Abstract
Absence of myocardial fibrosis on late gadolinium enhanced (LGE) magnetic resonance imaging (MRI) is associated with improvement of left ventricular systolic function after catheter ablation (CA) for atrial fibrillation (AF) with non-ischemic dilated cardiomyopathy (NIDCM). Extracellular volume fraction (ECV) by T1 mapping has emerges as a non-invasive mean to quantify severity of myocardial fibrosis. The aim of this study was to assess the incremental value of ECV over LGE-MRI for the improvement of LVEF(∆EF) after CA in NIDCM patients. A total of thirty-two patients with NIDCM and AF (mean age 67.4 ± 9.3 years; 29 (91%) male) were retrospectively studied. Using a 1.5 T MR scanner and 32 channel cardiac coils, LGE-MRI, pre- and post-T1 mapping images of LV wall at mid-ventricular level (modified look-locker inversion recovery sequence) were acquired. All patients successfully underwent CA for AF, and the improvement of LVEF after CA were evaluated by echocardiography. All patients restored sinus rhythm after CA at the time of echocardiography. The mean LVEF was 35.1 ± 9.7% before CA and 52.2 ± 10.2% after CA (p < 0.001), resulting an increase of 17.4 ± 12.6%. Significant correlation was found between ∆LVEF and % LGE (r = − 0.49, p = 0.004), ∆LVEF and extracellular volume fraction (ECV) (r = − 0.47, p = 0.010). Area under the receiver operating characteristics curve (AUC) of combination of %LGE and ECV for predicting improvement of LVEF > 10% was substantially higher than that of %LGE alone (AUC: 0.830 vs 0.602). In NIDCM patients with AF, ECV had incremental value over %LGE for predicting improvement of EF by CA, suggesting that the assessment of diffuse interstitial fibrosis may be important to forecast the response of CA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) trial has shown that catheter ablation (CA) for atrial fibrillation (AF) significantly reduced the risk of death and hospitalization for heart failure for patients with non-ischemic dilated cardiomyopathy (NIDCM) and AF [1]. However, some patients do not respond to CA; therefore, appropriate patient selection is necessary to avoid fatal procedural complications. The Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction (CAMERA-MRI) study demonstrated that the absence of myocardial fibrosis on late gadolinium enhanced (LGE) magnetic resonance imaging (MRI) is associated with improvement of left ventricular (LV) systolic function after CA in NIDCM patients with AF [2]. This study indicated the potential utility of LGE-MRI for decision making on indication of CA for NIDCM patients with AF.
Recently, T1 mapping has been widely used as an imaging method for measuring the myocardial extracellular volume fraction (ECV) as an index of diffuse interstitial fibrosis [3,4,5]. The cardiac magnetic resonance (CMR) derived ECV reflects the degree of myocardial fibrosis obtained by endocardial biopsy [3, 5], and abnormal ECV is associated with adverse clinical outcomes in patients with NIDCM [6]. In addition, T1 mapping can detect interstitial fibrosis which would be missed by LGE-MRI [7]. Therefore, we hypothesized that the combination of quantitative assessment of myocardial fibrosis using T1 mapping and LGE might predict improvement of LV systolic function after CA better than LGE MRI alone. Therefore, the aim of this study was to assess the incremental value of ECV over LGE-MRI for the improvement of LVEF(∆EF) after CA in NIDCM patients.
Materials and methods
Study population
A total of thirty-two patients with NIDCM and AF (mean age 67.4 ± 9.3 years; 29 (91%) male) were retrospectively studied. The definition of NIDCM was patients with a left ventricular ejection fraction (LVEF) of < 50% and no significant coronary artery stenosis on X-ray coronary angiography. Exclusion criteria included patients with severe valvular heart disease, an estimated glomerular filtration rate of < 30 mL/min/1.73 m2, contraindication to MRI examinations (claustrophobia etc.) and patients after a metallic device implantation. All CMR scans were acquired before the CA. Echocardiography was performed pre- and post-CA to evaluate the change in LVEF. This study was approved by the institutional review board, and written informed consent was waived because of the retrospective study design.
CMR image acquisition
Using a 1.5 T MR scanner, cine MRI, LGE MRI, and T1 mapping images were obtained (Achieva; Philips Healthcare, Best, The Netherlands). To calculate the LV volume and LVEF, vertical long-axis, horizontal long-axis, and short-axis cine-images of LV were imaged using a steady-state free precession sequence, (repetition time, 4.1 ms; echo time, 1.7 ms; flip angle, 55°; field of view, 350 × 350 mm2; acquisition matrix, 128 × 128; and number of phases per cardiac cycle, 20). Fifteen minutes after the injection of gadolinium contrast media (a total dose of 0.15 mmol/kg) (Gd-BTDO3A, Gadovist; Bayer, Berlin, Germany), LGE MRI images of LV were obtained in the same planes as the cine MR images using an inversion recovery-prepared gradient-echo sequence (repetition time, 4.3 ms; echo time, 1.3 ms; flip angle, 15°; field of view, 380 × 380 mm2; acquisition matrix, 256 × 180; and slice thickness, 10 mm). To acquire the pre-contrast and post-contrast T1 mapping images of the LV myocardium, the modified look locker inversion recovery (MOLLI) sequence was used (MOLLI 5s[3s]3s; TR, shortest; TE, shortest; FA, 35°; FOV, 350 × 350 mm2; acquisition matrix, 144 × 144; reconstruction matrix, 256 × 256, slice thickness, 10 mm; and acquisition voxel size, 2.43 × 2.65 × 10 mm). T1 mapping images were acquired in a mid-ventricular slice of the LV in each patient.
Image analysis
The cine MR images were analyzed using a dedicated workstation (the Extend MR WorkSpace, Philips Healthcare, Best, the Netherland). To determine the LV mass, the end-diastolic epi- and endocardial LV borders were manually traced on the short axis dataset. The LV mass was calculated as the sum of the myocardial volume multiplied by the specific gravity (1.05 g/mL) of the myocardial tissue [11]. To determine the amount of fibrosis on LGE-MRI, fibrosis was defined as region with > 5SD signal intensity than the remote myocardium (Vitrea, Canon medical systems corporation, Otawara, Japan). Percentage LGE was defined as the volume of fibrosis divided by the volume of total myocardium, multiplied by 100. To assess the T1 mapping, we performed a six-segmental analysis using commercially available software (Zaiostation, Zaiosoft Inc. Tokyo, Japan) (Fig. 1). To combine the pre- and post-contrast myocardial T1 time, the patient’s hematocrit, the ECV, was calculated using the following formula [8].
LGE MRI and ECV map. a NIDCM patients with negative LGE and low ECV (n = 10). ECV was 0.23 for this patient. b NIDCM patients with negative LGE and high ECV (n = 8). ECV was 0.30 for this patient. c NIDCM patients with positive LGE and high ECV (n = 10). ECV was 0.43 for this patient. ECV extracellular volume fraction, LGE late gadolinium enhancement, NIDCM non-ischemic dilated cardiomyopathy
ECV from six segments of mid ventricular level were averaged for each patient.
To evaluate the incremental value of ECV over %LGE, pre- and post-contrast T1 time of non-enhanced myocardium on LGE-MRI was manually traced.
CA procedure
Ablation procedure was performed under general anesthesia. If patients were under AF at the time of ablation, direct current cardioversion was carried out to restore sinus rhythm. Voltage and anatomical mapping of pulmonary vein and left atrium was done using a PentaRay® catheter (Biosense Webster, Irvine, California). Extensive encircling pulmonary vein isolation was performed using a 3.5 mm irrigated-tipped catheter (SmartTouch thermocool, Biosense Webster) with assistance of 3-dimensional mapping system (Carto, Biosense Webster). Posterior wall isolation, with roof and bottom line, was added if necessary.
Assessment of pre- and post-ablation LVEF
Measurement of LVEF was performed before and after PVI using echocardiography. LV volume and LVEF were measured by the modified Simpson’s method. We measured LV diastolic and systolic dimension, systolic left atrial dimension, and LA volume by biplane disc method. [9]
Statistical analysis
Data were analyzed using SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA). Continuous values are presented as means ± standard deviation. Categorical values are expressed as the number (%). The normality was determined by the Shapiro–Wilk test. Significance was evaluated using an unpaired t-test for normally distributed variables and the Mann–Whitney U test for skewed variables. Correlation between %LGE and change in ∆LVEF after CA, ECV value and ∆LVEF after CA were assessed using Spearman’s correlation coefficients. Patients were allocated into two groups based on median ECV value of 0.28 (low ECV group: ECV < 0.28; high ECV group: ECV ≧ 0.28). ∆LVEF was compared between high and low ECV groups. Increase of LVEF > 10% is considered as a threshold of LV reverse remodeling. [10, 11] Therefore, receiver operating characteristics curves (ROC) were generated to assess the predictive value of %LGE and ECV for the increase of LVEF > 10% after CA. The optimal cut-off value of %LGE and ECV were obtained by Youden index. To assess the incremental value of ECV over %LGE, we performed multiple regression analysis, then compared area under the ROC (AUC) of ECV + %LGE and %LGE alone. Intra- and inter-observer reproducibility for ECV measurement were assessed using intra class correlation coefficient (ICC). A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 summarizes the patient characteristics. In NIDCM patients, mean age was 67.4 ± 9.3 years and 29 (91%) patients were male. Sixteen (50%) patients had a New York Heart Association class II or III. Prevalence of hypertension and diabetes mellitus was 59% and 16%, respectively. Twenty-nine (91%) patients had a previous history of hospitalization due to heart failure. The mean CHA2DS2-Vasc score was 2.5 ± 0.9. The mean brain natriuretic peptide level was 240 ± 188 pg/mL. Thirty (94%) patients were prescribed beta blockers. Prevalence of paroxysmal AF, persistent AF, and longstanding persistent AF was 0%, 50%, 50%, respectively (Table 1). There was no significant difference between NIDCM patients ECV ≤ 0.28 and those with ECV > 0.28 in terms of demographics, blood test and echocardiographic findings.
Change in echocardiographic parameters after ablation
No patients did cardioversion before ablation. 30 patients (94%) had need cardioversion during ablation procedure. Table 2 shows the results of the echocardiography parameters before and after the CA. Duration between echocardiography before CA and echocardiography after CA was 321 ± 318 days (range: 1–567 days). All patients restored sinus rhythm at the time of echocardiography after CA. The mean LVEF was 35.1 ± 9.7% before CA and 52.2 ± 10.2% after CA (p < 0.001), resulting a ΔLVEF of 17.4 ± 12.6%. There is no significant correlation between “days from CA to post-CA echo” and “ΔEF” (Pearson’s r = 0.16, p = 0.41). LV end-systolic volume and stroke volume also showed significant improvement. LVEF showed significant improvement in the subgroup stratified by ECV of 0.28 (Table 2). Brain natriuretic peptide significantly reduced from 240 ± 188 pg/mL to 88.8 ± 50.7 pg/mL (p < 0.001) after CA. Intra- and inter-observer reproducibility for ECV measurement were assessed using intra class correlation coefficient (ICC).
Correlation between %LGE and ΔLVEF, ECV, and ΔLVEF
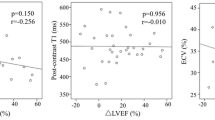
Among 32 patients, ECV was evaluated in 28 patients. Figure 2 shows the correlation between %LGE and ΔLVEF, ECV, and ΔLVEF. Significant correlation was found between ΔLVEF and %LGE (r = − 0.49, p = 0.004), ΔLVEF and ECV (r = − 0.47, p = 0.010) after CA. Figure 3 demonstrates the comparison of ΔLVEF between low and high ECV groups. ΔLVEF was significantly higher in the low ECV group compared to high ECV group (23.7 ± 10.9% vs 7.9 ± 9.2%, p < 0.001) (Fig. 3).
ROC curve of ECV and %LGE for the prediction of improvement of LVEF after CA
Figure 4 illustrates the ROC curve of ECV + %LGE and that of %LGE alone for the prediction of increase of LVEF ≧ 10% after CA. In 21 (65%) of 32 patients, LVEF increased ≧ 10% after CA. AUC was 0.602 (95% CI 0.368–0.837) for %LGE alone, 0.830 (95% CI 0.633–1.00) for combination of %LGE and ECV (p = 0.35) (Fig. 4). Sensitivity and specificity of %LGE for predicting increase of LVEF ≧ 10% after CA were 67% and 63% with a cut-off value of 6.5%. Sensitivity and specificity of combination of %LGE and ECV were 89% and 79% with a cut-off value of 1.71. Multivariable linear regression analysis demonstrated significant correlation between LVEF pre CA and ΔLVEF, ECV and ΔLVEF (Table 3).
Discussion
Our study compared the predictive value of %LGE and ECV for the improvement of LVEF after CA in NIDCM patients with reduced EF and AF. The major findings are as follows: (1) Change in absolute LVEF after CA (ΔLVEF) was significantly correlated with both %LGE and ECV; and (2) The AUC of combination of %LGE and ECV was higher than that of %LGE alone in terms of prediction of increase of LVEF > 10% after CA. These results indicated that ECV had incremental value over %LGE for predicting improvement of EF by CA in NIDCM patients with AF, and myocardial fibrosis would be a key pathophysiology to predict improvement of LVEF by CA.
Previously, a number of meta-analyses have examined the benefit, efficacy and safety of CA for patients with HFrEF. All of these studies have suggested AF ablation to be safe, effective and beneficial [12,13,14,15]. Successful CA results in improved LV function, clinical heart failure status, quality of life and mortality [16]. The CASTLE-AF study provided novel insight into the therapeutic strategy for patients with heart failure [1]. This prospective randomized trial enrolled high risk heart failure patients with LVEF ≦ 35% and AF, assigned to a CA group or medical therapy group. Implantable cardioverter-defibrillator or a cardiac resynchronization therapy defibrillator were implanted in all patients. The authors showed that the CA for AF significantly reduced the composite endpoint, death from any cause and hospitalization due to heart failure, compared with medical therapy (hazard ratio: 0.62, 95%CI 0.43–0.87, p = 0.007). Despite the favorable effect of CA for patients with NIDCM and AF, careful decision making to perform CA should be done, as the risks of intra-procedural fatal complications (cardiac tamponade, atrio-esophageal fistula etc.) are not negligible.
The CAMERA-MRI study shed light on the patient selection suitable to CA [2]. This study demonstrated that the absence of myocardial fibrosis on LGE-MRI is associated with the improvement of left ventricular systolic function after CA. On the other hand, patients with severe LV fibrosis on LGE-MRI rarely showed an increase of LVEF after CA. These results emphasized the utility of LGE-MRI for decision making purposes when deciding whether to perform CA for NIDCM patients with AF. However, one of the major limitations of LGE-MRI is that the quantitative assessment of fibrosis is difficult. T1 mapping has emerged as a non-invasive and accurate mean to quantify the severity of myocardial fibrosis [7]. ECV by T1 mapping is well-correlated to the degree of myocardial fibrosis by endocardial biopsy [4, 5]. With a cut-off value of > 25.8, ECV discriminates NIDCM patients from control subject’s sensitivity of 91.1 and specificity of 62.1% [4]. In a large multicenter study, ECV can predict all-cause mortality and heart failure hospitalization in NIDCM patients [6]. Another study has shown that ECV in the anteroseptal wall is the most predictive for adverse events for patients with NIDCM [17]. In addition, incremental or different prognostic value of ECV over LGE for other ischemic and non-ischemic cohorts, such as myocarditis [18], NIDCM [19] and general patient cohort [20], were reported. Based on these pieces of evidence, we hypothesized that the combination of ECV and %LGE would be important tools for predictive the improvement of LVEF after CA, and for forecast the response of CA more accurately. In our study, we used median ECV as cut-off value, as there is no established cut-off value for predicting good response by ablation in NIDCM patients. ΔLVEF after CA was significantly correlated with both %LGE and ECV, and the AUC of the combination of %LGE and ECV was higher than that of the %LGE alone in terms of prediction of increase of LVEF > 10% after CA. These results indicated that myocardial fibrosis would be a key pathophysiology to predict improvement of LVEF, and the assessment of ECV by T1 mapping may be useful for patient selection before the CA for NIDCM patient, to avoid less-effective CA for EF improvement. In addition, multivariable linear regression analysis has shown that LVEF pre CA and ECV were correlated with ΔLVEF. There may be multiple co-factors impacts the relationship between ECV and LVEF recovery, further study thus is necessary to clarify this point.
Study limitations
First, this study was a single center observational study. Therefore, a randomized clinical trial would be necessary to compare CA and medical therapy to evaluate the true effect of CA for LVEF improvement. Second, patients with severe renal dysfunction or patients with mechanical devices were excluded in this study. Third, some diabetic patients (16% of patients) can have an elevated ECV, which may bias the results of our study. In our cohort, one diabetic patient was negative LGE and elevated ECV, and 5 diabetic patients were LGE positive. Fourth, we do not have post-CA MRI data in all patients, therefore pre- and post-CA LVEF were compared using echocardiography, which is less accurate than cine MRI images. Fifth, T1-mapping is particularly sensitive to arrhythmia, however, we did not use any adjustment of the sequence for T1 calculation, such as arrhythmia-insensitive-rapid cardiac T1 mapping pulse sequence [21]. In addition, heart rate variability may impact the ECV value. A previous study has shown that the change of ECV by heart rate variability was very small (0.13% by every increase in 10 heart beats) [22]. Furthermore, there was no significant difference in heart rate between ECV ≤ 0.28 and those with ECV > 0.28 (101 ± 21 bpm vs 91 ± 13 bpm, p = 0.15, Table 1). Spearman’s correlation coefficient between ECV and HR was − 0.24 (p = 0.21). Therefore, the impact of HR on ECV in our cohort may be limited. Sixth, Due to the limit of total scan time, only mid-ventricular slice of T1 mapping was acquired in our institution.
Conclusion
Change in absolute LVEF after CA (ΔLVEF) was significantly correlated with both %LGE and ECV. AUC of combination of %LGE and ECV was higher than that of %LGE in terms of prediction of increase of LVEF > 10% after CA. These results indicated that ECV could be useful as a non-invasive imaging marker for the prediction of increase of LVEF after CA in NIDCM patients with AF.
Abbreviations
- AUC:
-
Area under the curve
- AF:
-
Atrial fibrillation
- CA:
-
Catheter ablation
- ECV:
-
Extracellular volume fraction
- EF:
-
Ejection fraction
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- MRI:
-
Magnetic resonance imaging
- NIDCM:
-
Non-ischemic dilated cardiomyopathy
- ROC:
-
Receiver operating characteristics
References
Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bansch D, Investigators C-A (2018) Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 378(5):417–427. https://doi.org/10.1056/NEJMoa1707855
Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM (2017) Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI Study. J Am Coll Cardiol 70(16):1949–1961. https://doi.org/10.1016/j.jacc.2017.08.041
Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA, Taylor AJ (2015) Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Heart J Cardiovasc Imaging 16(1):14–22. https://doi.org/10.1093/ehjci/jeu182
aus dem Siepen F, Buss SJ, Messroghli D, Andre F, Lossnitzer D, Seitz S, Keller M, Schnabel PA, Giannitsis E, Korosoglou G, Katus HA, Steen H (2015) T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging 16(2):210–216. https://doi.org/10.1093/ehjci/jeu183
Nakamori S, Dohi K, Ishida M, Goto Y, Imanaka-Yoshida K, Omori T, Goto I, Kumagai N, Fujimoto N, Ichikawa Y, Kitagawa K, Yamada N, Sakuma H, Ito M (2018) Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 11(1):48–59. https://doi.org/10.1016/j.jcmg.2017.04.006
Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, Hinojar R, Doltra A, Varma N, Child N, Rogers T, Suna G, Arroyo Ucar E, Goodman B, Khan S, Dabir D, Herrmann E, Zeiher AM, Nagel E, International TMCMROS (2016) T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging 9(1):40–50. https://doi.org/10.1016/j.jcmg.2015.12.001
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18(1):89. https://doi.org/10.1186/s12968-016-0308-4
Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB (2012) Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 126(10):1206–1216. https://doi.org/10.1161/CIRCULATIONAHA.111.089409
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39 e14. https://doi.org/10.1016/j.echo.2014.10.003
Lee K, Daimon M, Kuwabara Y, Hasegawa R, Toyoda T, Sekine T, Kawata T, Komuro I (2009) Prediction of the response to beta-blocker therapy in patients with dilated cardiomyopathy: comparison of 123I-MIBG scintigraphy and low-dose dobutamine stress echocardiography. J Echocardiogr 7(4):74–79. https://doi.org/10.1007/s12574-009-0022-4
Pinamonti B, Perkan A, Di Lenarda A, Gregori D, Sinagra G (2002) Dobutamine echocardiography in idiopathic dilated cardiomyopathy: clinical and prognostic implications. Eur J Heart Fail 4(1):49–61
Wilton SB, Fundytus A, Ghali WA, Veenhuyzen GD, Quinn FR, Mitchell LB, Hill MD, Faris P, Exner DV (2010) Meta-analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol 106(9):1284–1291. https://doi.org/10.1016/j.amjcard.2010.06.053
Anselmino M, Matta M, D’Ascenzo F, Bunch TJ, Schilling RJ, Hunter RJ, Pappone C, Neumann T, Noelker G, Fiala M, Bertaglia E, Frontera A, Duncan E, Nalliah C, Jais P, Weerasooriya R, Kalman JM, Gaita F (2014) Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 7(6):1011–1018. https://doi.org/10.1161/CIRCEP.114.001938
Dagres N, Varounis C, Gaspar T, Piorkowski C, Eitel C, Iliodromitis EK, Lekakis JP, Flevari P, Simeonidou E, Rallidis LS, Tsougos E, Hindricks G, Sommer P, Anastasiou-Nana M (2011) Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta-analysis. J Card Fail 17(11):964–970. https://doi.org/10.1016/j.cardfail.2011.07.009
Ganesan AN, Nandal S, Luker J, Pathak RK, Mahajan R, Twomey D, Lau DH, Sanders P (2015) Catheter ablation of atrial fibrillation in patients with concomitant left ventricular impairment: a systematic review of efficacy and effect on ejection fraction. Heart Lung Circ 24(3):270–280. https://doi.org/10.1016/j.hlc.2014.09.012
Liang JJ, Callans DJ (2018) Ablation for atrial fibrillation in heart failure with reduced ejection fraction. Card Fail Rev 4(1):33–37. https://doi.org/10.15420/cfr.2018:3:1
Vita T, Grani C, Abbasi SA, Neilan TG, Rowin E, Kaneko K, Coelho-Filho O, Watanabe E, Mongeon FP, Farhad H, Rassi CH, Choi YL, Cheng K, Givertz MM, Blankstein R, Steigner M, Aghayev A, Jerosch-Herold M, Kwong RY (2018) Comparing CMR mapping methods and myocardial patterns toward heart failure outcomes in nonischemic dilated cardiomyopathy. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2018.08.021
Grani C, Biere L, Eichhorn C, Kaneko K, Agarwal V, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY (2019) Incremental value of extracellular volume assessment by cardiovascular magnetic resonance imaging in risk stratifying patients with suspected myocarditis. Int J Cardiovasc Imaging 35(6):1067–1078. https://doi.org/10.1007/s10554-019-01552-6
Youn JC, Hong YJ, Lee HJ, Han K, Shim CY, Hong GR, Suh YJ, Hur J, Kim YJ, Choi BW, Kang SM (2017) Contrast-enhanced T1 mapping-based extracellular volume fraction independently predicts clinical outcome in patients with non-ischemic dilated cardiomyopathy: a prospective cohort study. Eur Radiol 27(9):3924–3933. https://doi.org/10.1007/s00330-017-4817-9
Yang EY, Ghosn MG, Khan MA, Gramze NL, Brunner G, Nabi F, Nambi V, Nagueh SF, Nguyen DT, Graviss EA, Schelbert EB, Ballantyne CM, Zoghbi WA, Shah DJ (2019) Myocardial extracellular volume fraction adds prognostic information beyond myocardial replacement fibrosis. Circ Cardiovasc Imaging 12(12):e009535. https://doi.org/10.1161/CIRCIMAGING.119.009535
Gunasekaran S, Lee DC, Knight BP, Fan L, Collins JD, Chow K, Carr JC, Passman R, Kim D (2020) Left ventricular extracellular volume expansion is not associated with atrial fibrillation or atrial fibrillation-mediated left ventricular systolic dysfunction. Radiol Cardiothorac Imaging 2(2):e190096. https://doi.org/10.1148/ryct.2020190096
Vassiliou VS, Heng EL, Gatehouse PD, Donovan J, Raphael CE, Giri S, Babu-Narayan SV, Gatzoulis MA, Pennell DJ, Prasad SK, Firmin DN (2016) Magnetic resonance imaging phantoms for quality-control of myocardial T1 and ECV mapping: specific formulation, long-term stability and variation with heart rate and temperature. J Cardiovasc Magn Reson 18(1):62. https://doi.org/10.1186/s12968-016-0275-9
Acknowledgements
We are grateful to Masanori Ito, RT and Yuki Yoshimura, RT for CMR image acquisition.
Funding
Research Grant, Japan Society for the Promotion of Science: Grant-in-Aid for Early-Career Scientists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest directly relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azuma, M., Kato, S., Sekii, R. et al. Extracellular volume fraction by T1 mapping predicts improvement of left ventricular ejection fraction after catheter ablation in patients with non-ischemic dilated cardiomyopathy and atrial fibrillation. Int J Cardiovasc Imaging 37, 2535–2543 (2021). https://doi.org/10.1007/s10554-021-02219-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02219-x