Abstract
Regional ejection fraction (REF) provides important functional information of the left ventricular regional myocardium. We aimed to test the diagnostic accuracy of computerized REF analysis for detecting the ischemia and significant stenosis with multidetector CT angiography (MDCT). This is a retrospective study including 155 patients who underwent MDCT scans for evaluation of coronary artery disease. Among them, 83 patients also underwent SPECT imaging and invasive coronary angiography (ICA). Two groups of patients were defined: Control group with 0 coronary artery calcium and normal global and regional ventricular function, and comparison group. REF measurement was performed on all patients using computerized software. Control group REF measurements will be used as reference standard (mean-2SD REF/mean global ejection fraction) to define abnormal REF. The sensitivity, specificity, positive and negative predictive value of REF in detecting perfusion defects (fixed and reversible) was 73, 80, 75 and 79 % respectively, in a patient based analysis of comparison group. The diagnostic accuracy of REF in predicting significant stenosis (>50 %) on ICA compared with SPECT was 72 versus 61 % and 85 versus 79 % in patient and vessel based analysis of comparison group, respectively. ROC curve analysis showed REF to be a better predictor of perfusion defects on SPECT compared with significant stenosis (>50 %) alone or stenosis combined with REF (P < 0.05). The computerized assessment of REF analysis is comparable to SPECT in predicting ischemia and a better predictor of significant stenosis than SPECT. This study also provides reference standard to define abnormal values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the US [1]. Improved sub-millimeter spatial resolution and faster acquisition times for cardiac images have resulted in increased use of multidetector computed tomography (MDCT) scans in the evaluation of CAD. Higher contrast resolution and better anatomical details allow accurate assessment of CAD which is comparable to the invasive coronary angiography. The negative predictive value of MDCT in CAD detection has been shown to be above 97 % in multiple studies [2–4]. The role of MDCT is limited not only to the evaluation of CAD, but it can also used for assessing structural and functional aspects of the heart [5, 6] providing important information regarding prognosis and choice of treatment options.
Ischemic heart disease may lead to decline in left ventricular function as measured by global left ventricular ejection fraction. However due to compensatory hyperkinesias in the normal segments [7, 8], global ejection fraction may be normal in certain patients, thereby requiring an index which may be more indicative of regional myocardial function such as regional ejection fraction (REF). REF can be determined using echocardiography, nuclear magnetic resonance imaging, contrast ventriculography, radionuclide techniques and SPECT [9–14]. Current study looks at the possibility of using objective criteria for the assessment of REF using contrast enhanced sub-millimeter resolution MDCT scans. We aimed to provide a reference standard for REF using automated software from patients without any CAD and normal global and regional wall function. This reference standard will then be used to define abnormal REF in patients with CAD. We will then determine the diagnostic accuracy of the REF in detecting myocardial perfusion defects (PD) seen on images acquired using single photon emission computed tomography (SPECT). We will also compare the diagnostic accuracy of REF and SPECT in predicting significant stenosis (>50 %) of the coronary arteries.
Materials and methods
Study population
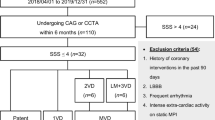
A retrospective analysis was performed on our data registry of patients who underwent MDCT angiography for evaluation of the CAD. Patients were divided into two groups: Control group used to determine reference values for the REF, Comparison group used to test the REF reference values derived from control group. Inclusion criteria for the control group were zero coronary artery calcium (CAC) score, retrospectively acquired MDCT images, normal wall motion, normal ejection fraction and absence of perfusion defects on the MDCT scans. Inclusion criteria for the comparison group were patients who underwent invasive coronary angiography (ICA), MDCT and SPECT imaging within 3 months time duration and presence of retrospectively acquired images. Exclusion criteria for the study were coronary artery bypass grafting, patients who failed to undergo all three tests in the comparison group, had interim revascularization between the studies. All subjects had signed the informed consent at the time of image acquisition for the use of their study for research purposes which was approved by the Institutional Review Board of our center.
MDCT image acquisition
All MDCT images were acquired using 64-multi-detector row Lightspeed VCT scanner (General Electric Healthcare, Milwaukie, Wisconsin). The details of the image acquisition were previously published [2]. All patients undergoing MDCT had non-contrast studies as a part of the protocol for CAC score measurement. Patients presenting with baseline heart rate >65 received oral beta blockers (metoprolol) in 50 mg increments up to a total dose of 100 mg. Intravenous administration was also allowed in the protocol at 5 mg increments to a total dose of 25 mg to achieve resting heart rate <65 beats per minute for better image quality. Following a scout X-ray of the chest, a timing bolus (usually 10–20 ml contrast) was performed to detect time to reach optimal contrast opacification in the axial image at a level immediately superior to the ostium of the left main artery. Nitroglycerine 0.4 mg sublingually was given immediately before contrast administration, Triple phase contrast protocol was used during image acquisition: 60 ml iodixanol, followed by 40 ml of 50:50 mixture of iodixanol and saline and followed by 50 ml of saline flush. The scans were acquired with 20 mm above the level of the left main artery to 20 mm below the inferior myocardial apex. The scan parameters for MDCT were 64 × 0.625 mm collimation, tube voltage 120 kV, and effective mA 350–780 mA. Radiation reduction algorithms using electrocardiography modulations were used to reduce radiation exposure (mA) during systole and end-diastole. After scan completion, multiphase reconstruction of the MDCT scans was performed, with reconstructed images from 5 to 95 % by 10 % increments. Estimated radiation exposure for retrospectively acquired images at our center is 8–12 mSV for retrospective studies.
All MDCT images were then transferred to workstation (AW Volume Share™, GE Medical Systems, Milwaukee, WI) for 3-dimensional image analysis. Volume rendering and curved multi-planar reformations were used to evaluate the coronary vessels. Each vessel was assessed as normal (no stenosis), non-obstructive (luminal stenosis <50 %), and obstructive (luminal stenosis ≥50 %). Vessels 1.5 mm in diameter or larger were assessed. Two skilled cardiologists blinded to the clinical data assessed the coronary arteries separately.
SPECT image acquisition and analysis
A standard imaging protocol as endorsed by American Society of Nuclear Cardiology (ASNC) was used for all patients [15]. All patients underwent rest and stress imaging with technetium-99 m sestamibi (MIBI). Patients who could exercise underwent treadmill stress using the Bruce protocol to reach 85 % of maximum predicted heart rate. Patients who could not exercise underwent pharmacological stress test using dipyridamole @ 142 mg/kg/min infused over 4 min. Patients were asked to terminate beta-blocker 24 h prior to testing when desirable. For resting studies patients were injected with 9–10 mCi of technetium-99 m sestamibi and SPECT images were acquired 30–45 min after resting injection. All SPECT studies were acquired on a commercially available camera and computer using a 180 degree arc, high-resolution parallel-hole collimator; and 64 stops with 20 s per stop for a total imaging time of 25 min. All images were acquired using a 64 × 64 matrix. Patients underwent symptom limited exercise on Bruce protocol. At peak heart rate, 25–30 mCi of technetium-99 m sestamibi (dose was weight based) was injected. Patient then underwent post-stress SPECT image acquisition.
The post-stress and rest MIBI scans were interpreted using visual assessment of perfusion abnormalities by the two skilled physicians. All the readers were nuclear board certified and blinded to the results of CT. The short axis data were displayed in polar map format, with the maps divided into 4 and/or 17 segments model. The 17 segments were allocated to the territories of the different coronary arteries [16]. Gating was not performed in all the studies and thus regional wall motion was not assessed.
For the 4 segment model, the anterior and septal wall perfusion defects were allocated to the left anterior descending (LAD) coronary artery, the lateral defects were allocated to the left circumflex (LCX) coronary artery, and the inferior defects were allocated to (RCA) the right coronary artery(5) or LCX (if the area was supplied by left circumflex artery). For current study, we used 4 segments myocardial model to compare with the REF. Reversible perfusion defects were considered to represent myocardial ischemia. Fixed perfusion defects were considered to represent myocardial scars following infarction [17]. A partially reversible defect was defined as a fixed defect that increased in size during stress. Fixed and partially reversible defects were both taken as fixed defects.
Regional and global ejection fraction measurement by MDCT
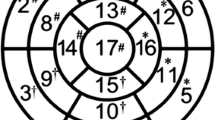
All REF and global left ventricular ejection fraction (LVEF) analyses were completed automatically using time volume analysis (TVA) protocol with TeraRecon computer (TeraRecon, Inc. CA). For control group, left ventricle was assessed visually for any wall motion abnormalities by an expert cardiologist. The abnormal wall motion was defined using a 4 point system as follows: normal wall motion-0, hypokinesis-1, akinesis-2 and dyskinesis-3. Patients with abnormal wall motion on visual assessment were excluded from further analysis. First, we identified images with the smallest and largest left ventricular diameter as end-systolic and end-diastolic images that were then uploaded to the TVA protocol. We used floating axis system to determine the REF. We identified the center of left ventricle for both end-systolic and end-diastolic images. With the TVA protocol, the short and long axial images can be displayed automatically. The LV top level was defined at the level of mitral valve annulus. After adjusting the long axial image to mid mitral valve level and depicting the level line, the LV can be separated from left atrium. The LV can depict from RV automatically by the difference of HU or manually. After defining the mitral valve annulus level, the level line was reset to exclude the left ventricular outflow tract on both end-systolic and end-diastolic images, so that the left ventricular myocardium is visible on 360° images on short axis images. After defining the left ventricle cavity on end-systolic and end-diastolic images, the computerized software provides end-systolic volume, end-diastolic volume, global LVEF and 17 myocardial segment REF values. The REF of 17 segment models can be revealed by polar graph. There was statistically significant difference in REF of individual segments as shown in Fig. 1. We took low limit border value of individual segments to derive a reference standard, represented by the ratio: individual low border REF/mean global LVEF whereas the low border REF value is derived from below two standard deviation value of corresponding segment (mean-2SD).
showing normal REF values of 17 segment myocardial model (blue line) derived from the control group, reference standard for REF derived from low border individual REF/mean global LVEF (red line) from control group and abnormal REF values determined for the comparison group using red line as the reference (green line)
For comparison group, REF and global LVEF were measured using the TVA protocol as mentioned above. For the sake of comparison with SPECT, the abnormal REF on MDCT images were extrapolated in three vessel territories (in RCA, LAD and LCX) as mentioned above for SPECT.
We selected 35 cases from control group to test the reproducibility of measurements by two experience readers. Reassessment of REF was performed by computerized analysis blinded to the each other’s assessment. Inter-reader variability was assessed by the Parson’s analysis (Fig. 2).
Showing 17 segments myocardial model used to define the myocardial segments along with the corresponding segment numbers used in Fig. 1
Invasive coronary angiography (ICA)
All patients in the comparison group had ICA on the basis of clinical presentation and/or imaging findings decided by their cardiologists. Conventional coronary angiography was performed according to standard clinical protocols [18]. Multiple projections were acquired to discern the maximal coronary artery luminal narrowing. The investigators recorded the maximum stenosis in each vessel. Angiographic abnormalities were considered significant for 2 cut points (>50 or >70 % luminal diameter stenosis) in any epicardial coronary vessel. Coronary segment narrowing was described as 0 if no lesion, <50 % stenosis, 50–70 % stenosis, and greater than 70 % stenosis by means of quantitative coronary angiography, using a 15-segment AHA coronary tree model. For current study, we utilized >50 % luminal narrowing cutoff for analysis (Fig. 3).
A 62 years old male with ICA, SPECT, and MDCT angiography studies (left, mid and right panels). ICA shows 80 % stenosis at proximal LAD and 50 % at mid LCX (white arrows). The SPECT shows fixed perfusion defect area points by yellow arrows (up two lines of short axial images), red arrows (mid two lines with horizontal images) and blue arrows (bottom of two vertical long axis images) in the mid panel. Left panel shows bull’s eye diagram of REF analysis using the MDCT images. Segment 1, 2, 3, 9 and 14 pointed by black arrows shows low REF with 32,−32, 31, 29 and 35 % respectively comparing a 59.4 % of mean LVEF
Statistics analysis
Demographic and clinical information of both groups will be compared using t test for continuous variables and Chi square test for the categorical variables. Values will be presented as mean ± standard deviation or percentages or numbers. The mean value of each segment REF for control group will then be plotted to determine the inter-segmental variation. The REF values two standard deviation below mean (mean-2SD) will be used as low border values for individual segment. To derive the reference standard, we will take ratio of individual segment mean low border value divided by the mean global LVEF. These reference standard values will then be used to define abnormal REF in comparison group patients. We will compute the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of REF for the detection of PD on SPECT images and, diagnostic accuracy of REF and SPECT in detecting significant stenosis (>50 %) detected on ICA, in both patient- and vessel-based analysis in comparison group. An ROC analysis curve will be generated to look at the diagnostic accuracy of REF alone, stenosis alone and REF and stenosis combined to predict PD on SPECT. Inter-observer variability for computerized REF analysis will be calculated using Pearson correlation coefficient. The analysis was performed using SAS software version 9.13 (SAS, Cary, North Carolina, USA)”.
Results
Table 1 provides clinical and demographic information of both groups. The mean time interval between ICA and MDCT was 14.5 ± 15.4 (range 1–69 days) whereas the time interval between MDCT and SPECT was 19.7 ± 9.2 (range 1–90 days). In the control group, all the patients had normal wall motion, no myocardial PD and zero CAC score. In the comparison group, there were 14 patients who were diagnosed as having no luminal narrowing and 22 patients with <50 % luminal narrowing on ICA. There were a total of 47 patients with >50 % luminal narrowing (18 patients with 50–70 % and 29 patients with >70 %) on ICA involving a total of 76 coronary arteries. On SPECT, there were 37 patients with PD on images; 22 with reversible defects and 17 with fixed defects with corresponding 49 vessels involved. Reversible defects were identified as ischemia, and fixed and partial fixed defects were identified as infarction. Both reversible and fixed defects were taken as ischemic disease. There were 36 patients involving 47 vessels with abnormal REF detected on MDCT images based on the reference standard derived from control group.
There was significant variation in the REF of control group patients in the 17 segment myocardial model (blue line) as shown in Fig. 1. REF was lower in the myocardial segments corresponding to the inter-ventricular septum (segments 2, 3, 8, 9 & 14). This figure also shows ratio of low border REF values of individual segments (mean-2SD) with mean global LVEF (red line) which will be used as reference to define the abnormal REF values for the comparison group. The abnormal REF values determined for comparison groups were significantly lower than the reference REF values determined by using control group.
The sensitivity, specificity, PPV and NPV of REF for predicting PD (ischemia and infarction) on SPECT for patient based and vessel based analysis are given in Table 2. REF had a good diagnostic accuracy in predicting PD on SPECT (both fixed and reversible defects). The diagnostic accuracy was higher for fixed defects compared with the reversible PD in both patient and vessel based analysis (70 vs. 66 %, 88 vs. 85 %) respectively. The negative predictive value of REF in predicting SPECT PD was significantly higher in both patient and vessel based analysis (79–98 %).
Table 3 gives sensitivity, specificity, PPV and NPV of REF and SPECT in detecting significant stenosis (>50 %) on ICA. The diagnostic accuracy was higher for REF in both patient and vessel based analysis in predicting significant stenosis on ICA compared with SPECT (72 vs. 61 %, 85 vs. 79 %), respectively.
Figure 4 shows an ROC curve for predicting PD (fixed and reversible) for REF, significant stenosis (>50 %) on ICA, and REF and significant stenosis (>50 %) combined. The diagnostic accuracy was lower for >50 % stenosis in predicting SPECT PD compared with REF alone. REF diagnostic accuracy was still higher when REF and >50 % stenosis were combined for predicting SPECT PD.
Inter-observer variability for REF assessment was performed on 35 patients by 2 observers blinded to each other measurements. There was good correlation observed for computerized REF assessment with r = 0.87 (P < 0.001).
Discussion
This study shows that REF assessment has a good diagnostic accuracy in predicting PD on SPECT. The reference standard derived from control group reliably distinguishes normal from abnormal values. There was significant inter-segmental variation of REF values in the control group. This underscores the importance of a reference standard for determining abnormal values. REF was significantly lower in the septal region. When we compared the REF values from base to apex, there was trend of increasing REF values from basal towards apical region. Cerqueira et al. [19] defined normal limits of REF using quantitative gated blood pool tomography in a group of 15 normal subjects. They used fixed versus floating axis system for determining the REF. In fixed axis system, geometric center of the left ventricle is defined for end-diastole which is used for end-systolic images without realignment. In floating axis system, geometric center of the left ventricle is defined for both end-diastolic and end-systolic images. There was significant variation observed in the REF of septal and lateral regions when measured using fixed axis system whereas with the floating axis system, the REFs were comparable for both the lateral and septal walls. The reason for this disparity mentioned was that the cardiac long axis translates towards the septum during contraction, producing a low ejection fraction in the septum if this translation is not corrected. In our opinion, lateral walls contribute more to the regional ejection fraction compared with the septum as they have more space to expand resulting in greater force generated as supposed by the Frank-Sterling law of force of contraction. This is supported by our results showing higher REF values in the lateral walls compared with the septum. For current study, we used the floating axis system and defined the ventricular center for both end-diastole and end-systole images and observed the variation between septal and lateral wall REF. We had a larger sample size to determine the normal reference standard compared to the previous study. They identified regions >2.5 SD below the mean as abnormal. We defined low border value as being 2SD below the mean and used ratio of low border value of individual REF to mean global LVEF to define the reference standard. This reference standard clearly distinguished normal from abnormal REF values which were comparable to SPECT in detecting perfusion defects and better predictor of significant stenosis than SPECT.
Global ejection fraction provides important diagnostic and prognostic information following myocardial infarction. The myocardium undergoes complex compensatory changes that help to restore the myocardial function [20]. As a result of these compensatory changes, global ejection may be normal in certain cases due to hyperkinesias of the normal segments [7, 8]; thereby global ejection fraction may not be true indicator of regional function. Information of REF may provide indirect evidence of improved myocardial function after revascularization and may be used to evaluate efficacy of new treatment strategies such as regenerative cell therapy [21, 22].
We also evaluated the role of REF in detecting myocardial perfusion defects seen on SPECT images of the heart. The negative predictive value of REF for detecting PD was 79 %–98 %; it was significantly higher in vessel based analysis (92–98 %). REF was also a better predictor of significant stenosis (>50 %) on ICA compared with SPECT. When we compared the stenosis with REF in predicting PD, REF was better than stenosis alone or stenosis with REF combined. This may be explained by the fact that degree of stenosis may not be a better predictor of PD as majority of myocardial infarctions occur in patients with non-obstructive lesions in the culprit vessels.
This study has limitations including its observational retrospective design. The MDCT, SPECT and ICA were performed at different times but within 3 months of each other. The control group was defined based on zero CAC score and normal global and regional myocardial function. These patients did not undergo SPECT imaging for myocardial perfusion defect. SPECT images were not gated, so we cannot get wall motion information and REF from SPECT images. We did not have detailed clinical information regarding myocardial infarctions. The study was performed on retrospectively acquired images. With increasing awareness about radiation exposure and recent advancements in radiation reduction techniques [23], prospectively acquired images are increasingly being utilized for CAD evaluation. But REF analysis will be helpful in patients where cardiac MDCT will be performed to rule out perfusion defects and functional consequences of a significant coronary luminal narrowing.
Conclusion
This study provides a reference standard for defining abnormal REF. This also shows that computerized assessment of REF analysis is comparable to SPECT in predicting ischemia and a better predictor of significant stenosis than SPECT. With current trends towards prospectively gated cardiac CT imaging and awareness regarding the radiation dosing, the number of helical acquired scans are decreasing. But in patients where helical MDCT scans are performed for LVEF and wall motion analysis, contrast enhanced CT scans will provide information not only for the degree of coronary luminal narrowing but can also be used to determine important functional information such as REF which may be helpful in detecting myocardial perfusion defects. With REF reference values available, REF analysis may help to provide information about functional consequences of significant coronary artery stenosis which should be further explored.
Abbreviations
- CAD:
-
Coronary artery disease
- CAC:
-
Coronary artery calcium
- MDCT:
-
Multi-detector computed tomography
- REF:
-
Regional ejection fraction
- SPECT:
-
Single photon emission computed tomography
- ICA:
-
Invasive coronary angiography
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- PD:
-
Perfusion defects
- LVEF:
-
Left ventricular ejection fraction
References
McGovern PG, Pankow JS, Shahar E, Doliszny KM, Folsom AR, Blackburn H et al (1996) Recent trends in acute coronary heart disease–mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med 334:884–890
Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E et al (2008) Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 52:1724–1732
Pugliese F, Mollet NR, Runza G, van Mieghem C, Meijboom WB, Malagutti P et al (2006) Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol 16:575–582
Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA (2005) Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 46:552–557
Cury RC, Nieman K, Shapiro MD, Butler J, Nomura CH, Ferencik M et al (2008) Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology 248:466–475
Lessick J, Dragu R, Mutlak D, Rispler S, Beyar R, Litmanovich D et al (2007) Is functional improvement after myocardial infarction predicted with myocardial enhancement patterns at multidetector CT? Radiology 244:736–744
Stadius ML, Maynard C, Fritz JK, Davis K, Ritchie JL, Sheehan F et al (1985) Coronary anatomy and left ventricular function in the first 12 hours of acute myocardial infarction: the Western Washington Randomized Intracoronary Streptokinase Trial. Circulation 72:292–301
Martin GV, Sheehan FH, Stadius M, Maynard C, Davis KB, Ritchie JL et al (1988) Intravenous streptokinase for acute myocardial infarction. Effects on global and regional systolic function. Circulation 78:258–266
Sheehan FH, Stewart DK, Dodge HT, Mitten S, Bolson EL, Brown BG (1983) Variability in the measurement of regional left ventricular wall motion from contrast angiograms. Circulation 68:550–559
Moynihan PF, Parisi AF, Feldman CL (1981) Quantitative detection of regional left ventricular contraction abnormalities by two-dimensional echocardiography. I. Analysis of methods. Circulation 63:752–760
Lotan CS, Cranney GB, Bouchard A, Bittner V, Pohost GM (1989) The value of cine nuclear magnetic resonance imaging for assessing regional ventricular function. J Am Coll Cardiol 14:1721–1729
Starling MR, Walsh RA, Lasher JC, Lancaster JL, Blumhardt R (1987) Quantification of left-ventricular regional dyssynergy by radionuclide angiography. J Nucl Med 28:1725–1735
Masci PG, Dymarkowski S, Rademakers FE, Bogaert J (2009) Determination of regional ejection fraction in patients with myocardial infarction by using merged late gadolinium enhancement and cine MR: feasibility study. Radiology 250:50–60
Hashimoto A, Nakata T, Wakabayashi T, Kyuma M, Takahashi T, Tsuchihashi K et al (2002) Validation of quantitative gated single photon emission computed tomography and an automated scoring system for the assessment of regional left ventricular systolic function. Nucl Med Commun 23:887–898
Hansen CL, Goldstein RA, Berman DS, Churchwell KB, Cooke CD, Corbett JR et al (2006) Myocardial perfusion and function single photon emission computed tomography. J Nucl Cardiol 13:e97–e120
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Fleischmann S, Koepfli P, Namdar M, Wyss CA, Jenni R, Kaufmann PA (2004) Gated (99 m)Tc-tetrofosmin SPECT for discriminating infarct from artifact in fixed myocardial perfusion defects. J Nucl Med 45:754–759
Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS et al (1975) A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51:5–40
Cerqueira MD, Harp GD, Ritchie JL (1992) Quantitative gated blood pool tomographic assessment of regional ejection fraction: definition of normal limits. J Am Coll Cardiol 20:934–941
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101:2981–2988
Qureshi U, Nagueh SF, Afridi I, Vaduganathan P, Blaustein A, Verani MS et al (1997) Dobutamine echocardiography and quantitative rest-redistribution 201Tl tomography in myocardial hibernation. Relation of contractile reserve to 201Tl uptake and comparative prediction of recovery of function. Circulation 95:626–635
Mathur A, Martin JF (2004) Stem cells and repair of the heart. Lancet 364:183–192
Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL et al (2011) SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 5:198–224
Conflict of interest
None of the authors have received any funding for this study from any institution. As for industrial financial disclosure, there is none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeb, I., Li, D., Nasir, K. et al. Computerized left ventricular regional ejection fraction analysis for detection of ischemic coronary artery disease with multidetector CT angiography. Int J Cardiovasc Imaging 29, 685–692 (2013). https://doi.org/10.1007/s10554-012-0121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0121-6