Abstract
Hypertrophic cardiomyopathy (HCM) is a relatively common disease that exhibits heterogeneous phenotypes with an autosomal dominant Mendelian pattern of inheritance. It is characterized by diverse phenotypic expressions and variable natural progression. Accurate diagnosis is of great importance for the perioperative management of these patients. Traditionally, the echocardiographic assessment and cardiac magnetic resonance imaging were both the methods of choice for evaluating HCM, which includes assessing the extent and location of left ventricular hypertrophy, left ventricular outflow tract gradients, systolic and diastolic function, anatomic as well as functional abnormalities of the mitral valve and papillary muscles. Recently, the rapid development of cardiac CT imaging technique provides more possibilities for its value in the differential diagnosis and condition evaluation of HCM patients. In this chapter, based on a case of apical HCM, we will discuss the cardiac CT imaging manifestations of HCM, and further possibly promising role of new cardiac CT technology in HCM.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
26.1 Case of HCM
26.1.1 History
A 61-year-old male patient felt progressive exertional chest tightness which relieved by rest for the past 11 years. He had a recent dizziness in 10 days and appointed to cardiac CT for suspected coronary artery disease (CAD).
Physical Examination
-
Blood pressure: 142/74 mm Hg; Breathing rate: 18/min; heart rate: 81 bpm without arrhythmia.
-
3/6 grade systolic murmurs are audible in the apical region
Electrocardiograph
Standard 12-lead electrocardiograph (ECG) revealed ST-T segment changes on leads V2–V6 and T wave inversion.
Laboratory
Serum myocardial enzyme spectrum showed negative results.
26.1.2 Imaging Examination
CT Images
A coronary CT angiography (CTA) combined with adenosine triphosphate (ATP)-stress myocardial CT perfusion was requested to investigate the coronary artery status and myocardial blood flow (Figs. 26.1, 26.2, and 26.3).
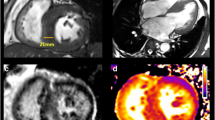
Cardiac CT imaging of myocardium: four-chamber view (a) and long-axis two-chamber view (b) of left ventricular. Delayed scan imaging of long-axis views in the systolic phase (c, MPR image; e, dual-energy iodine maps) and diastolic phase (d) showed a thickened apical myocardium with partially patchy mid-wall delayed enhancement
Conventional Coronary Angiography
See Fig. 26.4
26.1.3 Imaging Findings and Diagnosis
The coronary CTA images results showed there were mixed plaques in the proximal-middle segments of LAD and noncalcified plaques in the proximal-middle segments of LCX, resulting in both mild lumen stenosis. No significant stenosis has been found in all the coronary arteries. All the myocardial perfusion of left ventricular (LV) images, including bull-eye polar-maps, four-chamber views, two-long chamber views, and two-short chamber views, showed the extensive reduction of myocardial blood flow in apical portion compared to the basal area, which do not correspond to the coronary blood supply region of lesion vessels. The four-chamber views and two-long chamber views of both systolic and diastolic LV delayed CT scan results show a thickened apical myocardium. Partially in the delayed phase of dual-energy iodine maps, the apparent enhancement of the apical portion suggested local fibrosis. The invasive coronary angiography images confirmed only several mild stenosis on the LAD and RCA.
26.1.4 Management
-
Conventional medical therapy for Secondary Prevention of CAD
-
Out-patient follow-up observations for HCM
26.2 Discussion
HCM is one of the most common monogenic cardiovascular disorders, affecting 1 of every 500 adults. It is characterized by diverse phenotypic expressions, including focal basal septum HCM, diffuse septum HCM, concentric and diffuse HCM, burned-out phase HCM, mid-ventricular HCM, apical HCM, focal mid-septum HCM, free-wall HCM, and crypts in genotype-positive and phenotype-negative HCM.
The asymmetric septal form is the most common morphologic variant of HCM and accounts for up to 60–70% of cases [1]. It is typically presented with the disproportionate enlargement of the ventricular septum, with the anteroseptal myocardium most commonly involved. The diagnostic criteria consist of basal septal thickness ≥15 mm; the ratio of septal thickness to thickness of inferior wall at mid-ventricular level >1.5.
The symmetrical or concentric HCM ranked the second most common phenotype and is characterized by diffuse left ventricular wall thickening with an associated decrease in left ventricular cavity size [1, 2]. Diagnosis should be made in the absence of a secondary cause like hypertension, aortic stenosis, or the patient being an endurance athlete.
Apical HCM accounts for approximately 2–15% of all the HCM cases [1, 3]. It is typically presented with giant negative T waves on the electrocardiogram and a spade-like configuration of the LV cavity, which resulted from the apical portion hypertrophy of the LV. Diagnosis of the apical HCM depends on LV thickening (predominantly confined to the apex measuring 15 mm or more) and the ratio of apical LV wall thicknesses to basal LV wall thicknesses (1.3–1.5) [1].
Clinically, patients with apical HCM may present with exertional angina or dyspnea and may have electrocardiographic findings similar to those with CAD or in acute coronary syndromes [1, 4]. Differential diagnosis is important.
26.3 Current Technical Status and Applications of CT
Although cardiac MR imaging has advantages for accurate diagnosis, Cardiac Computed Tomographic (CT) scan technology has showed a complementary role for these patients [3, 5,6,7]. Cardiac CT is able to identify the hypertrophy of the myocardium and as well as the asymmetry of the ventricular hypertrophy [7, 8]. When a 4D cardiac CT imaging was performed, the left ventricular outflow tract obstruction (LVOTO) and the mitral valve and papillary muscles situation can be clearly showed with a dynamic imaging as MR [9]. The geometric predictors of LVOTO measured by cardiac CT in HCM patients have been demonstrated as independent predictors, including Spiral pattern of LV hypertrophy, the length of the anterior mitral leaflet (AML), and the distance between lateral papillary muscle (PM) base and left ventricular (LV) apex [10]. Furthermore, the CT angiography planning improves the localization of infarct and procedural success at the first attempt in Alcohol septal ablation (ASA) when compared to traditional methods. Follow-up to 6 months suggests a symptomatic, functional, and hemodynamic improvement [11]. Besides, the cardiac CT is possibly contributing for revealing the enlarged mitral valve and systolic anterior motion (SAM) of the mitral valve or mitral valve regurgitation [8].
Some of these HCM patients were admitted for PCI because of suspected CAD. A coronary CTA is always clinically referred for before the invasive angiography. Recently, with the rapid development of CT technology, the perfusion imaging approach has become more and more prevalent in the clinical application. Coronary CTA combined with ATP-stress myocardial CT perfusion can be organized as pre-opreative examination to investigate the coronary artery stenosis and myocardial blood flow. Mild lumen stenosis and unmatched local extensive reduction of myocardial blood flow combined with myocardium thickness on Cardiac CT can highly suggest the diagnosis of HCM [1, 3, 4]. Apart from apical hypertrophy, other atypical forms of HCM including concentric hypertrophy or sometimes mid-ventricular hypertrophy can also be demonstrated by CT imaging [8].
In addition, dual-energy cardiac CT imaging can be utilized for a delayed scan of the HCM patients. The delayed enhancement mostly focal but can be diffuse, which is particularly obvious in the iodine maps, suggesting fibrosis of the myocardium. It can be related to life-threatening arrhythmia and cardiac death [4, 8].
Lately, the fractional flow reserve derived from CT (FFRCT) has shown its potential value for HCM patients. It offers a noninvasive method for evaluating the coronary artery volume to myocardial mass ratio (V/M), which demonstrated significantly greater coronary volume yet decreased V/M for HCM patients [12].
Appropriate cardiac CT examination contributes a lot for correct differential diagnosis thinking and prompt treatment choice, as well as accurate prognosis assessment.
26.4 Key Points
-
HCM may present with exertional angina or dyspnea, or have electrocardiographic findings similar to those with CAD or in acute coronary syndromes.
-
Appropriate cardiac CT examination with developing imaging technologies may contribute to correct differential diagnosis of HCM from CAD and myocardial viability and fibrosis evaluation.
References
Baxi AJ, Restrepo CS, Vargas D, et al. Hypertrophic cardiomyopathy from A to Z: genetics, pathophysiology, imaging, and management. Radiographics. 2016;36(2):335–54.
Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: part I, MRI appearances. AJR Am J Roentgenol. 2007;189(6):1335–43.
Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381(9862):242–55.
Ho CY, Lopez B, Coelho-Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363(6):552–63.
Brouwer WP, Baars EN, Germans T, et al. In-vivo T1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2014;16:28.
Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2012;14:13.
Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112(6):855–61.
Khalil H, Alzahrani T. Cardiomyopathy imaging. Treasure Island, FL: StatPearls; 2019.
Rajiah P, Fulton NL, Bolen M. Magnetic resonance imaging of the papillary muscles of the left ventricle: normal anatomy, variants, and abnormalities. Insights. Imaging. 2019;10(1):83.
Song Y, Yang DH, Harrtaigh BO, et al. Geometric predictors of left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy: a 3D computed tomography analysis. Eur Heart J Cardiovasc Imaging. 2018;19(10):1149–56.
Cooper RM, Binukrishnan SR, Shahzad A, et al. Computed tomography angiography planning identifies the target vessel for optimum infarct location and improves clinical outcome in alcohol septal ablation for hypertrophic obstructive cardiomyopathy. EuroIntervention. 2017;12(18):e2194–203.
Sellers SL, Fonte TA, Grover R, et al. Hypertrophic Cardiomyopathy (HCM): New insights into Coronary artery remodelling and ischemia from FFRCT. J Cardiovasc Comput Tomogr. 2018;12(6):467–71.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yi, Y., Lin, L., Wang, Y. (2020). Hypertrophic Cardiomyopathy. In: Jin, Zy., Lu, B., Wang, Y. (eds) Cardiac CT. Springer, Singapore. https://doi.org/10.1007/978-981-15-5305-9_26
Download citation
DOI: https://doi.org/10.1007/978-981-15-5305-9_26
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5304-2
Online ISBN: 978-981-15-5305-9
eBook Packages: MedicineMedicine (R0)