Abstract
By using late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) imaging, we compared left atrial late gadolinium enhancement (LA-LGE) quantification methods based on different references to characterize the left atrial wall in patients with atrial fibrillation (AF). Thirty-eight patients who underwent three-dimensional LGE-CMR imaging before catheter ablation for AF were classified into three groups depending on their clinical AF type: (1) paroxysmal AF (PAF; n = 12); (2) persistent AF (PeAF; n = 16); and (3) recurrent AF after catheter ablation (RAF; n = 10). To quantify LA-LGE on LGE-CMR imaging, we used the thresholds of 2 standard deviations (2-SD), 3-SD, 4-SD, 5-SD, or 6-SD above the mean signal from the unenhanced left ventricular myocardium, and we used the full width at half maximum (FWHM) technique, which was based on the maximum signal from the mitral valve with high signal intensity. The 6-SD threshold and FWHM techniques were statistically reproducible with an intraclass correlation coefficient >0.7. On applying the FWHM technique, the normalized LA-LGE volume by LA wall area showed a significant difference between the RAF, PeAF, and PAF groups (0.22 ± 0.04, 0.16 ± 0.06, and 0.09 ± 0.03 mL/cm2, respectively) (P < 0.05). Furthermore, most of the fibrotic scarring and low-voltage tissue on the electroanatomic map corresponded well with the extent of LA-LGE. The FWHM technique based on the mitral valve can provide a reproducible quantification of LA-LGE related to AF in the thin LA wall.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In clinical practice, atrial fibrillation (AF) is the most common arrhythmia that can lead to morbidity and mortality [1]. Subsequent explorations of the left atrial (LA) substrate suggest that AF may be triggered and aggravated by progressive electrical and tissue fibrosis [2]. Furthermore, LA fibrosis may be arrhythmogenic, leading to more aggressive symptoms and affecting patient outcomes in the management of AF [3, 4]. Therefore, a noninvasive technique to evaluate the LA wall composition has been investigated to understand and properly manage atrial fibrillation.
Late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) imaging is an established method for visualizing myocardial infarction or fibrosis [5–7]. Hyperenhancement by LGE-CMR occurs because of altered washout kinetics of gadolinium relative to normal tissue, which may reflect the increased interstitial space of the myocardium with fibrosis [7]. In patients with AF, LGE-CMR has been considered to be a promising method for noninvasively characterizing the composition of the LA wall. However, the thin LA wall with adjacent structures such as blood, spine, and esophagus may limit the correct selection of the left atrial myocardium on LGE-CMR images. When analyzing LGE-CMR images, the two important areas of focus are (1) the left ventricular (LV) myocardium of nulled signal intensity and (2) the mitral valve with high signal intensity. For the first finding, the LGE-CMR technique enhances a relative signal difference between the injured tissue and the reference normal myocardium by using an inverse recovery pulse to null the signal from the reference LV myocardium [8]. For the second area of focus, the mitral valves have a fibrous skeleton, which can show dense hyperenhancement on an LGE-CMR image [9]. Therefore, it can be hypothesized that the hyperenhanced mitral valve or the unenhanced LV myocardium may be useful references in the quantification analysis by LGE-CMR of thin LA walls.
To the best of our knowledge, a left atrial late gadolinium enhancement (LA-LGE) quantification method based on the signal intensity from the LV myocardium or the mitral valve has not yet been studied. Therefore, we assessed the feasibility of LA-LGE quantification methods based on the LV myocardium and mitral valve signal intensity to analyze the LA wall composition associated with atrial fibrillation by using LGE-CMR.
Materials and methods
Patient selection
The study protocol was approved by the Institutional Review Board. All patients provided written, informed consent. Forty-two patients were selected; they underwent LGE-CMR examination for imaging of the left atrium before catheter ablation for symptomatic AF at the Korea University Anam Hospital. In this study, the exclusion criteria included the presence of a contraindication for CMR (e.g. severe claustrophobia, automatic implantable defibrillators, pacemakers, or intracranial aneurysm clips). Of the 42 selected patients, 4 patients were excluded because they showed an inappropriately nulled LV myocardial signal (n = 3) or prominent LV myocardial hyperenhancement (n = 1) on the LGE-CMR images. During the LGE image acquisition, the mean heart rate of all of the patients was 70.5 ± 17.5 beats per minute (BPM). The AF attack could not be diagnosed by electrocardiography (ECG) monitoring from the CMR console.
The remaining 38 enrolled patients were classified into three AF groups, depending on the clinical AF manifestation: (1) paroxysmal AF (PAF) group, (2) persistent AF (PeAF) group, and (3) recurrent AF (RAF) group. The PAF group consisted of patients who had a history of one or more episodes of self-terminating AF that lasted <7 days without catheter ablation [10]. The PeAF group consisted of patients who had an AF episode that lasted longer than 7 days or required termination by cardioversion without catheter ablation [10]. The RAF group included the patients who underwent previous catheter ablation for pulmonary vein isolation, but still had symptomatic AF lasting over 5 min [11].
CMR protocol
For patients with AF, a pre-procedural CMR examination was performed using a 3-T magnetic resonance system (Achieva; Philips Medical Systems, Best, The Netherlands) with a 32-element phased-array cardiac coil. Because all enrolled patients were referred for catheter ablation in order to perform the pulmonary vein isolation, a three-dimensional (3D) spoiled gradient-echo contrast-enhanced timing robust angiography (CENTRA) was performed to define the anatomy of the left atrium and the pulmonary veins after the injection of 0.2 mmol/kg gadolinium contrast (Dotarem; Guerbet, S.A., Villepinte, France).
To depict the injured area of the left atrium, high spatial resolution LGE-CMR images were acquired approximately 20 min after the injection of the contrast agent using a 3D inversion recovery-prepared, respiration-navigated, ECG-gated, gradient-echo pulse sequence after the Look–Locker sequence to identify the optimal nulling time for the LV myocardium. Typical acquisition parameters were free-breathing using a respiratory navigator with a 6-mm acceptance window; a transverse imaging volume with a voxel size of 1.5 × 1.5 × 1.5 mm; TR/TE of 4.7/1.4 ms; TI of 230–270 ms; flip angle of 25°; bandwidth of 127 Hz/pixel; 1 R wave to R wave (RR) interval between inversion pulses; phase encoding in the right-left direction; and parallel imaging using the sensitivity encoding (SENSE) technique with R = 2. The LGE-CMR image data were acquired within a window of 100–150-ms for each RR interval. Respiratory navigator inflow artifacts were reduced by lowering the navigator rescale factor and positioning the navigator away from the right-sided pulmonary vein. Free-breathing images that were acquired 15–25 min post-injection, depending on the successful leading navigator placement, aimed for a navigator efficiency of >30 %. All LGE-CMR images were transferred into the workstation for quantification analysis.
Electroanatomic mapping
At the beginning of the catheter ablation procedure for AF, a detailed voltage map of the LA was obtained in 33 (87 %) of the 38 enrolled patients with the 3D electroanatomic mapping system CARTOMERGE (Biosense Webster, Diamond Bar, CA, USA). Of these 33 patients, 6 (18 %) had a history of catheter ablation before the acquisition of the voltage map. Electroanatomic (EA) mapping based on the tissue voltage measurement was performed during sinus rhythm. Efforts were made to distribute the measurement points evenly throughout the LA, and the bipolar voltage was measured peak-to-peak with the signal filtered from 30 to 400 Hz. Endocardial contact of the mapping catheter (Navistar-ThermoCool, Biosense Webster) was visually confirmed with fluoroscopy and intracardiac echocardiography through the CARTO 3D navigation system to indicate that the catheter was stable in space and in good contact with the LA wall. The electroanatomic map consisted of color-coded pixels according to tissue voltage: the healthy tissue (voltage >1 mV, purple), low-voltage tissue (voltage >0.1 to <0.5 mV, red), and fibrotic scar (voltage <0.1 mV, gray).
Analysis of the LGE-CMR images
To quantify the LA wall composition, all LGE-CMR images were analyzed using commercial software (Terarecon iNtuition; TeraRecon, Foster City, CA, USA) by a reader (S.H.H) who had 6 years of experience in CMR imaging. On every transverse high resolution late gadolinium enhancement (LGE) image, the pixels showing hyperenhancement in the LA wall were identified using the full width at half maximum (FWHM) technique and the six-standard deviation (6-SD), 5-SD, 4-SD, 3-SD, and 2-SD thresholds above the mean signal in the unenhanced LV wall [12]. This quantification method involved the manual delineation of 2 regions of interest (ROIs) on the LGE images that included, to the greatest possible extent, the unenhanced LV myocardium and the mitral valve leaflet with high signal intensity. The first ROI was dictated by the boundaries in the unenhanced LV wall to generate the mean and SD values of the nulled myocardial signal for the 6-SD, 5-SD, 4-SD, 3-SD, and 2-SD thresholds. The second ROI was drawn around the mitral valve leaflet of high signal intensity to define the maximum signal for the FWHM technique, which provided the signal threshold as one-half of the maximum signal from the mitral valve. Then, the LA wall pixels with signal intensities greater than each of the established thresholds were automatically selected (Fig. 1). Additional manual correction was required for any obvious pools of blood that needed to be manually removed from all automated ROIs and assigned to the LA-LGE pixels. The summation of the pixels for the LA-LGE over all slices was defined as the LA-LGE volume (in mL). After the semiautomatic identification of the LA-LGE pixels, the left atrium was semiautomatically segmented to reformat the 3D volume-rendered LGE-CMR image based on a gray color scale. The segmented LA-LGE pixels were then applied with a color look-up table mask on the 3D volume-rendered LGE-CMR images for better differentiation between the LA-LGE and the unenhanced LA wall tissue (Fig. 2).
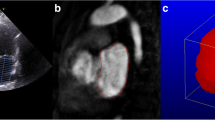
The analysis process of the left atrial LGE in basis of different tissue references. a A semiautomated algorithm uses the voxels corresponding to the LGE, based on each quantification method, through a transverse LGE-CMR slice that includes the unenhanced LV myocardium or the hyperenhanced mitral valve. b, c The threshold algorithms for identifying LGE voxels are established based on the measured signal intensity from (b) the left ventricular wall or (c) the mitral valve. d The LGE voxels within the epicardial and endocardial borders of the left atrium are manually selected and assigned to the LA-LGE, depending on the quantification method. e, f All voxels assigned to the LA-LGE are (e) color-coded and (f) summated to measure the LA-LGE volume. LA-LGE left atrial late gadolinium enhancement, LG-CMR late gadolinium enhancement cardiac magnetic resonance, LGE late gadolinium enhancement, LV left ventricular
Three-dimensional volume-rendered LGE-CMR images are color-coded by each quantification method [i.e. the 2-SD, 3-SD, 4-SD, 5-SD, 6-SD thresholds, and the full width at half maximum (FWHM) technique]. The yellow or reddish left atrial wall areas show variable distribution and extent, depending on the quantification method selected for LGE
To assess the interobserver and intraobserver reproducibility of each quantification method, the LA-LGE volumes were measured twice, separated by a 1-month interval, by two independent readers (S.H.H and D.I.L). In addition, to compare the LA-LGE measurements between the different AF groups, the normalized LA-LGE volumes by LA wall area were calculated. In the assessment of the LA wall area value (in cm2), the three diameters (i.e., the radii of the ellipse) of the left atrium on the LGE-CMR images were measured along the three orthogonal planes. The anterior-posterior and longitudinal diameter were measured at the midpoint of the transverse diameter by using the oblique axial and sagittal LGE images of the left atrium. By using an oblique axial LGE-CMR image, the transverse diameter of the left atrium was defined as the distance between the midpoint of the pulmonary veins on the right and left sides of the left atrium. The LA wall area was then calculated by using the formula for a scalene ellipsoid (i.e. the Knudsen formula) [13]:
in which “a”, “b”, and “c” are the transverse, anterior-posterior, and longitudinal diameters, respectively, of the left atrium and “p” is 1.6075.
Comparison between LGE-CMR and the electroanatomic map
To compare the extent of LA-LGE with the EA map results (Fig. 3), the left atrial model for each of the EA maps and the 3D volume-rendered LGE-CMR image was subdivided into 18 sectors: 9 on the posterior wall and nine on the anterior and septal walls of the LA in accordance with the previously reported method [14]. Using the statistically reproducible quantification method to define the LA-LGE, the visual extent of LA-LGE on the 3D volume-rendered LGE-CMR image was evaluated by two readers (S.H.H and D.I.L) with consensus. Depending on the extent of LA-LGE, all 18 sectors were assigned into two sector groups: LGE sectors (LA-LGE involved ≥50 % of the corresponding sector area) and less-extensive LGE sectors (LA-LGE involved <50 % of the corresponding sector area). The extent of low-voltage tissue and fibrotic scaring on the EA map was evaluated by two independent catheter ablation experts (J.M.S and S.W.P) with consensus. Depending on the extent of the fibrotic scarring or low-voltage tissue, all 18 sectors on the EA map were classified into two sector groups: injured sectors (gray or red pixels involved ≥50 % of the corresponding sector area) and the less-injured sectors (gray or red pixels involved <50 % of the corresponding sector area).
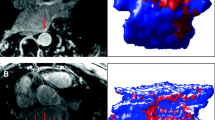
Three-dimensional left atrial models from the electroanatomic (EA) map and the volume-rendered LGE-CMR images in patients with persistent atrial fibrillation. a The EA map shows multiple patchy regions of electrically fibrotic scarring (gray) interspersed with electrically low-voltage tissue (red) in the posterior left atrial wall. b The LGE-CMR image reveals a large amount of color-coded hyperenehancement in the posterior left atrial wall
Statistical analysis
Statistical calculations were performed by SPSS software (version 19.0; IBM, Somers, NY, USA). All continuous data were expressed as the mean ± SD and all categorical data were presented as the number (%). Analysis of variance (ANOVA) with Tukey`s test was used to compare the LA-LGE volumes of the different quantification methods. For each quantification method, the interobserver and intraobserver reproducibility were assessed using the intraclass correlation coefficient (ICC) [15]. Methods with an ICC >0.7 were considered statistically acceptable [12]. The significance of the interobserver and intraobserver mean differences in LA-LGE volume was assessed using a paired t test. By applying a statistically accepted quantification method for the LA-LGE, the ANOVA with Tukey`s test was again used to compare the left atrial measurements by LGE-CMR among the three different AF groups. The Chi square test was used to compare categorical variables between the LGE-CMR and EA map. A P value <0.05 was considered statistically significant.
Results
Table 1 summarizes the baseline characteristics of the enrolled patients. Among the 38 patients, 32 (84 %) were men, the mean patient age was 58 ± 10 years, 28 (74 %) patients had no history of catheter ablation for AF, and 12 (43 %) patients and 16 (57 %) patients were assigned into the PAF group and PeAF group, respectively. In contrast, 10 (26 %) patients had a history of catheter ablation before the CMR examination for this study and were assigned to the RAF group. The mean interval between the CMR and the last catheter ablation was 14 ± 7 months, with an interval ranging from 7 to 23 months.
The 3D volume-rendered LA-LGE images (Fig. 2) show the variable extent of LA-LGE depending on the selection of the six quantification methods (i.e. 2-SD, 3-SD, 4-SD, 5-SD, 6-SD, and the FWHM technique). The ANOVA with Tukey`s test showed that the LA-LGE volume measurements could be statistically different based on the quantification method (Fig. 4). However, there was no statistical difference (P > 0.05) in the LA-LGE volume measurements between the 5-SD threshold, the 6-SD threshold, and the FWHM technique. Table 2 summarizes the reproducibility of the LA-LGE volume measurements. Of the 6 quantification methods evaluated in this study, the 6-SD threshold and FWHM technique were statistically accepted (i.e. the ICC was >0.7 for both), and showed no significant difference in the LA-LGE volume measurements assessed by different readers on different days (P > 0.05).
A comparison of the left atrial late gadolinium enhancement (LA-LGE) volume measurements between the different quantification methods for late gadolinium enhancement (LGE) shows that increasing the signal threshold to define the LGE contributes to a decrease in the LA-LGE volume measurements. However, there is no significant difference in the LA-LGE volume measurements between the full width at half maximum (FWHM) technique, the 5-standard deviation (5-SD) threshold, and the 6-SD threshold
Table 3 summarizes all measurements, including the LA-LGE volume and LA wall area, by patient group. The mean LA wall area was significantly less in the RAF group than in the PAF group (16.5 ± 1.7 vs. 21.7 ± 5.0 cm2, respectively; P < 0.01) or in the PeAF group (16.5 ± 1.7 vs. 21.7 ± 5.0 cm2, respectively; P < 0.01). However, the PAF and PeAF groups showed no significant differences in the LA wall area (P > 0.05). By applying the statistically accepted 6-SD threshold and the FWHM technique in comparing LA-LGE (Fig. 5), the mean LA-LGE volume was significantly less (P < 0.05 for both methods) in the PAF group than in the PeAF and in the RAF groups, which showed no significant difference in LA-LGE volume (P > 0.05). Furthermore, in comparing the normalized LA-LGE volumes by LA wall area between the patient groups (Fig. 6), the mean normalized LA-LGE volumes obtained by the 6-SD threshold technique in the RAF, PeAF, and PAF groups were found to be 0.22 ± 0.04, 0.13 ± 0.05, and 0.08 ± 0.04 mL/cm2, respectively, which were significantly different (P < 0.01). In addition, the mean normalized LA-LGE volumes obtained by the FWHM technique in the RAF, PeAF, and PAF groups were 0.22 ± 0.04, 0.15 ± 0.06, and 0.09 ± 0.03 mL/cm2, respectively, which were also significantly different (P < 0.01).
A comparison of high resolution LGE-CMR images between the three atrial fibrillation patient groups. a, b, c Transverse LGE-CMR images. d, e, f The segmentation of left atrial hyperenhancement (green color) on the LGE-CMR images applied to the full width at half maximum technique for quantification of the LGE pixels. The paroxysmal AF (PAF) image shows small foci of hyperenhancement in the LA wall and mitral valve leaflet. The persistent AF (PeAF) image shows subendocardial linear hyperenhancement in the posterior LA wall. Furthermore, the recurrent AF (RAF) image shows multiple thickened LA wall regions of hyperenhancement
A comparison of the normalized left atrial late gadolinium enhancement (LA-LGE) volumes between the three patient groups. a, b When applied to (a) the full width at half maximum (FWHM) technique and (b) the 6-standard deviation (6-SD) threshold, the patient groups showed a significant difference in the normalized LA-LGE volume. The recurrent atrial fibrillation (RAF) group has the highest normalized LA-LGE volume. AF atrial fibrillation, PAF paroxysmal AF, PeAF persistent AF, RAF recurrent atrial fibrillation, SD standard deviation
Table 4 summarizes a sector-based comparison of the extent of LA-LGE depending on the low-voltage tissue and fibrotic scarring on the EA map. Of a total of 594 sectors on the EA map, 50 (8 %) were assigned to the injured sector and 544 (92 %) were assigned to the less-injured sector. The injured sector group had an increased prevalence of LGE sectors by the 6-SD threshold technique and by the FWHM technique (P < 0.01, respectively) than the less-injured sector group. Using the 6-SD threshold technique to define the LA-LGE, the LGE sectors corresponded to 82 % (41 out of 50) of the injured sectors and 4 % (22 out of 523) of the less-injured sectors. Using the FWHM technique to define the LA-LGE, the LGE sectors corresponded to 86 % (43 out of 50) of the injured sector and 4 % (21 out of 523) of the less-injured sector.
Discussion
The aim of this study was to establish the feasibility of a new quantification method for LA-LGE in patients with AF. We used the unenhanced LV myocardium and the fibrotic component of the mitral valve as the reference to evaluate the signal intensities from the thin LA wall on LGE-CMR images. In this study, the selection of the quantification technique to define the hyperenhancement could affect the LA-LGE volume measurement and its reproducibility. When using the unenhanced LV myocardium as the reference, a high interval (i.e. the 6-SD threshold) above the mean signal intensity from the reference promoted the reproducibility of the LA-LGE volume assessment. When applying the high signal intensity mitral valve as the reference, the FWHM technique based on the maximum signal of the mitral valve provided a statistically reproducible assessment of LA-LGE volume. By applying the 6-SD threshold and the FWHM techniques on different references, the quantified measurements of the normalized LA-LGE volume by LA wall area were statistically different depending on the AF type (i.e., PAF, PeAF, and recurrent AF after catheter ablation). Furthermore, most of the fibrotic scarring and low-voltage tissue on the EA map corresponded well to the extent of LA-LGE defined using the 6-SD threshold and FWHM techniques.
In this study, the LGE-CMR technique we used for imaging the left atrium is based on a 3D high-resolution sequence [16]. This technique achieves a much greater spatial resolution compared to 2D LGE-CMR, which is used to evaluate left ventricular wall viability [16, 17]. In addition, the post-processing of the images obtained by the 3D LGE-CMR sequence greatly facilitates a more comprehensive evaluation of the LA wall status and can provide a view of the entire LA wall volume rendered in 3D from the slice data set [14]. However, the greatest difficulty in the analysis of an LGE-CMR image is establishing the pathophysiologic status of the myocardium that corresponds to the LGE [17, 18]. Quantitative assessment of myocardial LGE usually needs an objective and definite threshold because LGE is determined by the relative difference of signal intensity between the injured myocardium and the Ref. [17, 18]. The blood pool in the left atrium and the unenhanced left atrial wall itself are commonly selected as the reference to define the LA-LGE [14, 17, 19]. However, it is not easy to confine the normal myocardium in the thin LA wall, and the blood signal intensity can change with time. Therefore, we suggest using the mitral valve leaflet and the left ventricular wall as the reference for LA-LGE. The results of the current study demonstrated that these different tissues can act as an acceptable reference in the quantification of LA-LGE.
The FWHM technique is one of the most reproducible methods in quantifying myocardial LGE [12, 20]. However, the FWHM technique has potential limitations with regard to its accuracy if a myocardial lesion is homogeneously gray on the LGE-CMR image [20]. The FWHM with a threshold of half the maximal signal necessarily assumes that a LA wall injury has a composition similar to the dense fibrosis of the mitral valve leaflet. Therefore, the LA wall pixel of a homogenously gray signal intensity corresponding to the injured myocardium may be less accurately delineated using the FWHM technique. In our experience, the maximum signal intensity from the mitral valve is nevertheless easily detected on multiple 3D LGE-CMR slices with thin slice thickness. Therefore, a FWHM technique that is based on the maximum mitral valve signal intensity seems to be more straightforward in the quantification of LA-LGE.
Different signal thresholds that are based on low-signal intensity reference tissue also need objective criteria to determine the impact interval of signal intensity between the threshold and the reference on the LGE-CMR image. In general, the standard deviation of signal intensity from the reference tissue is used for the signal intensity interval criteria [12, 19]. When a target tissue shows at least a 2-SD higher signal intensity than the mean signal intensity of the reference tissue on LGE-CMR image, the difference can be statistically accepted [12, 21]. Bondarenko et al. [21] studied the mean 2-SD to 6-SD threshold techniques for analyzing myocardial infarct size by using unenhanced myocardium. They reported that the 6-SD signal threshold was a predictive technique. Malcolme-Lawes et al. [19] selected the blood pool as the reference tissue for the definition of LA-LGE. They reported that the LA regions with signal intensities that were above 3-SD, 4-SD, and 5-SD from the mean of the referenced blood pool were correlated with significant reductions in bipolar and unipolar voltages in the LA wall. The current study results showed that using a high SD interval above the mean signal intensity from the unenhanced myocardium effectively resulted in a reproducible measurement of the LA-LGE volume.
Multiple classification schemes for AF have been proposed, with a consensus driven by the desire for simplicity and clinical relevance [10]. In a person with 2 or more episodes, AF is considered “recurrent”; if AF terminates spontaneously, then recurrent AF is designated as “paroxysmal”; if AF is sustained, it is designated to be “persistent” [1]. Furthermore, PeAF usually has worse clinical manifestations and higher risk of AF recurrence after catheter ablation compared to PAF [1]. Left atrial remodeling promotes the occurrence and maintenance of AF, typically manifesting as atrial dilatation and atrial wall fibrosis [22, 23]. Some authors insist that LA remodeling is closely associated with the advancement and chronicity of AF [22, 23], and that PeAF with LA remodeling commonly results in a poor prognosis after catheter ablation [14, 22, 23]. If PeAF and PAF can be considered to be on different ends of the AF spectrum with respect to atrial remodeling, it can be hypothesized that aggravated LA remodeling with myocardial injury will contribute to the advancement and chronicity of AF into PeAF. Our study results also revealed that patients with PeAF had a greater mean LA-LGE volume as compared to patients with PAF.
After catheter ablation for AF, the reverse remodeling of the left atrium commonly manifests as a decrease in the atrial volume and an improvement in the left atrial ejection fraction [24, 25]. These manifestations of reverse LA remodeling can be considered indexes of the effective injury to the atrial wall and markers of procedural success after the catheter ablation [25, 26]. The artificial tissue injury in the left atrium after catheter ablation also shows hyperenhancement, which can be considered the LGE on an LGE-CMR image. In this study, the mean LA-LGE volume divided by the LA wall area was higher in the RAF group after catheter ablation than in the PeAF and PAF groups, which had no history of catheter ablation. The results of this study showed that the comprehensive assessment of LA remodeling, including the LA-LGE and the LA volume, could eventually help characterize the left atrium in patients with AF. Invasive electroanatomical mapping is an important tool to help determine LA wall scarring or fibrosis during the catheter ablation procedure [27]. The noninvasive evaluation of the LA wall with advancements in the LGE-CMR technique nevertheless has been considered a promising method for providing a comprehensive evaluation of LA remodeling in patients with AF.
This study had several limitations. First, the sample size in this study was small. A large follow-up study will help to better define the clinical significance of the quantification methods used in this study for LA-LGE volume measurements in patients with AF. Second, although the LA-LGE defined using the 6-SD threshold and the FWHM techniques showed a significant correlation to the fibrotic scarring and low-voltage tissue on the EA map, this study did not provide pathologic proof of a LA wall scar or fibrosis that corresponded with the LA-LGE. A LA wall injury may have had a signal intensity below the selected thresholds for the definition of LA-LGE. Furthermore, the detection of small LA wall injury may depend on the overall LGE-CMR image quality. Third, there were no AF patients who underwent LGE-CMR before and after the catheter ablation. Therefore, it is possible that some LA wall injury may have been present because of preexisting left atrial myopathy. However, this study showed that the LA-LGE volume changes depending on the chronicity of the AF as well as the history of catheter ablation, and the LA-injured myocardium following catheter ablation showed greater LA-LGE volume and smaller LA wall area as compared to the patients groups who had no history of catheter ablation. Fourth, detail studies using electroanatomic maps will be necessary to compare endocardial voltage and LA-LGE in the corresponding pixels. Malcolme-Lawes et al. [19] report that low endocardial voltages in the LA wall have been associated with LA-LGE, as determined by a high signal threshold. In this study, we performed visual assessment to correlate the LGE-CMR image with an electroanatomic map. However, the visual assessment is limited in its detail and its accurate registration between the LGE-CMR image and the electroanatomic map.
In conclusion, this study emphasizes that the quantification measurements of LA-LGE is associated with the chronicity of AF and myocardial injury after catheter ablation for AF. In this study, the appropriate selection of a quantification method for LA-LGE could affect the measurement of LA-LGE and promote the reproducibility of the LA-LGE quantification. Therefore, we recommend a high SD threshold (e.g. 6-SD) that is based on the unenhanced LV wall and the FWHM technique (using the maximum signal intensity from the mitral valve) to establish the signal threshold in the quantification of LA-LGE associated with myocardial status in patients with atrial fibrillation.
References
Estes NA III, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG, American College of Cardiology/American Heart Association Task Force on Performance M, Physician Consortium for Performance I, Heart Rhythm S (2008) ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): developed in collaboration with the Heart Rhythm Society. Circulation 117(8):1101–1120
Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR Jr, Ilstrup DM, Frye RL (1987) The natural history of lone atrial fibrillation: a population-based study over three decades. N Engl J Med 317(11):669–674
Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A (2005) Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol 45(2):285–292
Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, Kottkamp H, Dhein S (2004) Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 90(4):400–405
Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA (1995) Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI potential mechanisms. Circulation 92(5):1117–1125
Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF (1999) Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology 211(3):698–708
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343(20):1445–1453
Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM (2001) An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218(1):215–223
Misfeld M, Sievers HH (2007) Heart valve macro- and microstructure. Philos Trans R Soc Lond B Biol Sci 362(1484):1421–1436
European Heart Rhythm Association; Heart Rhythm Society, Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation (2006) ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). J Am Coll Cardiol 48(4):854–906
Shah AN, Mittal S, Sichrovsky TC, Cotiga D, Arshad A, Maleki K, Pierce WJ, Steinberg JS (2008) Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol 19(7):661–667
Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC (2011) Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 4(2):150–156
Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S (2000) Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation 102(21):2619–2628
Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF (2009) Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 119(13):1758–1767
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420–428
Akcakaya M, Rayatzadeh H, Basha TA, Hong SN, Chan RH, Kissinger KV, Hauser TH, Josephson ME, Manning WJ, Nezafat R (2012) Accelerated late gadolinium enhancement cardiac MR imaging with isotropic spatial resolution using compressed sensing: initial experience. Radiology 264(3):691–699
Tatli S, Zou KH, Fruitman M, Reynolds HG, Foo T, Kwong R, Yucel EK (2004) Three-dimensional magnetic resonance imaging technique for myocardial-delayed hyperenhancement: a comparison with the two-dimensional technique. J Magn Reson Imaging 20(3):378–382
Huber AM, Schoenberg SO, Hayes C, Spannagl B, Engelmann MG, Franz WM, Reiser MF (2005) Phase-sensitive inversion-recovery MR imaging in the detection of myocardial infarction. Radiology 237(3):854–860
Malcolme-Lawes LC, Juli C, Karim R, Bai W, Quest R, Lim PB, Jamil-Copley S, Kojodjojo P, Ariff B, Davies DW, Rueckert D, Francis DP, Hunter R, Jones D, Boubertakh R, Petersen SE, Schilling R, Kanagaratnam P, Peters NS (2013) Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: a 2-center study. Heart Rhythm 10(8):1184–1191
Kim HW, Farzaneh-Far A, Kim RJ (2009) Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol 55(1):1–16
Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG, Visser CA, van Rossum AC (2005) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7(2):481–485
Nattel S, Burstein B, Dobrev D (2008) Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1(1):62–73
Akkaya M, Higuchi K, Koopmann M, Damal K, Burgon NS, Kholmovski E, McGann C, Marrouche N (2013) Higher degree of left atrial structural remodeling in patients with atrial fibrillation and left ventricular systolic dysfunction. J Cardiovasc Electrophysiol 24(5):485–491
Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ (2005) Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation 112(14):2089–2095
Tops LF, Bax JJ, Zeppenfeld K, Jongbloed MR, van der Wall EE, Schalij MJ (2006) Effect of radiofrequency catheter ablation for atrial fibrillation on left atrial cavity size. Am J Cardiol 97(8):1220–1222
Reant P, Lafitte S, Jais P, Serri K, Weerasooriya R, Hocini M, Pillois X, Clementy J, Haissaguerre M, Roudaut R (2005) Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation 112(19):2896–2903
Taclas JE, Nezafat R, Wylie JV, Josephson ME, Hsing J, Manning WJ, Peters DC (2010) Relationship between intended sites of RF ablation and post-procedural scar in AF patients, using late gadolinium enhancement cardiovascular magnetic resonance. Heart Rhythm 7(4):489–496
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, S.H., Oh, YW., Lee, D.I. et al. Evaluation of quantification methods for left arial late gadolinium enhancement based on different references in patients with atrial fibrillation. Int J Cardiovasc Imaging 31 (Suppl 1), 91–101 (2015). https://doi.org/10.1007/s10554-014-0563-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-014-0563-0