Abstract
To assess the accuracy of cardiac magnetic resonance (CMR) for the diagnosis of angiographic stenosis after percutaneous coronary intervention (PCI) of left main coronary artery (LMCA). Patients undergone in the last year PCI of unprotected LMCA and scheduled for conventional X-ray coronary angiography (CXA) were evaluated with stress perfusion CMR within 2 weeks before CXA. Main contraindications to CMR were exclusion criteria. Stress perfusion CMR was performed to follow a bolus of contrast Gadobutrol after 3 min of adenosine infusion. Between the 50 patients enrolled, only 1 did not finish the CMR protocol and 49 patients with median age 71 (65–75) years (38 male, 11 female) were analyzed. Between 784 coronary angiographic segments evaluated we found 75 stenosis or occlusions (prevalence 9.5%), but only 13 stenosis or occlusions in proximal segments (prevalence 6.6%). Patients with coronary stenosis (n = 12, 24%) showed a significantly (p = 0.002) higher prevalence of diabetes (7 of 12, 58%). At CMR examination, late gadolinium enhancement was present in 25 (51%), reversible perfusion defects in 12 (24%), and fixed perfusion defects in 6 subjects (12%). The only patient with LMCA restenosis resulted positive at perfusion CMR. The accuracy of stress perfusion CMR in diagnosis of coronary stenosis was higher when the analysis was performed only in proximal coronary arteries (95%, CI 86–99) compared to overall vessels (84%, CI 70–92). Stress perfusion CMR could strongly reduce the need for elective CXA in follow up of LMCA PCI and should be validated in further multicenter prospective studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Significant unprotected left main coronary artery (LMCA) stenosis occurs in approximately 5% of coronary angiographies [1].
The medical management of the LMCA stenosis is associated with a higher mortality when compared to coronary artery bypass (CABG) treatment [2, 3]. In LMCA stenosis CABG is the first choice treatment, but percutaneous coronary intervention (PCI) can be an alternative to CABG in selected patients. According to the recent guidelines, PCI could be considered in LMCA stenosis when either anatomic conditions associated with a low risk of PCI procedural complication are present and a good long-term outcome is expected, or when clinical characteristics predict a significantly increased risk of adverse CABG-related outcomes [4].
The most important disadvantage of the PCI procedure, despite the introduction of drug-eluting stents (DES), is the phenomenon of restenosis requiring reinterventions within the LMCA. The incidence of restenosis within the LMCA is estimated at 4.5–42%, depending on the type of stent implanted, definition of restenosis, and lesion location within the LMCA [5, 6].
Most of LMCA restenosis are asymptomatic and, despite high numbers of PCIs performed within the LMCA, the prognostic value of angiographic follow-ups has never been evaluated in prospective randomized trials and remains unclear [7].
In recent years, several studies [8,9,10,11] documented a high diagnostic performance of stress perfusion-cardiac magnetic resonance (CMR) vs. conventional X-ray coronary angiography (CXA) and showed its prognostic value [12].
To date, despite the increasing utilization of stress perfusion-CMR in clinical practice and its impact on clinical patient management [13], the utility of CMR in clinical follow-up after PCI of LMCA is poorly established.
The aim of the present prospective pilot study was to assess the feasibility and accuracy of a combined examination of adenosine first-pass stress perfusion with late gadolinium enhancement (LGE) for the diagnosis of significant stenosis in patients who had undergone LMCA PCI and who were clinically scheduled for invasive coronary angiography.
Methods
This study is a prospective single centre analysis of 50 consecutive subjects. All subjects were selected from November 2010 to September 2013 at St. Orsola Malpighi Hospital, a tertiary care centre located in Bologna, Northern-Italy.
Local ethical committee (Comitato Etico Azienda Ospedaliero-Universitaria Policlinico S.Orsola- Malpighi) approved the research protocol and all subjects provided informed written consent before study participation.
Men and women older than 40 years were eligible for the study if they had undergone in the last year percutaneous coronary intervention of the LMCA and if they were scheduled for conventional CXA.
Stress perfusion CMR had to be performed within 2 weeks before the CXA.
Exclusion criteria were recent myocardial infarction or unstable angina pectoris, history of coronary artery bypass graft (CABG) or severe congestive heart failure, and contraindications to DE-CMR such as implantable devices or not DE-CMR safe prosthetic materials, claustrophobia, severe renal insufficiency and history of allergic reactions to DE-CMR contrast agents. Substantial mental disorder, including severe dementia or any disorder that interfered with a patient’s ability to comply with the CMR protocol, was also an exclusion criterion. Detailed inclusion and exclusion criteria are provided in Table 1.
The patients had to refrain from coffee, tea, chocolate, or other caffeinated beverages and food for at least 24 h before the CMR exam.
In all subjects included in this prospective research protocol we recorded clinical data before stress CMR, including ECG, standard transthoracic echocardiography and main laboratory parameters.
Body mass index (BMI) was weight in kilograms divided by the square of the height in meters. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or use of antihypertensive medications [14]. Diabetes mellitus was defined as fasting plasma glucose concentration ≥126 mg/dl (7.0 mmol/L), use of insulin or other anti-diabetic medicine, self-reported disease, or HbA1c level ≥7.0% [15]. Smoking status was self-reported. Individuals were defined as smokers if they smoked in lifetime more than 100 cigarettes.
eGFR was calculated by Cockcroft-Gault formula:
Total cholesterol levels ≥200 mg/dl (5.17 mmol/L) were considered elevated and high density lipoprotein (HDL) ≤40 mg/dl (1.03 mmol/L) were low [16].
Family history of CAD was defined by the history of myocardial infarction (MI) in a male 1st degree relative before the age 55 or a female 1st degree relative before the age of 65.
Known heart failure, stroke, symptomatic peripheral artery disease and chronic obstructive pulmonary disease were defined by documented prior hospitalization for each pathology.
During CMR, blood pressure and cardiac frequency were recorded before, after 2 min of adenosine infusion, and at the end of the adenosin infusion.
All patients were examined in supine position using a 1.5-T scanner (Signa Horizon, GE Medical Systems, Milwaukee, Wisconsin, USA) equipped with a cardiac phased array coil.
A rapid gradient echo sequence allowed for localization of the heart in the three standard planes.
A breath-hold MR first-pass perfusion examination was performed to follow a bolus of contrast Gadobutrol 1 mmol/mL solution (Gadovist, Bayer Schering Pharma) administered intravenously 0.1 mmol/kg at 4 ml/s after 3 min of adenosine infusion (140 mcg/min/kg i.v.).
During bolus arrival, three short-axis slices were acquired every heart beat at one-fourth, half, and three-fourth of the left ventricular (LV) long axis (non-slice selective 90°-preparation, fast gradient-echo acquisition, spatial resolution: 2–3 mm × 2–3 mm, slice thickness 8–10 mm).
After stress perfusion, a complete set of short axis cine images covering the whole heart were acquired with the patient performing a series of breath-holds in end-expiration.
Standard 2-chamber and 4-chamber long-axis series conform to standard cardiac protocols [17]. Imaging parameters of the 2-chamber and 4-chamber long-axis series and the short axis cine acquisitions by a balanced fast-field echo sequence [18] were the following: repetition time (TR) 3.0 ms, echo time (TE) 1.5 ms, flip angle = 50°, field of view (FOV) 350 mm, and gated cardiac triggering with retrospective reconstruction of 30 phases. For the short axis cine acquisitions covering the heart 15 to 22 (mean 18) parallel oriented slices were acquired without a slice gap, one or two slices per breath-hold.
After a 10–15 min delay from the MR contrast, a segmented inversion recovery fast gradient echo sequence was performed in short-axis views and the standard long-axis views. Optimal inversion times to null the normal myocardial signal were determined for each patient using short axis scout sequences.
Finally, using the same locations of stress perfusion, we acquired a rest perfusion imaging at the same Gadobutrol dose (0.1 mmol/kg), approximately 20 min after the stress imaging.
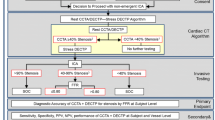
The CMR study protocol is shown in Fig. 1.
CMR protocol. Survey: transversal, sagittal, and coronal view for the localization of the heart. Adenosine infusion: 3 min of adenosine infusion (140 mcg/kg/min) in peripheral vein. Stress perfusion: first-pass perfusion in 3 short-axis views 3 min after the start of adenosine. Short axis cine: short-axis views for cardiac function. LGE (late gadolinium enhancement): inversion recovery technique 10–12 min after stress perfusion. Rest perfusion: first-pass perfusion in the same 3 short-axis views of stress perfusion
Functional assessment: left ventricle (LV) and right ventricle (RV) volumes and ejection fraction, parietal thickness and mass were measured by tracing epicardial or endocardial borders manually with commercially available software (Mass Analysis Plus; Medis, Leiden, the Netherlands). The endocardial and epicardial borders were manually drawn in the end-diastolic and end-systolic short-axis cine images. Papillary muscles and trabeculatures were not included in the myocardium. The standard-16 segment model (17-segment model minus the apical segment) [19] was used to assess myocardial perfusion, thickness, kinesis, and contrast enhancement of the LV. The standard-16 segment model was applied since the vessels supplying the apex are <2 mm in diameter in most cases and are rarely treated [20].
Perfusion defects and delayed contrast hyperenhancements in which any portion involved at least 75% of the thickness of the myocardial wall was considered transmural [21].
The presence and transmural extent of a perfusion defect was determined from the dynamic images at the time of myocardial enhancement showing the maximum extent of regional hypoenhancement. To determine if a defect is stress induced and to detect artefacts, the perfusion images were visually compared side by side with rest perfusion and LGE images. Ischemia was defined as any regional stress-induced hypoenhancement in the absence of LGE, hypoenhancement larger than LGE, if present (partially reversible defect), whereas a defect of the same size as LGE (transmural or subendocardial) was considered nonischemic (fixed defect). Matched stress-rest perfusion defects in the absence of infarction by LGE were considered artefactual [22].
Segments with a transmural infarct supplied by a stenosed or occluded vessel will not show ischemia in a non-invasive test (fixed defect) and will therefore be classified as false negative. To overcome this problem, the analysis was carried out by classifying these segments as true negative, but only if the area of transmural infarction matched the area supplied by the vessel, as defined by angiography retrospectively [9].
All perfusion CMR studies were visually analysed off-line by two radiologists with >10 years of experience in CMR blinded to CXA results. Contrasts were solved by consensus.
All coronary X-ray angiographies (CXA) were performed within 2 weeks after CMR examination. Clinically significant coronary heart disease was defined as 70% or more area stenosis of a first order coronary artery measuring 2 mm or greater in diameter, or left main stem stenosis 50% or more, as was used in previous studies [10, 23].
Vessels of <2 mm diameter were not considered for definition of CAD, since such small vessels are rarely treated (e.g. no stents available for <2 mm vessels).
Coronary angiograms were analysed and divided in proximal, mid and distal segments following Syntax classification [24].
Two experienced interventional cardiologists blinded to the results of the CMR imaging examinations visually evaluated the angiograms. Contrasts were solved by quantitative coronary angiography (QCA), as in previous studies [25].
Statistical analyses were performed using Stata/SE 14.1 for Windows; continuous variables were expressed as medians and interquartile range; categorical data were expressed as numbers (percentages). The Two-sample Wilcoxon rank-sum (Mann–Whitney) test and χ2 test or Fisher exact test were used to compare respectively continuous and categorical variables between groups.
All p values refer to two-tailed tests of significance. P < 0.05 was considered significant.
Sensitivity, specificity, and accuracy (including the confidence intervals) were calculated according to standard definitions.
Results
From November 2010 to September 2013 83 subjects fulfilled the inclusion criteria and 33 were ruled out because they met the exclusion criteria (n = 28) or refused to participate (n = 5). Between the 50 patients enrolled in the study, only 1 patient did not finish the stress perfusion CMR protocol because of claustrophobic reaction and was not included in the analysis.
Forty-nine patients with median age 71 (65–75) years (38 male, 11 female) were included for analysis. Table 2 summarizes main clinical characteristics.
Mean time between LMCA PCI and scheduled coronary angiography was 228 ± 108 days. Overall, we evaluated 784 coronary angiographic segments and found 75 stenosis or occlusions (prevalence 9.5%). In the 196 proximal coronary segments there were 13 stenosis or occlusions (prevalence 6.6%) and only 1 LMCA stenosis. On a patient based analysis, at least one significant coronary stenosis or occlusion was present in 12 subjects (24%).
Patients with coronary stenosis (n = 12, 24%) showed a significantly (p = 0.002) higher prevalence of diabetes (7 of 12, 58%) compared to subjects without stenosis (4 of 37, 11%). The other clinical and electrocardiographic characteristics were similar between the two groups (all p ≥ 0.1). Only 2 patients presented atrial fibrillation. At echocardiographic examination, only 2 patients had severe mitral regurgitation and 10 had moderate mitral and/or aortic regurgitation. As shown in Table 3, main echocardiographic parameters and laboratory data were not significantly different in patients with and without angiographic coronary stenosis (all p ≥ 0.07).
Stress perfusion CMR was conducted without any serious adverse event. During adenosine first-pass stress perfusion, heart rate increased significantly (p < 0.001) from 63 (58–66) bpm to 75 (66–85) bpm, while systolic and diastolic blood pressure did not vary (all p > 0.3).
Table 4 describes main CMR findings. Only 25 patients had LGE, with transmural distribution in 11. Reversible perfusion defects were present in 12 patients (24%), while fixed perfusion defects were present in six subjects (12%).
Patients with angiographic coronary lesions showed at CMR examination lower LV ejection fraction (53 vs. 61%, p = 0.03) and thicker interventricular tele-diastolic septum (1.0 vs. 0.8 cm, p = 0.03) compared to patients without coronary lesions.
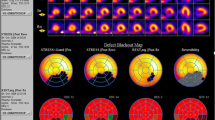
Figure 2 shows CMR stress perfusion, delayed enhancement and coronary angiography images of true positive (a), false positive (b) and false negative (c) CMR cases.
CMR stress perfusion, delayed enhancement and coronary angiography images of true positive (a), false positive (b) and false negative (c) CMR cases. Basal basal short axis view, mid mid short axis view, apical apical short axis view, Perfusion stress perfusion CMR images acquired in short axis views after 3 min of adenosine infusion, Delay delay gadolinium enhancement images acquired 10–12 min after stress perfusion, coronary angiography two orthogonal images of coronary angiograms
Table 5 shows specific performance of stress perfusion CMR in diagnosis of coronary stenosis. The diagnostic accuracy was higher when the analysis was performed only in proximal coronary arteries (95%, CI 86–99) compared to overall vessels (84%, CI 70–92).
Between the four false negative patients only one had proximal coronary stenosis that was a significant (70%) right coronary artery stenosis. In the other three cases, the coronary obstruction was in the mid or distal tract.
Three of the four false positive patients presented reversible perfusion defects in the septal segments and only one in the inferior wall.
The only patient with significant LMCA stenosis resulted positive at stress perfusion CMR.
Discussion
The present study shows the feasibility and suggests the utility of stress perfusion CMR in follow up of patients after PCI of LMCA. In this selected population adenosine stress perfusion CMR showed a good accuracy (84%, CI 70–92%) in diagnosis of significant coronary stenosis. Accuracy was higher (95%, CI 86–99) when the analysis was restricted to proximal coronary segments (Table 5). Sensitivity of stress perfusion CMR was 89% (CI 75–97%) for diagnosis of proximal coronary artery stenosis, and the only false negative patient (one over five) presented a 70% stenosis of the right coronary artery. The only patient with significant restenosis of LMCA resulted positive on stress perfusion CMR.
In this study 12 (24%) patients had significant coronary stenosis, but only 5 (10%) presented proximal lesions. The relatively small number of patients with significant stenosis can be due to selection bias and to the late timing of scheduled CXA (mean time from prior LMCA PCI was 228 ± 108 days). In fact we know from literature that more severe patients present some of the exclusion criteria for CMR (Table 1) or die in the first months after the LMCA PCI [26].
Overall, our stress perfusion sensitivities of 67% for total segment analysis and 80% for proximal segments are comparable to those of published CMR data [10].
Despite the elderly study population (mean age 70 ± 9 years), CMR was well tolerated without any serious adverse event. Only one patient over fifty interrupted the examination for claustrophobic reaction and was not included in the final analysis.
In LMCA stenosis CABG is still the first choice treatment, but PCI can be a valid alternative for a select proportion patients [4, 27]. Improvements in both devices and techniques have made LMCA PCI an effective and safe procedure [1], but restenosis is still unpredictable and carries a risk of sudden death [28]. For that reason, most centres performing this procedure mandate angiographic follow-up at 9–12 months, although no single prospective study is available on the impact of angiographic follow-up after LMCA PCI. Patients undergoing unprotected LMCA PCI often are elderly, have frequent serious comorbidities and consequently have high event rates [29]. In these fragile patients elective CXA remains a safe procedure, but presents increased risks of complications [30,31,32].
Stress perfusion CMR is a non-invasive test that has a high diagnostic performance compared to conventional CXA [8,9,10,11] and a high prognostic value [12]. Other non-invasive diagnostic techniques, such as single-photon-emission-computed-tomography [33] or cardiac computed tomography angiography [34], showed a good accuracy for stent restenosis, but CMR has the advantage of avoiding radiations, although it is a time consuming procedure which requires prolonged patient collaboration.
To the best of our knowledge, no published report exist evaluating stress perfusion CMR in follow-up of patients who had undergone LMCA PCI. In this specific setting, we showed for the first time in a pilot prospective study that this diagnostic imaging technique is feasible, safe and accurate.
We have included in the study a significant proportion of elderly patients (mean age 70 ± 9 years), with prior myocardial infarction (n = 31, 63%) and with multivessel PCI (n = 39, 76%), which may complicate the diagnosis of ischemia, but overall diagnostic accuracy was not impaired compared with literature data [8,9,10,11].
To reproduce current clinical practice, we performed visual analysis of stress CMR perfusion defects and angiographic stenosis and we compared a functional imaging test like CMR stress perfusion with the angiography of the coronary arteries as the gold standard. In a small subgroup (n = 22) of patients with LMCA stenosis from CE-MARC study [35] it has been demonstrated that quantitative CMR analysis of myocardial perfusion compared to visual CMR analysis did not increase diagnostic sensitivity. We know from literature [10] that comparing a functional test with the morphology of the coronary arteries as the gold standard without additional functional assessment has its limitations, since myocardial perfusion is not determined only by epicardial coronary stenoses, but also by collateral flow and microcirculatory conditions. These discrepancies could explain some of the stress CMR false negative and false positive cases and could be solved by future studies comparing in this specific patients population stress perfusion CMR with functional measures, like fractional flow reserve [36].
The small sample size and the single-centre design are the major drawbacks of this study. Given the limited number of patients with significant stenosis, we could evaluate the diagnostic accuracy of stress perfusion CMR only on a per patient basis, but not on a vessel basis. On the other hand, the strict enrolment criteria and the rigorous methodology of this pilot prospective study limit the presence of confounding factors.
Additionally, no prognostic information can be drawn from our data, as the CMR perfusion results were unknown to the interventional cardiologist, and therefore did not influence patient management in the catheterization laboratory.
Adenosine stress perfusion CMR is a non-invasive and reliable method for diagnosis of significant coronary artery disease.
In this prospective study, we showed for the first time the feasibility and accuracy of adenosine stress perfusion CMR in follow up of patient undergone LMCA PCI. Accuracy for diagnosis of proximal coronary stenosis (95%, CI 86–99%) was higher than for all coronary lesions (84%, CI 70–92%).
Stress perfusion CMR could strongly reduce the need for elective CXA in follow up of LMCA PCI and should be validated in further multicenter prospective studies.
References
Fajadet J, Chieffo A (2012) Current management of left main coronary artery disease. Eur Heart J 33:36–50
Cohen MV, Gorlin R (1975) Main left coronary artery disease. Clinical experience from 1964 to 1974. Circulation 52:275–285
Taylor HA, Deumite NJ, Chaitman BR, Davis KB, Killip T, Rogers WJ (1989) Asymptomatic left main coronary artery disease in the Coronary Artery Surgery Study (CASS) registry. Circulation 79:1171–1179
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A (2014) ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35:2541–2619
Cherradi R, Ouldzein H, Zouaoui W, Elbaz M, Puel J, Carrié D (2008) Clinical and angiographic results of angioplasty with a paclitaxel-eluting stent for unprotected left main coronary artery disease (a study of 101 consecutive patients). Arch Cardiovasc Dis 101:11–17
Price MJ, Cristea E, Sawhney N, Kao JA, Moses JW, Leon MB, Costa RA, Lansky AJ, Teirstein PS (2006) Serial angiographic follow-up of sirolimus-eluting stents for unprotected left main coronary artery revascularization. J Am Coll Cardiol 47:871–877
Biondi-Zoccai GGL, Giraudi E, Moretti C, Sciuto F, Omedè P, Sillano D, Garrone P, Trevi GP, Sheiban I (2010) Impact of routine angiographic follow-up after percutaneous coronary drug-eluting stenting for unprotected left main disease: the Turin Registry. Clin Res Cardiol Off J Ger Card Soc 99:235–242
Schwitter J, Arai AE (2011) Assessment of cardiac ischaemia and viability: role of cardiovascular magnetic resonance. Eur Heart J 32:799–809
Klein C, Nagel E, Gebker R, Kelle S, Schnackenburg B, Graf K, Dreysse S, Fleck E (2009) Magnetic resonance adenosine perfusion imaging in patients after coronary artery bypass graft surgery. JACC Cardiovasc Imaging 2:437–445
Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schonberg SO, Luchner A, Strohm O, Ahlstrom H, Dill T, Hoebel N, Simor T, for the MR-IMPACT Investigators (2013) MR-IMPACT II: magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 34:775–781
Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S (2012) Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 379:453–460
Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I (2007) Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation 115:1769–1776
Bruder O, Schneider S, Nothnagel D, Dill T, Hombach V, Schulz-Menger J, Nagel E, Lombardi M, Rossum AC van, Wagner A, Schwitter J, Senges J, Sabin GV, Sechtem U, Mahrholdt H (2009) EuroCMR (European Cardiovascular Magnetic Resonance) registry: results of the German pilot phase. J Am Coll Cardiol 54:1457–1466
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee (2003) Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42:1206–1252
Deckers JGM, Schellevis FG, Fleming DM (2006) WHO diagnostic criteria as a validation tool for the diagnosis of diabetes mellitus: a study in five European countries. Eur J Gen Pract 12:108–113
Boden WE (2003) Therapeutic implications of recent ATP III guidelines and the important role of combination therapy in total dyslipidemia management. Curr Opin Cardiol 18:278–285
Van Rossum AC, Visser FC, Van Eenige MJ, Valk J, Roos JP (1987) Oblique views in magnetic resonance imaging of the heart by combined axial rotations. Acta Radiol 28:497–503
Steenbeck J, Pruessmann K (2001) Technical developments in cardiac MRI: 2000 update. Rays 26:15–34
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 9:240–245
Seiler CH, Kirkeeide RL, Gould KL (1992) Basic structure-function relations of the epicardial coronary vascular tree. Basis of quantitative coronary arteriography for diffuse coronary artery disease. Circulation 85:1987–2003
Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S (2004) Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation 110:1535–1541
Klem I, Heitner JF, Shah DJ, Sketch MH Jr, Behar V, Weinsaft J, Cawley P, Parker M, Elliott M, Judd RM, Kim RJ (2006) Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol 47:1630–1638
Schwitter J, Wacker CM, Rossum AC van, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HBW, Flamm SD, Marquardt M, Johansson L (2008) MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 29:480–489
Farooq V, Klaveren D van, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, Colombo A, Holmes DR, Mack M, Feldman T, Morice M-C, Ståhle E, Onuma Y, Morel M, Garcia-Garcia HM, Es GA van, Dawkins KD, Mohr FW, Serruys PW (2013) Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 381:639–650
Giang T, Nanz D, Coulden R, Friedrich M, Graves M, Alsaadi N, Luscher T, Vonschulthess G, Schwitter J (2004) Detection of coronary artery disease by magnetic resonance myocardial perfusion imaging with various contrast medium doses: first european multi-centre experience. Eur Heart J 25:1657–1665
Khattab AA, Abdel-Wahab M, Röther C, Liska B, Toelg R, Kassner G, Geist V, Richardt G (2008) Multi-vessel stenting during primary percutaneous coronary intervention for acute myocardial infarction. A single-center experience. Clin Res Cardiol Off J Ger Card Soc 97:32–38
Tan WA, Tamai H, Park S-J, Plokker HT, Nobuyoshi M, Suzuki T, Colombo A, Macaya C, Holmes DR, Cohen DJ, others (2001) Long-term clinical outcomes after unprotected left main trunk percutaneous revascularization in 279 patients. Circulation 104:1609–1614
Roques F, Nashef SA, Michel P, Gauducheau E, Vincentiis C de, Baudet E, Cortina J, David M, Faichney A, Gabrielle F, Gams E, Harjula A, Jones MT, Pintor PP, Salamon R, Thulin L (1999) Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 15:816-822-823
Mencel G, Kowalczyk J, Lenarczyk R, Chodór P, Wąs T, Świerad M, Honisz G, Świątkowski A, Woźniak A, Kalarus Z, Sredniawa B (2016) The impact of routine angiographic follow-up in a population of patients undergoing percutaneous coronary intervention within the left main coronary artery. Angiology 67:742–748
Dai X, Busby-Whitehead J, Alexander KP (2016) Acute coronary syndrome in the older adults. J Geriatr Cardiol JGC 13:101
Rosengren A, Wallentin L, Simoons M, Gitt AK, Behar S, Battler A, Hasdai D (2006) Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J 27:789–795
Bach RG, Cannon CP, Weintraub WS, DiBattiste PM, Demopoulos LA, Anderson HV, DeLucca PT, Mahoney EM, Murphy SA, Braunwald E (2004) The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann Intern Med 141:186–195
Giedd KN, Bergmann SR (2004) Myocardial perfusion imaging following percutaneous coronary intervention. J Am Coll Cardiol 43:328–336
Graaf FR de, Schuijf JD, Velzen JE van, Boogers MJ, Kroft LJ, Roos A de, Reiber JHC, Sieders A, Spanó F, Jukema JW, Schalij MJ, Wall EE van der, Bax JJ (2010) Diagnostic accuracy of 320-Row multidetector computed tomography coronary angiography to noninvasively assess in-stent restenosis Invest Radiol 45(6):331–340
Greenwood JP, Kidambi A, Maredia N, Mohee K, Sourbron S, Motwani M, Uddin A, Ripley DP, Herzog BA, Zaman A, Dickinson CJ, Brown J, Nixon J, Everett C, Plein S (2013) Visual and quantitative perfusion analysis in left main stem disease: a CE-MARC substudy. J Cardiovasc Magn Reson 15:P195
Hussain ST, Chiribiri A, Morton G, Bettencourt N, Schuster A, Paul M, Perera D, Nagel E (2016) Perfusion cardiovascular magnetic resonance and fractional flow reserve in patients with angiographic multi-vessel coronary artery disease. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 18:44
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No relevant conflict of interest in this manuscript to disclose.
Rights and permissions
About this article
Cite this article
Nanni, S., Lovato, L., Ghetti, G. et al. Utility of stress perfusion-cardiac magnetic resonance in follow-up of patients undergoing percutaneous coronary interventions of the left main coronary artery. Int J Cardiovasc Imaging 33, 1589–1597 (2017). https://doi.org/10.1007/s10554-017-1149-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1149-4