution to the work has not been previously published elsewhere, or that if it has been published in whole or in part, any permission necessary to publish it in the work has been obtained.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, X., Chen, W., Shi, Z. et al. Three-dimensional transesophageal echocardiography measurement of mitral valve area in patients with rheumatic mitral stenosis: multiplanar reconstruction or 3D direct planimetry?. Int J Cardiovasc Imaging 37, 99–107 (2021). https://doi.org/10.1007/s10554-020-01950-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01950-1