Abstract
Apical variant hypertrophic cardiomyopathy (AHCM) is characterized by asymmetric hypertrophy of the left ventricular (LV) apex. T wave inversions of variable degree, particularly in the left precordial leads, and left ventricular hypertrophy (LVH) are common EKG findings in AHCM. Echocardiography is typically the initial imaging modality used in the diagnosis and evaluation of AHCM. The diagnosis is made when the LV apex has apical wall thickness of ≥ 15 mm or a ratio of apical to basal LV wall thickness of ≥ 1.3 at end-diastole. The use of microbubble contrast agents with echocardiography is helpful for visualization of the apex. Cardiac magnetic resonance (CMR) has the advantage of a large field of view and the ability to perform tissue characterization. Late gadolinium enhancement (LGE) sequences are essential in the assessment of potential areas of myocardial scarring. Cardiac computed tomography (CCT) has the advantage of being able to evaluate coronary arteries in addition to assessing cardiac anatomy and function. A “Solar Polar” map pattern is the characteristic feature of AHCM on myocardial perfusion imaging (MPI) in cases not associated with apical aneurysm (APA). Recognition of typical perfusion patterns in AHCM patients is not only important in the diagnostic evaluation of this disease process, but also for avoiding unnecessary and costly tests. The purpose of this article is to review the imaging features of AHCM from different imaging modalities and assess the value added of each modality in the diagnosis of AHCM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Epidemiology and pathophysiology
AHCM, also known as Yamaguchi syndrome, is an uncommon variant of HCM, characterized by asymmetric hypertrophy of the LV apex [1]. Asian countries exhibit the highest prevalence of this phenotype, reported to be as high as 41% of HCM patients in China and more than 15% of HCM patients in Japan, compared with a prevalence of 1–3% of HCM patients in the United States [2, 3].
The pathophysiology is believed to be related to mutations in the sarcomere gene, but a direct genetic link has yet to be established. AHCM is considered to be a benign form of HCM, with a total cardiovascular mortality rate of 1.9%. However, cardiovascular complications, including atrial fibrillation (AF), myocardial infarction, stroke, heart failure (HF), and ventricular arrhythmias, may nonetheless occur. Most patients with AHCM present with no or mild symptoms, although a minority of patients may have refractory dyspnea, angina, presyncope, or syncope due to diastolic dysfunction and low cardiac output. A typical feature of AHCM on physical exam is an audible and palpable fourth heart sound, reflecting impaired LV relaxation [1].
Electrocardiography
Workup of AHCM usually begins with an ECG, with classic findings of “giant” negative T-waves (≥ 1 mV), particularly in the left precordial leads, and ECG findings suggestive of left ventricular hypertrophy (LVH). T wave inversions of any degree were found to be present in 93% of patients with AHCM (Fig. 1). However, the presence of “giant” negative T-waves has been much more variable, with prevalence ranging from 2 to 47% in a North American population [1, 3]. Additionally, only 65% of patients with AHCM have been found to have signs of LVH in a Canadian cohort [1]. Due to the nonspecific nature of these findings, an imaging modality such as echocardiography, cardiac computed tomography (CCT), or the most accurate imaging modality, cardiac magnetic resonance imaging (CMR), are performed to confirm the diagnosis [4].
Echocardiography
Echocardiography is typically the initial imaging modality used in the diagnosis and evaluation of AHCM due to its widespread availability and low cost. A complete echocardiographic examination includes assessment of the presence and magnitude of LVH, systolic and diastolic dysfunction, midventricular obstruction (MVO), and intraventricular gradient [5].
An “ace-of-spades” configuration of the LV cavity during diastole represents the diagnostic hallmark of AHCM [1]. On transthoracic echocardiography (TTE), AHCM is defined as LVH confined predominantly to the LV apex (Fig. 2) (the four apical segments and apical cap with reference to the 17-segment model from the American Heart Association) with maximal apical wall thickness of ≥ 15 mm or a ratio of maximal apical to posterior LV wall thickness of ≥ 1.3 at end-diastole, regardless of systemic hypertension [6, 7]. However, because the apex is the thinnest portion of the LV, a threshold apical wall thickness of 13 to 14 mm may be used to diagnose AHCM in the presence of other compelling information (e.g., family history of HCM) [8].
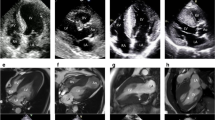
Apical hypertrophic cardiomyopathy in an 83-year-old Asian woman who presented with exertional dyspnea. a Echocardiography during systole shows myocardial thickening at the apex. b Cardiac MR shows left ventricular myocardial thickening with formation of small aneurysm at the true apex (arrowhead) with an “Hour Glass” pattern. There is a signal-void jet flow (small black arrow) through the mitral valve from regurgitation of the flow towards the left atrium. c Myocardial perfusion Rb-82 PET Vertical longitudinal axis (VLA) images and polar map show apical ischemia (white arrow) with fixed perfusion defect at the apex due to apical aneurysm (yellow arrows)
Prior studies have demonstrated that the extent of myocardial hypertrophy and the degree of thickening are important prognostic factors in patients with AHCM. According to patterns of hypertrophy, AHCM has been described to contain distinct phenotypes with distinct clinical outcomes. Eriksson et al divided AHCM into two groups: “pure” AHCM, where isolated asymmetric apical hypertrophy is present, and “mixed” AHCM, where there is hypertrophy extending to the interventricular septum [1]. In a study of 182 AHCM patients, Choi et al. further subdivided the “pure” AHCM group into “pure focal” type, where hypertrophy is confined to one or two apical segments, and “pure diffuse” AHCM, in which hypertrophy is displayed in more than two apical segments. Detailed morphological subtyping of AHCM was found to be important in the prediction of prognosis and clinical manifestations, including systolic and diastolic dysfunction, left atrial volume index (LAVI), and the presence of AF [9].
Measurement of LAV is important, because not only is LAV a barometer of diastolic dysfunction, it is also an independent predictor of HF and stroke [10, 11]. The most recent guidelines for cardiac chamber quantification by echocardiography uses 34 mL/m2 as the upper normal indexed LA volume [12]. Using this cutoff value, Yang et al. showed that HCM patients with LA enlargement had a higher incidence of serious cardiovascular events and demonstrated greater LV hypertrophy, more diastolic dysfunction, and higher filling pressures [13]. The extent and degree of LV hypertrophy has been shown to be directedly related with sudden death, as well [14]. As a result, the guidelines for the diagnosis and treatment of HCM that followed provided a Class IIa recommendation for the placement of an implantable cardioverter-defibrillator (ICD) in HCM patients with a maximum LV wall thickness ≥ 30 mm [8].
LV twist and apical longitudinal strain, other measures that are associated with systolic and diastolic function, have also been found to be reduced in patients with AHCM [15, 16]. A recent study utilized speckle tracking echocardiography to measure systolic strain in 20 patients with AHCM. These patients exhibited a decrease in mesocardial strain, predominantly in the apex, while myocardial deformation in the endocardium was preserved. A possible explanation for this transmural heterogeneity in systolic strain is that myofiber disarrangement and interstitial fibrosis are localized primarily in the mesocardium [17].
Although LV global systolic function is usually normal to hyperdynamic in patients with AHCM, regional dysfunction involving the apical segments is common, with these regions appearing hypokinetic, akinetic, or dyskinetic [18]. Apical aneurysms (APA), defined as a discrete, thin-walled akinetic or dyskinetic segment of the most distal portion of the LV chamber, in the setting of HCM has been demonstrated to portend a high risk for arrhythmic sudden death and thromboembolic events [19]. Matsubara, et al. demonstrated that the development of APA is related to severe LV cavity obliteration during systole [20]. End-systolic LV obliteration was shown to be a predictor of adverse cardiovascular events, including new onset AF, stroke, HF, and CV death, as well [21].
Minami et al. investigated the relationship between AHCM, HCM with MVO, and HCM with APA with respect to prevalence, overlap, and outcomes—probability of the combined endpoint of sudden death and potentially lethal arrhythmic events [22]. This study found that regardless of APA history, AHCM patients without MVO had strikingly good outcomes of < 5%, while APA patients without a history of apical hypertrophy had extremely poor outcomes of ≥ 50%. The authors hypothesized that two different mechanisms of APA formation—apical infarction due to apical hypertrophy vs. apical scarring due to MVO and increased wall stress—portend different prognoses, and that ICD implantation for primary prevention of sudden death may be indicated for the treatment of APA patients without a history of apical hypertrophy [23].
While diastolic dysfunction can be observed in approximately 80% of HCM patients [24, 25], studies from the Mayo Clinic found Doppler indices of diastolic function—deceleration time, transmitral flows, and mitral annular velocities—to correlate modestly, at best, with direct measurement of left atrial pressure [26, 27]. However, incorporating tissue Doppler imaging has been found to allow for accurate estimation of LV filling pressures and risk stratification in HCM patients [28,29,30]. The main limitation preventing adequate assessment of some or all of the echocardiographic parameters for diastolic dysfunction are technical in nature. Even if the parameters can be assessed, overlap between indices in healthy individuals and individuals with diastolic dysfunction can render the findings indeterminate [31].
Doppler evaluation of potential diastolic intraventricular gradients has been found to play a prognostic role in HCM patients and is thus of clinical importance. In particular, patients with HCM and LV cavity obliteration have been shown to exhibit paradoxical diastolic jet flow from the obliterated LV apex toward the base. This phenomenon was found to carry a higher risk of systemic embolism, ventricular tachycardia, and perfusion abnormalities compared to cavity obliteration alone [32].
Patients with technically difficult echocardiograms may benefit from the utilization of second-generation microbubble contrast agents to enhance LV endocardial border definition. The use of microbubble contrast agents is particularly helpful for visualization of the apex, as structural abnormalities in this region are often difficult to define clearly [33]. For example, the diagnostic accuracy for the detection of APA was found in one study to be 57% for non-contrast echocardiography compared with 80% for contrast echocardiography [34]. Despite the use of microbubble contrast agents, imaging with TTE may still result in poor detection of the endocardial border due to technical artifacts and variability of image quality [35]. Therefore, other imaging modalities have been developed to aid in the diagnosis and characterization of AHCM.
Cardiac MR imaging (CMR)
CMR is the modality of choice for the evaluation of AHCM due to its high sensitivity and specificity. CMR has the advantage of a large field of view and the ability to perform tissue characterization, thus making it a more accurate imaging modality in the detection of AHCM when compared with echocardiography (Fig. 3) [36]. Additionally, CMR is less operator dependent when compared with echocardiography. Multiple planes of the heart can be obtained with CMR, as well. It can also reliably identify heart segments with thickened myocardium; hence it can identify the various subtypes of AHCM. CMR can easily detect the presence or absence of apical aneurysm, which is a common finding in AHCM, as noted previously [37].
Apical hypertrophic cardiomyopathy in a 59-year-old woman, who presented with atypical chest pain. a Four-chamber view SSFP CMR at end-diastole and b during systole show left ventricular myocardial thickening (white arrows) more pronounced at the apex with almost obliteration of the cavity during systole. c Myocardial perfusion scan with evidence of mild ischemia during stress at the inferior wall and at the apex. Notice more counts at the apex during rest (white arrows) with “solar polar map pattern” on the Bull’s eye image
Apical myocardial wall thickness of more than 15 mm is one of the diagnostic criteria for AHCM. Normally, there is progressive tapering of myocardial wall thickness towards the apex. In AHCM, however, there is loss of this progressive tapering, and the ratio of apex to base is usually greater than 1.3 to 1.5 [38].
A spade-like configuration of the LV cavity at end-diastole, best appreciated on ventricular longitudinal axis view (VLA), suggests localized hypertrophy of the myocardium at the apex. Another feature is complete cavity obliteration during systole, best appreciated on short-axis view (SAX) [38].
Late gadolinium enhancement (LGE) sequences are essential in the assessment of potential areas of myocardial scarring. It can be present in up to 40% of patients with AHCM [39]. LGE is recommended by the ACC for risk stratification in HCM (IJC). A study done by Villa et al. showed that the presence of LGE on CMR is associated with a larger ischemic burden, and possibly more severe disease, in patients with AHCM. Thus, LGE can predict the presence of ventricular tachyarrythmias, which can be present in 30% of the patients with AHCM. Overall, the presence of LGE of more than 15% of the LV mass increases the risk of sudden cardiac death by twofold [5]. Olivotto et al. proposed a framework for systematic clinical staging of HCM based on clinical, echocardiographic, and CMR evidence of disease progression, including the percentage of myocardium occupied by LGE (Table 1) [40].
Mimics of AHCM on CMR include hypertrophic myocardium related to exercise (athlete’s heart) or hypertension. However, the distribution of thickened myocardium is even throughout the heart in these examples and does not exceed 15 mm in thickness [5].
Cardiac computed tomography
CCT has the advantage of being able to evaluate epicardial coronary arteries in addition to assessing cardiac anatomy and function. Although the temporal resolution of CCT is not as high as that of echocardiography or CMR, CCT can confirm or exclude the presence of co-existent coronary artery disease (CAD). Retrospective-gating of the CCT study is helpful in the assessment of myocardial thickening at different stages of the cardiac cycle (Fig. 4). The morphologic imaging criteria of AHCM on CT is similar to CMR [5].
AHCM -pure apical hypertrophy variant without aneurysm in a 72-year-old man who presented with exertional dizziness. a Contrast enhanced CT shows prominence of the left ventricular apex myocardium (arrows). b and d Contrast echocardiography show hypertrophy of myocardium (arrows) “ace-of spade” configuration. c Myocardial perfusion Tc99m-tetrofosmin SPECT images shows relative ischemia limited to the apex (arrows)
Myocardial perfusion imaging
Although less well described in literature, as opposed to imaging modalities such as echocardiography and CMR, myocardial perfusion imaging (MPI) has been also been used in the diagnosis of AHCM [41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Patients are often initially referred for MPI because of ECG abnormalities and chest pain that raise concerns for CAD. Recognition of typical perfusion patterns in AHCM patients is not only important in the diagnostic evaluation of this disease process, but also for avoiding unnecessary and costly tests such as CMR or invasive testing.
Stress MPI findings in AHCM patients have ranged from normal perfusion to reversible and fixed apical perfusion defects, often in the presence of normal epicardial coronary arteries [37]. Morishita et al. described increased apical uptake of technetium-99 m tetrofosmin on resting SPECT polar maps in patients with AHCM [41]. Ward et al. further characterized this perfusion pattern using dual-isotope rest and stress SPECT perfusion imaging. This “Solar Polar” map pattern on the rest polar maps showed an intensely bright spot of counts in the apical segment surrounded by a circumferential ring of decreasing counts [42]. In a study of 20 patients with AHCM, Cianciulli et al. identified three patterns on myocardial perfusion SPECT characteristic of AHCM: an increased apical tracer uptake, a spade-like configuration of the LV chamber, and the ‘‘Solar Polar” map pattern [43]. A recent study by Zhou et al. demonstrated a new method in the diagnosis of AHCM based on the integral quantitative analysis of myocardial perfusion and wall thickening from gated SPECT MPI that is highly accurate (95%) when compared with CMR [44].
F18-FDG PET
On F18-FDG PET scan images, a pattern of focal non-suppression of myocardial FDG uptake (glucose metabolism) at the apex has been described in patients with AHCM [45,46,47, 55]. This pattern could represent a pitfall when F18-FDG PET is performed for staging of oncologic patients (Fig. 5). Further evaluation with echocardiography or CMR is thus recommended to avoid calling this focal non-suppression as a mass or metastasis.
Apical hypertrophic cardiomyopathy mimicking a mass in a 72-year-old woman with history of treated lung cancer. a F18 FDG PET/CT coronal and b axial fused images show focus of intense activity at the apex of the heart (arrow). c CT of the chest shows prominence of the left ventricular myocardium at the apex (arrow)
Conclusion
In conclusion, AHCM is characterized by apical hypertrophy of the myocardium. Echocardiography is the initial imaging modality used in the diagnosis and evaluation of AHCM. CMR has the advantage of a large field of view and the ability to perform tissue characterization, thus making it a more accurate imaging modality in the detection of AHCM when compared with echocardiography. CCT can confirm or exclude the presence of co-existent epicardial coronary artery disease. It is important to recognize the imaging pattern of AHCM on MPI, including patterns on myocardial perfusion SPECT characteristic of AHCM: an increased apical tracer uptake, a spade-like configuration of the LV chamber, and the ‘‘Solar Polar” map pattern.
References
Eriksson MJ et al (2002) Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 39(4):638–645
Ho HH et al (2004) Clinical characteristics of and long-term outcome in Chinese patients with hypertrophic cardiomyopathy. Am J Med 116(1):19–23
Kitaoka H et al (2003) Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol 92(10):1183–1186
Moon JC et al (2004) Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 90(6):645–649
Jan MF et al (2016) Apical hypertrophic cardiomyopathy: present status. Int J Cardiol 222:745–759
Cerqueira MD et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542
Kim EK et al (2016) Differences in apical and non-apical types of hypertrophic cardiomyopathy: a prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur Heart J Cardiovasc Imaging 17(6):678–686
Gersh BJ et al (2011) ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011. 58(25):e212–e260
Choi EY et al (2008) Phenotypic spectrum and clinical characteristics of apical hypertrophic cardiomyopathy: multicenter echo-Doppler study. Cardiology 110(1):53–61
Di Tullio MR et al (1999) Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke 30(10):2019–2024
Takemoto Y et al (2005) Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol 96(6):832–836
Lang RM et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39.e14
Yang H et al (2005) Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr 18(10):1074–1082
Spirito P et al (2000) Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342(24):1778–1785
Biswas M et al (2013) Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography 30(1):88–105
Chang SA et al (2010) Left ventricular twist mechanics in patients with apical hypertrophic cardiomyopathy: assessment with 2D speckle tracking echocardiography. Heart 96(1):49–55
Saccheri MC et al (2017) Speckle tracking echocardiography to assess regional ventricular function in patients with apical hypertrophic cardiomyopathy. World J Cardiol 9(4):363–370
Binder J et al (2011) Apical hypertrophic cardiomyopathy: prevalence and correlates of apical outpouching. J Am Soc Echocardiogr 24(7):775–781
Rowin EJ et al (2017) Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 69(7):761–773
Matsubara K et al (2003) Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J Am Coll Cardiol 42(2):288–295
Kim H et al (2016) Significance of apical cavity obliteration in apical hypertrophic cardiomyopathy. Heart 102(15):1215–1220
Minami Y, Haruki S, Hagiwara N (2014) Phenotypic overlap in hypertrophic cardiomyopathy: apical hypertrophy, midventricular obstruction, and apical aneurysm. J Cardiol 64(6):463–469
Maron MS et al (2008) Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 118(15):1541–1549
Nagueh SF et al (2011) American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 24(5):473–498
Parato VM et al (2016) Echocardiographic diagnosis of the different phenotypes of hypertrophic cardiomyopathy. Cardiovasc Ultrasound 14(1):30
Geske JB et al (2007) Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation 116(23):2702–2708
Nishimura RA et al (1996) Noninvasive doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol 28(5):1226–1233
Kitaoka H et al (2013) Tissue Doppler imaging and prognosis in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 14(6):544–549
Kitaoka H et al (2011) Tissue doppler imaging and plasma BNP levels to assess the prognosis in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 24(9):1020–1025
Nagueh SF et al (1999) Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 99(2):254–261
Nagueh SF et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29(4):277–314
Nakamura T et al (1992) Diastolic paradoxic jet flow in patients with hypertrophic cardiomyopathy: evidence of concealed apical asynergy with cavity obliteration. J Am Coll Cardiol 19(3):516–524
Mulvagh SL et al (2008) American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr 21(11):1179–201 (quiz 1281)
Minami Y et al (2011) Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 57(23):2346–2355
Prasad K et al (1999) Echocardiographic pitfalls in the diagnosis of hypertrophic cardiomyopathy. Heart 82:Iii8–Iii15
Amano Y et al (2018) Cardiac MR imaging of hypertrophic cardiomyopathy: techniques, findings, and clinical relevance. Magn Reson Med Sci 17(2):120–131
Parisi R et al (2014) Multimodality imaging in apical hypertrophic cardiomyopathy. World J Cardiol 6(9):916–923
Baxi AJ et al (2016) Hypertrophic cardiomyopathy from A to Z: genetics, pathophysiology, imaging, and management. Radiographics 36(2):335–354
Yamada M et al (2009) Frequency and distribution of late gadolinium enhancement in magnetic resonance imaging of patients with apical hypertrophic cardiomyopathy and patients with asymmetrical hypertrophic cardiomyopathy: a comparative study. Int J Cardiovasc Imaging 25(Suppl 1):131–138
Olivotto I et al (2012) Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail 5(4):535–546
Morishita S et al (2001) Impaired retention of technetium-99m tetrofosmin in hypertrophic cardiomyopathy. Am J Cardiol 87(6):743–747
Ward RP et al (2003) Resting "Solar Polar" map pattern and reduced apical flow reserve: characteristics of apical hypertrophic cardiomyopathy on SPECT myocardial perfusion imaging. J Nucl Cardiol 10(5):506–512
Cianciulli TF et al (2009) Myocardial perfusion SPECT in the diagnosis of apical hypertrophic cardiomyopathy. J Nucl Cardiol 16(3):391–395
Zhou Y et al (2019) Development and validation of a new method to diagnose apical hypertrophic cardiomyopathy by gated single-photon emission computed tomography myocardial perfusion imaging. Nucl Med Commun 40(3):206–211
Yamamoto H et al (2017) Occasionally increased (18)F-fluorodeoxyglucose uptake in apical hypertrophic cardiomyopathy with mid-ventricular obstruction. J Cardiol Cases 16(2):44–47
Norikane T et al (2019) Occasionally increased (18)F-FDG uptake in apical hypertrophic cardiomyopathy on serial follow-up PET/CT. J Nucl Cardiol. https://doi.org/10.1007/s12350-019-01623-0
Takeishi Y et al (2017) Cardiac imaging with (18)F-fluorodeoxyglucose PET/MRI in hypertrophic cardiomyopathy. J Nucl Cardiol 24(5):1827–1828
Akbulut A et al (2019) The detection of apical variant of hypertrophic cardiomyopathy in myocardial perfusion imaging. Acta Cardiol 74:1–3
Zein RK et al (2017) An uncommon variant of an uncommon disease: a Caucasian adolescent with apical hypertrophic cardiomyopathy diagnosed with myocardial perfusion imaging. World J Nucl Med 16(3):251–254
Masrur S et al (2016) SPECT myocardial perfusion imaging in the diagnosis of apical hypertrophic cardiomyopathy. Tex Heart Inst J 43(5):467–468
Jouni H, Geske JB, Miller TD (2013) The diagnosis of apical hypertrophic cardiomyopathy with myocardial perfusion imaging. Heart 99(14):1064–1065
Hsieh BP, Travin MI (2012) Myocardial perfusion image findings in apical hypertrophic cardiomyopathy. J Nucl Cardiol 19(1):172–176
Russo RR et al (2010) Severe ischaemia on SPECT myocardial perfusion imaging secondary to microvascular dysfunction and apical hypertrophic cardiomyopathy. Clin Nucl Med 35(12):937–940
Irwin RB, Arumugam P, Khattar RS (2010) Incidental detection of apical hypertrophic cardiomyopathy by myocardial perfusion imaging. Nucl Med Commun 31(4):286–293
Minamimoto R et al (2013) Incidental focal FDG uptake in heart is a lighthouse for considering cardiac screening. Ann Nucl Med 27(6):572–580
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests or financial relations to disclose. The manuscript does not contain clinical studies or patient’s data. The authors comply with international, national and institutional ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, G., Fadl, S.A., Sukhotski, S. et al. Apical variant hypertrophic cardiomyopathy “multimodality imaging evaluation”. Int J Cardiovasc Imaging 36, 553–561 (2020). https://doi.org/10.1007/s10554-019-01739-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01739-x