Abstract
Cardiac Resynchronization Therapy (CRT) constitutes an established way of treatment for patients suffering from severe heart failure. However, this technologically based and expensive mode of therapy may not be effective for a substantial number of individuals. Recent research and advanced echocardiographic modalities have provided new insight on the proper patient selection, lead implantation, optimization and reasons for non-response. Based on this evidence, an overall assessment of parameters that appear to contribute significantly to the outcome of CRT in addition to electrical or mechanical dyssynchrony, seems to be a reasonable approach for more effective resynchronization therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the prevalence of heart failure sharply increases in contemporary society [1], a new mode of treatment, that of cardiac resynchronization, has emerged at the forefront of medical therapy, offering hope and showing significant clinical improvement in a selected patient population [2]. The rationale behind cardiac resynchronization therapy (CRT) resides on the observation that the latter can revert asynchronous mechanical events, appearing mainly in heart failure patients with wide QRS complex on surface ECG, leading to clinical improvement and lower risk of death [3]. For that reason, the current recommendations for the management of patients with heart failure have suggested CRT as an additional therapeutic modality in individuals classified as NYHA III–IV who are symptomatic despite optimal medical therapy, and who have a reduced EF (LVEF ≤ 35%) and QRS prolongation (QRS width ≥ 120 ms) [4]. However, patient selection based on electrical criterion (wide QRS) may lead to non-improvement of more than 30% of individuals after CRT [5, 6]. Moreover, recent evidence suggests that other important parameters, such as the site of pacing lead placement, the degree of scar formation, the availability of coronary venous access, the contractile reserve and A-V or V–V optimization may actually affect the final outcome of CRT and warrant particular attention when resynchronization therapy is going to be applied. In this review, echocardiographic modalities with cut-off values to assess mechanical dyssynchrony are highlighted, and an overall assessment of issues related to CRT in addition to electrical or mechanical selection criteria is demonstrated as a reasonable approach for more effective resynchronization therapy.
Mechanical dyssynchrony: an important determinant
It is well known that prolongation of QRS (mainly LBBB) is an expression of abnormal electrical activation of the myocardium (electrical dyssynchrony) that can be translated into alteration of right and left ventricular mechanical events [7], leading to increased morbidity and higher risk of sudden cardiac death [8]. However, although mechanical dyssynchrony is present in 64% of patients with wide QRS, it also appears with relatively less prevalence (43%) in heart failure patients with narrow QRS complexes [9]. Thus, selection of candidates for CRT based only on electrical criterion (QRS duration) may actually deprive from a substantial number of patients the benefits of cardiac resynchronisation therapy. Furthermore, the evidence of electromechanical dissociation as expressed by differences in electromechanical coupling intervals between earlier and later electrically activated regions may result in various degrees of mechanical dyssynchrony in patients with similar QRS durations [10]. The above observations imply that, in fact, echocardiographic assessment of mechanical dyssynchrony may provide a better insight of the interventricular and intraventricular synchronicity than prolongation of QRS alone. This is important, as it has been shown that heart failure patients with minimal to no dyssynchrony appear to have a poor prognosis and a lower probability of CRT response [11].
Echocardiographic assessment of mechanical dyssynchrony
Mechanical asynchrony of the cardiac function has been mainly described in terms of (A) atrioventricular (A-V) dyssynchrony, (B) (V-V) interventricular dyssynchrony, and (C) intraventricular dyssynchrony. In fact, assessment of A-V dyssynchrony is currently used mainly in optimization procedures and most evidence suggests that only intraventricular dyssynchrony can effectively predict response after resynchronisation therapy [12]. However, there are studies demonstrating that each of those types of dyssynchrony may actually affect the outcome of resynchronisation therapy and should be kept in mind when CRT assessment is carried out [13].
Atrioventricular (A-V) dyssynchrony
A-V dyssynchrony physiologically reduces the duration of left ventricular filling and thus decreases the preload, stroke volume and cardiac output. The synchronicity of atrial with ventricular mechanical events can be assessed by the evaluation of left ventricular filling time (LVFT) ratio, which is the ratio of LV filling divided by the RR interval from the surface ECG.
LV filling can be evaluated by pulsed Doppler recording of transmitral inflow (from the beginning of E to the end of A wave), with the sample volume placed at the tips of the mitral valve leaflets. A LVFT < 40% is typically observed in the presence of atrioventricular Dyssynchrony [14].
Interventricular dyssynchrony (RV-LV interaction)
Interventricular dyssynchrony occurs when there is a significant delay between RV and LV activation, which can be crudely denoted by the presence of a wide QRS on the surface electrocardiogram [15]. The LV-RV synchronicity can be assessed by two methods, as follows:
The interventricular mechanical delay (IVMD)
Pulsed Doppler-derived pulmonary and aortic blood flow waves are recorded from the RV and LV outflow tracts. The time from the onset of the QRS to the start of pulmonary and aortic blood flow is measured, giving rise to RV and LV pre-ejection time intervals respectively. The subtraction of RV from LV pre-ejection intervals is an index of interventricular mechanical delay. A cut-off value of IVMD: ≥ 40 ms is regarded as an indication of interventricular dyssynchrony [15, 16].
Difference of electromechanical coupling times (RV-LV) by tissue Doppler imaging
Interventricular (LV-RV) asynchrony can also be determined as the difference between electromechanical coupling times (from onset of the QRS complex up to the beginning of regional velocity of myocardial systolic shortening), considered in the basal lateral segment of the RV and in the most delayed LV segment.
A cut-off value of 56 ms had an area under the curve (AUC) = 0.69 in order to predict functional recovery after resynchronization. This index was better than QRS duration (AUC = 0.63) in predicting CRT response, but worse than LV asynchrony (AUC = 0.77) and sum asynchrony (AUC = 0.84) [17].
IntraVentricular dyssynchrony
Several methods have been described in order to assess intraventricular dyssynchrony (Table 1), most of which are presented as follows.
M-mode-derived septal to posterior motion delay (SPWMD)
Synchronicity of radial motion of the anterior septal and posterior walls can be assessed by m-mode echocardiography from the parasternal short-axis view at the mid-ventricular level. The time difference from the onset of QRS to peak of septal and posterior wall motion can be measured, and a cut-off value of SPWMD ≥ 130 ms is regarded as an indication of intraventricular dyssynchrony to predict CRT response [18]. However, ambiguity in septal motion measurement has been also demonstrated to be a reason for failure of prediction of CRT response [19]. As a result, LV dyssynchrony assessment was feasible in 59% of patients with M-mode echocardiography compared with 96% when colour-coded TDI was used [20].
An opposing wall delay by tissue Doppler imaging (TDI)
Tissue Doppler imaging can be used to record longitudinal systolic velocities of any opposing LV wall. A 2-site (septal–lateral), 4-site (septal–lateral and anterior–inferior) and six-site method (septal–lateral and anterior–inferior and anterior septal-posterior) from the 4-, 3- and 2-chamber views have been described. The time difference from the onset of QRS to peak systolic velocity of the basal regions can be measured. The presence of significant intraventricular dyssynchrony to predict CRT response is documented when there is a time difference of opposing walls: Time to Peak Delay ≥ 65 ms [11] (Fig. 1).
Standard deviation of time-to-peak longitudinal systolic velocities by TDI (dyssynchrony index)
Coloured-tissue Doppler has also been used for detection of LV synchronicity, taking into account the standard deviation (SD) of the average time from the onset of QRS to peak longitudinal velocity in the ejection phase (Ts), recorded from the basal and mid segments of the lateral, inf. septal, anterior, inferior, ant. septal and posterior walls in a 12-segment model. A cut-off value of dyssynchrony index ≥ 32.6 ms is regarded as an indication of intraventricular dyssynchrony to predict CRT response [6]. Similarly, a 6-segment model has also been used, including the six basal LV segments, with a SD cut-off ≥ 36.5 ms predicting response [21].
Peak velocity difference (PVD)
A 6-segment model in coloured-tissue Doppler imaging can been used, measuring the time from the onset of QRS to the highest peak longitudinal velocity in either the ejection phase or post-systolic shortening of the six basal walls recorded from the 4-, 3- and 2-chamber apical views. Calculation of the maximal time difference from the above walls generates the PVD, with a cut-off value > 110 ms predicting CRT response [22]. Similarly, a maximum delay in a 12-segment model (including mid also segments) has also been used with a cut-off ≥ 100 ms predicting CRT response (Table 1).
Displacement mapping
For displacement mapping, the velocity–time integral of tissue velocity is computed and the regional long-axis movement curve is displayed. A 6- or 12-segment model can be used, measuring the SD of time to peak displacement (Td) or the maximum delay. A cut-off ≥ 75 ms for the SD of Td and a cut-off ≥ 205 ms for the max delay of Td have been reported to predict CRT response [23].
Sum of maximal electromechanical delay RV-LV
A 3-segment model has been proposed using spectral-tissue Doppler, calculating the time period from the onset of QRS to the onset of longitudinal velocity curve recorded from the basal lateral, septal and posterior walls, from the 4- and 3-chamber apical views. The time difference between the longest and shortest intervals generates the maximal electromechanical delay (MED). A sum of LV MED with LV-RV MED, the last calculated as the difference between electromechanical coupling times in the basal lateral segment of the RV and in the most delayed LV segment, have been found to predict CRT response with a cut-off ≥ 102 ms [17].
Tissue synchronization imaging (TSI)
Colour-coded myocardial velocity [24, 25] based on colour-tissue Doppler imaging has also been used for qualitative assessment of LV synchronicity. The TSI algorithm automatically calculates and colour-codes the time to peak tissue velocity in every region in the image within a specified time interval with reference to the QRS signal. Event timing markers corresponding to aortic valve opening and closure can be incorporated in order to define the ejection phase. The severity of the delay for each myocardial region is depicted in a colour-coded 2D image in a sequence of green, yellow, orange or red for immediate visual identification of regional delay. Quantitative evaluation of time delay based on velocity curves can subsequently be made from the 4-, 3- or 2-chamber apical views.
Systolic dyssynchrony index by three-dimensional (3D) echocardiography
Regional volume changes in respect to timing of the cardiac cycle from a 16-segment model can be provided by three-dimensional echocardiography. The time to the minimum regional volume can be calculated for each myocardial segment. As normally each segment is expected to achieve the minimum volume at almost the same point in the cardiac cycle, the degree of dispersion of time to the minimum regional volume can reflect the severity of dyssynchrony [26]. LV dyssynchrony can be defined as the standard deviation of the average time taken to reach the minimum systolic volume for 16 LV segments (referred as the systolic dyssynchrony index, SDI). A cut-off value for SDI ≥ 5.6% has been found as evidence of intraventricular dyssynchrony predicting CRT response [27].
Septal to posterior wall delay of radial strain based on 2D speckle tracking
Speckle tracking strain analysis from the short axis mid-ventricular level has also been used to assess LV dyssynchrony. Radial strain values from multiple circumferential points are calculated and data are averaged into six segmental time-strain curves. A time difference ≥130 ms of peak strain between the anterior-septal and posterior wall has been defined as a cut-off for intraventricular dyssynchrony for prediction of CRT response [28].
Opposing wall longitudinal delay by velocity vector imaging
Tissue velocities are determined by the automated tracking of periodic B-mode image patterns on digital cine loops from standard apical 4-, 2- or 3-chamber views, with the user tracing the endocardial surface of the left ventricular wall from a single frame. The velocity vector images can facilitate a rapid qualitative visual assessment of LV synchronicity, but can also provide quantitative assessment. Dyssynchrony has been determined as the greatest opposing wall peak longitudinal velocity delay at ejection or post-ejection phase from the three apical views, with a cut-off value ≥ 75 ms to predict CRT response [29].
Time-to-peak longitudinal strain standard deviation (TPS-SD)
Regional longitudinal Doppler-derived strain profiles can be extracted from the basal and mid-segments of each LV wall in the standard 4-, 3- or 2-chamber apical views for a total of 12 segments. The time period from the beginning of Q on the ECG to peak negative strain can be measured for each segment and the standard deviation (SD) of the average has been used as a strain dyssynchrony index. A cut-off value TPS-SD ≥ 60 ms can identify CRT response [30]. Although some evidence suggests that strain-derived dyssynchrony index is a better measurement than the tissue velocity dyssynchrony index for monitoring changes in mechanical dyssynchrony after CRT and for predicting CRT response [31], other studies have reported contradictory results demonstrating that longitudinal strain-derived dyssynchrony analysis failed to predict CRT response compared to velocity-derived dyssynchrony indices, with the latter showing good sensitivity for the same prediction [32]. Those differences may be attributed to variability issues of the examined modes of dyssynchrony, and for that reason, further studies are needed to clarify that discrepancy.
Combined radial 2D speckle tracking and longitudinal colour-TDI dyssynchrony
A combined approach has been used based on 2D speckle tracking, with a cut-off ≥130 ms between the anterior-septal and posterior wall peak radial strain delay and a 2-site (septal–lateral) systolic longitudinal velocity delay with a cut-off ≥ 60 ms. Patients having both longitudinal and radial dyssynchrony demonstrated the greatest improvement in NYHA functional class compared to those having either radial or longitudinal Dyssynchrony [33].
Triplane tissue Doppler imaging
A novel three-dimensional (3-D) tissue Doppler imaging (TDI) modality [34], which permits simultaneous acquisition of TDI from all LV segments during the same heartbeat, in addition to 3-D volumes, has been used to assess intraventricular dyssynchrony. The triplane colour-coded TDI dataset can be used to analyse myocardial velocity curves from 12 LV segments and the SD of time to peak systolic velocities (Ts) is calculated as the dyssynchrony index. Data can be reported in a bulls’ eye plot, and a cut-off value of Ts-SD ≥ 33 ms can predict clinical response to CRT [35]. Similarly, a 6-basal LV segment model can be used (Table 1).
Longitudinal strain delay index by speckle tracking imaging
Londitudinal Strain based on 2D speckle tracking in a 16-segment model has been used in order to calculate the sum of the difference between peak and end-systolic strain defined as Strain Delay index (SDI). A cut-off of SDI ≥ 25% can predict CRT response in both ischemic and non-ischemic Cardiomyopathy [36].
Septal flash
The direct mechanical consequence of dyssynchronous contraction induced by LBBB can be identified by detecting the early activation of the septum as an early septal thickening (Septal Flash) within the isovolumic contraction period. The septal flash can be visualised on the SAX or PLAX either using grey scale or tissue Doppler colour M-mode. The timing and extent of septal flash can be measured by the amplitude of early radial septal velocities. The presence of septal flash as a single parameter of intraventricular dyssynchrony appeared to have a 64% sensitivity and 55% specificity to predict CRT response [13].
Representative measurements of Dyssynchrony based on colour tissue Doppler. Left: Colour tissue Doppler imaging with regions of interest at the basal septal and lateral walls. Middle: Velocity curves are shown from the respective sites with time durations from the onset of QRS to the highest peak systolic velocity at the ejection phase (Red and Green arrows) (AVC: Aortic Valve Closure). The yellow arrow represents the degree of dyssynchrony between the two opposing walls. Right: Longitudinal strain curves from the same regions. Time durations from the onset of the QRS to the peak negative strain in the ejection or post-ejection phase are depicted. The yellow arrow again represents the degree of dyssynchrony between the two opposing walls
Three-dimensional velocities, strain and displacement mapping based on 3D speckle tracking
A novel application based on the procession of 4D data, by speckle tracking of three-dimensional cubic templates throughout the cardiac cycle, has recently evolved. This modality is able to analyse regional 3D wall motion, using components of regional displacement in different directions and subsequently calculating 3D strain and other parameters of LV function for the entire left ventricle [37]. Qualitative assessment of dyssynchrony based on parametric imaging of regional LV displacement can be obtained, using colour-coded dyssynchrony mapping of regional delays (Fig. 2). Quantitative assessment is also feasible, and cut-off values of regional velocities, displacement or 3D strain to predict CRT response are expected.
Dyssynchrony imaging map by 3D speckle tracking. Parametric Imaging (lower left) and polar map (centre) displaying regional colour coded time delays of displacement of the entire left ventricle based on 3D speckle tracking, identifying a post systolic shortening (PSS) event located at inferior septal and inferior wall. End-systolic and end-diastolic volumes, as well as ejection fraction based on 3D measurements are also presented at the same time. Regional curves of longitudinal displacement can also be seen
Lead implantation, scar formation and coronary venous system
The role of the exact site of implantation of the pacing lead on CRT response, has been under focus by many research groups lately. Recent evidence suggests that the site of the latest mechanical activation (most often posterolateral), identified by any of the tissue Doppler, speckle tracking Echo or 3D modalities, is the preferable pacing region, as patients appear to have better acute or long-term response and better prognosis than those with discordant lead implantation [28, 38, 39]. However, other evidence suggests that LV lead concordance to the delayed contraction segment may not be a major determining factor for favourable echocardiographic and clinical outcomes after CRT [40]. In fact, other parameters may also determine the outcome of CRT, such as the presence of scar formation at the region of lead implantation. This is of great importance in patients with ischemic heart disease, especially if this scarring is extensive. Even if the lead is implanted at the site of latest activation, the presence of extensive scarring in that region is associated with low response rates [41, 42]. For that reason, gadolinioum-enhanced cardiovascular magnetic resonance may provide significant information for the selection process and decision making, identifying any scar formation and determining the total scar burden and its distribution [43, 44].
Another important step for the CRT process is the exploration of the coronary venous system and the correct location of the branch that will be used for lead implantation. The coronary transvenous approach through the tributaries of the coronary sinus is the usual way of LV lead placement. Subsequently, the feasibility of appropriate transvenous lead positioning should be determined exploring the anatomy of the venous system by retrograde venography or multislice computed tomography [45]. If suitable veins for pacing a particular site cannot be found, surgical LV lead positioning may be considered, using limited left-lateral thoracotomy with direct epicardial lead placement [46].
Optimization
Optimization of pacemaker settings entails adjustments in the atrio-ventricular (A-V) and right-left ventricular (V–V) synchronicity, in order to have the highest benefit from the resynchronization therapy.
A-V optimization
The aim of A-V optimization is to find the optimal A-V delay in order to have higher diastolic filling time and thus an increased stroke volume and a reduction of a probable pre-systolic MR [47]. Using transmitral pulsed-wave Doppler, diastolic filling time shown by A and E wave is recorded at different A-V delays (iterative method). In particular, starting from a long A-V delay (160–200 ms), an incremental shortening of 20 ms is applied until A-wave truncation is visualised. Then, A-V delay again is incrementally lengthened until truncation no longer exists, which normally corresponds with the longest LV filling time. The minimal AV delay that allows for adequate E and A wave separation, without A wave truncation and termination of the A wave at approximately 40–60 ms before the onset of the QRS is considered the optimal AV delay.
Aortic pulse wave Doppler may also be applied in order to assess velocity time integral (VTI), as an index of stroke volume. The highest VTI should correspond with the optimal A-V delay and the longer filling time. A simplified rule for screening A-V delay is first to check if the A-wave is absent or truncated and second if the E and A waves are merged. In the first case the A-V delay is probably too short, whereas in the second it is too long.
Although in the common clinical setting, a standard short A-V delay (100–120 ms) is usually applied, A-V optimization seems to be of great importance in cases of non-response. However, unresolved issues related to the benefit of fixed or dynamic shortening or lengthening of A-V delay remain to be further elucidated by future studies. Optimization of atrioventricular interval after CRT appears necessary during follow-up as it needs to be readjusted in up to 55% of patients [48].
V–V optimization
Accumulating evidence suggests that sequential LV-RV pacing (LV paced first) is associated with improvement of EF and exercise capacity, and reduction of mitral regurgitation in the majority of patients, compared to simultaneous or RV pre-activated biventricular pacing [49–51]. However, in other studies, simultaneous or RV pre-activation are still of benefit in a considerable cohort of patients, and equal degree of benefit among patients with LV and RV pre-activation may exist [47]. Those findings dictate the need for a patient-tailored approach for optimal V–V pacemaker settings. For that purpose, incremental change by 20 ms of V–V pacing interval, starting from −80 ms (RV first) to +80 ms (LV first) is applied. At the same time, aortic velocity time integral (VTI), as an index of stroke volume, is also measured for each V–V pacing interval. The optimal V–V offset is that of producing the greatest VTI.
CRT response and non-responders
Several clinical and echocardiographic parameters have been used in order to define acute or long-term CRT response.
The clinical/functional end points most often include changes in: (1) NYHA functional class; (2) quality-of-life score; (3) 6-min walk distance; (4) exercise capacity peak VO2; (5) heart failure hospitalizations; and (6) cardiac mortality [52–54].
The echocardiographic markers may include changes in: (1) left ventricular ejection fraction ≥ 15%; (2) reverse LV remodelling (LV end-systolic dimensions/volumes ≥ 15%); (3) degree of mitral regurgitation; (4) dp/dt; and (5) cardiac output [11, 25, 55].
Differences in the response rate can be attributed to the certain end point/marker selected (higher rates of functional response probably due to placebo effect), but also to the fact that clinical and echocardiographic response to CRT may not appear simultaneously at the same time. In fact, improvement of LV function appears to be a gradual process over time [56]. Unfortunately, there is lack of consensus for standard criteria that should be selected to define non-responders, and this may be one of the reasons of the variable rates of non-response reported in the literature. A number of other important factors though have also been described as possible reasons for non-response [57], such as: (1) The limitation of ECG-based selection criterion as opposed to echocardiographic-based mechanical Dyssynchrony; (2) Suboptimal location of the pacing lead, away from the site of the latest activation [28]; (3) The presence of scarring at the region of LV lead placement [41]; (4) Suboptimal A-V and/or V–V optimisation [47, 50]; (5) Unsuitable coronary venous system for appropriate lead implantation; (6) Suboptimal medical therapy [58]; (7) Lead dislodgment; (8) Ischemic heart disease.
The role of myocardial contractile reserve by DSE
The role of dobutanine stress echocardiography (DSE) in patient selection for CRT has also been explored recently. A cutoff value of >7.5% increase in dobutamine-induced LVEF exhibited a sensitivity of 76% and a specificity of 86% to predict response after 6 months of CRT. In addition, contractile reserve in the region in the LV pacing lead was present only in responders (delta strain during low-dose dobutamine 6% ± 5% in responders [59]).
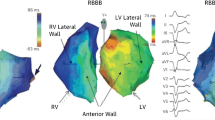
CRT in RBBB
Current guidelines do not discriminate LBBB from RBBB on the basis of long QRS. Furthermore, the evidence of RBBB represents an ‘electrical’ criterion with probably the same limitations as LBBB. Initial studies suggested that patients with RBBB may get a benefit from CRT but probably less than those with LBBB [52]. More recently, it has been demonstrated that in failing hearts, less mechanical dyssynchrony is induced by RBBB than LBBB, and the corresponding impact of CRT on the former is reduced, suggesting that right ventricular-only pacing may be equally as efficacious as biventricular CRT in hearts with pure right bundle branch conduction delay [60]. Finally, other studies again propose the ‘mechanical’ criterion for patient selection, dictating that among patients with RBBB, only those with LV intraventricular mechanical dyssynchrony are likely to benefit from CRT [61].
CRT in short QRS duration
CRT on patients with short QRS duration < 120 ms, remains one of the hot issues in the contemporary research and clinical arena. In this case the patient selection again is based on the controversial electrical criterion. As already mentioned, evidence suggests that patients with short QRS duration may have up to 30–50% incidence of intraventricular mechanical Dyssynchrony [9, 62] and may benefit from CRT [63, 64]. However, the first randomised trial of CRT in patients with heart failure with narrow QRS complexes failed to show a therapeutic effect of CRT on the primary end point of peak myocardial oxygen consumption. A positive effect of CRT was observed though on the secondary end point of improvement in the NYHA functional class. In addition, subgroup analysis showed that particular patients, like those with non-ischemic disease and those with borderline QRS duration (120–130 ms), appeared to have a benefit from CRT on 6-min walk test and peak oxygen consumption, accordingly [65]. More studies are unquestionably needed to clarify the benefit of CRT on patients with short QRS duration and those are expected in the near future.
Dyssynchrony in less severe heart failure (EF > 35%)
The beneficial effect of CRT has been mainly shown in patients with severe heart failure (EF ≤ 35%) in the majority of studies. Recent evidence suggests that up to 55% of patients with less severe heart failure (EF 35–50%) may also demonstrate mechanical asynchrony based on time to peak systolic velocity (Ts) in a 12-segmental model (Ts-SD), denoting that the prevalence of mechanical systolic dyssynchrony is independent of the severity of LV systolic dysfunction [66]. This finding also underlines the potential role of cardiac resynchronization therapy for patients with moderate to mild systolic dysfunction, provided that mechanical Dyssynchrony is present. More evidence is expected from future studies.
CRT in AF
Atrial Fibrillation is common in patients with heart failure, with the prevalence of AF increasing in parallel with the severity of the disease [67]. Available data suggest that CRT is beneficial to patients with AF [68–70]; however the degree of response appears to be largely dependent on the implementation of A-V nodal ablation. As a result, CRT response has been reported to be 54% in patients with AF without nodal ablation, and 71% in those with ablation, compared to 80% in those with sinus rhythm [71]. Furthermore, it has been shown that in patients with severe heart failure, improvement of LA function after CRT is associated with a lower incidence of AF and lower mortality [72].
The impact of CRT on longitudinal, circumferential and radial deformation and torsion
Recent findings based on 2D speckle tracking suggest that short-term improvement of global systolic function and LV reverse remodelling is primarily associated with improvement of the short-axis function (circumferential and radial strain), but not the long-axis function (longitudinal strain) or LV torsion. A favourable response was partially dependent on the relative preservation of the short-axis function, with a preserved baseline mean circumferential strain of >6.5% to be able to predict the gain in ejection fraction > 5% and LV reverse remodelling [73]. The role of radial function has also been demonstrated in another study, showing that differences in baseline LV dyssynchrony between responders and nonresponders were noted only for radial strain, whereas no differences were noted for circumferential and longitudinal strain [74]. With the advancements of 2D speckle tracking, more studies are expected to explore the role of the different components of LV function on CRT in the following years.
Ischemic versus non-ischemic cardiomyopathy
Several studies have demonstrated that CRT response is more likely in patients with non-ischemic than ischemic Cardiomyopathy [75, 76] probably due to a more heterogeneous pattern of asynchrony in the latter. However, other studies have found a comparable degree of response to CRT in those two entities [77, 78]. In a recent study, although it was found that left ventricular reverse remodelling, after CRT, occurred in similar degrees in both non-ischemic and ischemic groups, there was higher cardiovascular mortality in patients with ischemic Cardiomyopathy [79]. In another study [21], the number of segments with post-systolic shortening (PSS) appeared to be a good predictor for CRT response only in patients with non-ischemic cardiomyopathy, but more interestingly, the predictive values of all the TDI parameters were consistently higher in the non-ischemic than the ischemic group.
For how long CRT?
This issue has not been addressed by many studies, but initial evidence suggests that despite the presence of LV reverse remodelling, interruption of CRT results in worsening of LV function and desynchronization. Therefore, continuous long-term pacing may be warranted to maintain the beneficial effects [80].
Current limitations and future perspectives
Modern echocardiography is a versatile imaging modality, but at the same time, suffers from its own limitations. As a result, those limitations may have a deleterious effect on the proposed parameters of mechanical dyssynchrony and may dominate their clinical applicability.
In particular, M-mode (motion mode) provides an excellent temporal resolution, but as the m-mode tracing represents only one anatomical dimension along a single axis established by the location of a stationary ultrasound beam, the magnitude of the spatial resolution is limited [81]. As a consequence, the proposed SPWMD index is applied only in limited areas from the SAX view, evaluating only radial motion. Furthermore, ambiguity in motion measurement, especially when there is an extensive scar formation, may also become a reason of failure in assessing dyssynchrony in certain patients.
Systolic velocities based on colour or spectral tissue Doppler imaging have been subsequently proposed to overcome the shortcomings of the limited spatial resolution of the m-mode and to provide an index of regional longitudinal dyssynchrony. Indexes such as those described above, assessing intraventricular dyssynchrony (2nd, 3rd, 4th and 6th), have been used with success in many single-centre studies. However, as velocities subdued to tethering effects and translational motion of adjacent regions, the interpretation of the mechanical properties of the heart may sometimes become uncertain. Specifically, completely passive segments can show motion relative to the transducer due to tethering, but without any active mechanical deformation, consisting velocity and displacement information probably unreliable for the characterization of such regions [82]. In addition, all TDI data are amenable to angle dependency, and particularly for indexes based on colour tissue Doppler, high image quality is usually needed.
The theoretical advantage of deformation imaging based on TDI data, which calculates the spatial gradients in myocardial velocities and extracts regional deformation (strain) [83], gave birth to another promising index of intraventricular dyssynchrony (11th), in order to surpass the deficiencies of m-mode and velocity-based imaging. However, as the strain Doppler-derived technique is extremely vulnerable to incorporation of noise, reverberations, and artefacts, high variability consists a serious potential limitation, and contradictory publications have reduced the initial enthusiasm. For those reasons, operator experience is extremely important for calculating deformation indexes, and additional studies may be needed to clarify the value of Doppler-Strain in assessing myocardial dyssynchrony. Novel techniques based on 2D speckle tracking have also generated new intraventricular dyssynchrony indices (9th, 10th). The angle independence, the automated tracking algorithm, the lower variability, and the lesser incorporation of noise within the data, definitely give more confidence for what it is measured, however, reverberations and artefacts and more importantly the lower temporal resolution compared to TDI, remain significant shortcomings that need to be solved. Special equipment and operator experience are also significant considerations. 3D echocardiography—3D volumes, has also evolved as a promising method for the evaluation of LV dyssynchrony (8th). This modality also suffers from significant limitations, such as the low spatial and especially the low temporal resolution. Again, special equipment and operator experience are important matters.
There is also another issue that should be underlined at this point. The cut-off values published in the literature regarding the ability of certain indices to predict CRT response are in fact the optimal compromise to discriminate responders from non-responders based on ROC analyses. For that reason, absolute devotion on the exact value for decision making is rather inappropriate and therapy should not be denied to all patients who do not fulfil precisely the described cut-offs. Furthermore, more than one index of dyssynchrony based on different echo-modality should probably be used in order to overcome the potential limitations of certain techniques and to increase the probability of accurately assessing the degree of myocardial asynchrony. As in reality, the motion of the heart is characterized by longitudinal, radial, and circumferential motion, a combination of indices also describing different spatially-oriented planes of motion will also provide a better insight into the mechanical synchronicity. Finally, in an attempt to overcome some limitations of the echo-based indices, complementary imaging modalities such as cardiac MRI, may also significantly contribute to the evaluation of myocardial dyssynchrony, as recent studies have shown [84].
Although single-centre trials have shown the benefit of evaluation of mechanical dyssynchrony in CRT, more prospective multicentre studies are definitely needed in order to verify the role of echo-based dyssynchrony indices in decision-making, and to prove that a certain combination of parameters is indeed able to discriminate responders from non-responders. This is even more challenging because one of the first multicentre trials [85] failed to prove the need for echo-parameters of mechanical dyssynchrony, concluding that no single echocardiographic measure of dyssynchrony may be recommended to improve patient selection for CRT beyond current guidelines based on EF and QRS duration. It should be underlined though, that in the last study, a large variability in the analysis of the dyssynchrony parameters arising from technical and interpretative factors was regarded as an important reason for that failure. Certain facts should be taken into account in relation to echo-based indices of mechanical dyssynchrony, especially in a multicentre setting: (1) The quality of the measured tracings of the advanced echo parameters is probably not similar for different vendors; (2) All the indices have their own limitations based on the modality used, as mentioned before, and (3) The evolution of novel sophisticated echo parameters based on advanced technology may not have been accompanied by certified training and expertise in different centres. As a result, appropriate index selection, adequate training, and refinements in the technology of the ultrasound equipment appear to be the main challenges for the years to come.
Conclusion
From the current literature review it is clear that an overall approach of cardiac resynchronization therapy should be taken into consideration, focusing not only on electrical or mechanical dyssynchrony, but also on several other covariates that appear to contribute significantly to the outcome of that particular mode of treatment.
References
Ho KKL, Pinsky JL, Kannel WB, Levy D (1993) The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 22(Suppl A):6A–13A
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T et al (2004) Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350:2140–2150
Cleland J, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L et al (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352:1539–1549
The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. (2008). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J 29: 2388–2442
Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, Van Erven L, Boersma E et al (2003) Left ventricular Dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol 92:1238–1240
Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP (2003) Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 91:684–688
Grines C, Bashore T, Boudoulas H, Olson S, Shafer P, Wooley C (1989) Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 79:845–853
Padelettia L, Giaccardia M, Turrenia F, Musillia N, Colellaa A, Pieragnolia P et al (2004) Influence of QRS prolongation on the natural history of CHF. Eur Heart J Suppl 6:D79–D82
Yu CM, Lin H, Zhang Q et al (2003) High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart 89:54–60
Asirvatham S (2007) Cardiac resynchronization: is electrical synchrony relevant? J Cardiovasc Electrophysiol 18:1028–1031
Bax JJ, Bleeker GB, Marwick TH et al (2004) Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 44:1834–1840
Bax J, Abraham T, Barold S, Breithardt O, Fung J, Garrigue S et al (2005) Cardiac resynchronization therapy. J Am Coll Cardiol 46:2153–2167
Parsai C, Bijnens B, Sutherland GR, Baltabaeva A, Claus P, Marciniak M et al. (2009) Toward understanding response to cardiac resynchronization therapy: left ventricular dyssynchrony is only one of multiple mechanisms. Eur Heart J 8:940–949
Cazeau S, Bordachar P, Jauvert G, Lazarus A, Alonso C, Vandrell M et al (2003) Echocardiographic modeling of cardiac dyssynchrony before and during multisite stimulation: a prospective study. Pacing Clin Electrophysiol 26:137–143
Rouleau F, Merheb M, Geffroy S, Berthelot J, Chaleil D, Dupuis J et al (2001) Echocardiographic assessment of the interventricular delay of activation and correlation to the QRS width in dilated cardiomyopathy. Pacing Clin Electrophysiol 24:1500–1506
Ghio S, Constantina C, Klersy C, Serioa A, Fontana A, Campana C et al (2004) Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J 7:571–578
Penicka M, Bartunek J, de Bruyne B, Marc Vanderheyden M, Goethals M, Marc De Zutter M et al (2004) Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation 109:978–983
Pitzalis M, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G et al (2002) Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 40:1615–1622
Marcus G, Rose E, Viloria E, Schafer J, De Marco T, Saxon L et al (2005) Septal to posterior wall motion delay fails to predict reverse remodeling or clinical improvement in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol 46:2208–2214
Bleeker GB, Schalij MJ, Boersma E, Holman ER, Steendijk P, Van der Wall EE et al (2007) Relative merits of M-mode echocardiography and tissue Doppler imaging for prediction of response to cardiac resynchronization therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1:68–74
Yu CM, Fung JW, Zhang Q, Chan C, Chan Y, Hong Lin H et al (2004) Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 110:66–73
Notabartolo D, Merlino JD, Smith AL, DeLurgio D, Vera F, Easley K et al (2004) Usefulness of the peak velocity difference by tissue Doppler imaging technique as an effective predictor of response to cardiac resynchronization therapy. Am J Cardiol 94:817–820
Yu CM, Zhang Q, Chan YS, Chan CK, Yip GW, Kum LC et al (2006) Tissue Doppler velocity is superior to displacement and strain mapping in predicting left ventricular reverse remodelling response after cardiac resynchronisation therapy. Heart 92:1452–1456
Van de Veire NR, Bleeker GB, De Sutter J, Ypenburg C, Holman ER, Van DerWal EE et al (2007) Tissue synchronisation imaging accurately measures left ventricular dyssynchrony and predicts response to cardiac resynchronisation therapy. Heart 93:1034–1039
Yu CM, Zhang Q, Fung J, Chi-Kin Chan H, Chan Y, Yip G et al (2005) A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol 45:677–684
Kapetenakis S, Siva A, Corrigan N, Cooklin M, Kearney MT, Monaghan MJ (2005) Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 112:992–1000
Marsan NA, Bleeker GB, Ypenburg C, Ghio S, Van de Veire NR, Holman ER et al (2008) Real-time three-dimensional echocardiography permits quantification of left ventricular mechanical dyssynchrony and predicts acute response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol 4:392–399
Suffoletto M, Dohi K, Cannesson M, Saba S, Gorcsan J (2006) Novel speckle-tracking radial strain from routineblack-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 113:960–968
Cannesson M, Tanabe T, Suffoletto M, Schwartzman D, Gorcsan J (2006) Velocity vector imaging to quantify ventricular dyssynchrony and predict response to cardiac resynchronization therapy. Am J Cardiol 98:949–953
Mele D, Pasanisi G, Capasso F, Simone A, Morales M, Poggio D et al (2006) Left intraventricular myocardial deformation dyssynchrony identifies responders to cardiac resynchronization therapy in patients with heart failure. Eur Heart J 27:1070–1078
Miyazaki C, Lin G, Powell B, Espinosa R, Bruce C, Miller F et al (2008) Strain dyssynchrony index correlates with improvement in left ventricular volume after cardiac resynchronization therapy better than tissue velocity dyssynchrony indexes. Circ Cardiovasc Imaging 1:14–22
Yu CM, Gorcsan J III, Bleeker GB, Zhang Q, Schalij MJ, Suffoletto MS et al (2007) Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol 8:1263–1270
Gorcsan J, Tanabe M, Bleeker G, Suffoletto M, Thomas N, Saba S et al (2007) Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol 50:1476–1483
Van de Veire NR, Bleeker GB, Ypenburg C, De Sutter J, Ajmone Marsan N, Holman ER et al (2007) Usefulness of triplane tissue Doppler imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol 100:476–482
Van de Veire NR, Yu CM, Ajmone-Marsan N, Bleeker GB, Ypenburg C, De Sutter J (2008) Triplane tissue Doppler imaging: a novel three dimensional imaging modality that predicts reverse left ventricular remodelling after cardiac resynchronisation therapy. Heart 94:e9
Lim P, Buakhamsri A, Popovic ZB, Greenberg NL, Patel D, Thomas JD et al (2008) Longitudinal strain delay index by speckle tracking imaging: a new marker of response to cardiac resynchronization therapy. Circulation 11:1130–1137
Abe Y, Kawagishi T, Ohuchi H, Takeguchi T, Nishiura M (2008) Accurate detection of regional contraction using novel 3-dimensional speckle tracking technique. J Am Coll Cardiol 51:903–953
Ypenburg C, Van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E et al (2008) Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol 17:1402–1409
Van de Veire NR, Marsan NA, Schuijf JD, Bleeker GB, Wijffels MC, Van Erven L et al (2008) Noninvasive imaging of cardiac venous anatomy with 64-slice multi-slice computed tomography and noninvasive assessment of left ventricular dyssynchrony by 3-dimensional tissue synchronization imaging in patients with heart failure scheduled for cardiac resynchronization therapy. Am J Cardiol 7:1023–1029
Fung JW, Lam YY, Zhang Q, Yip GW, Chan WW, Chan GC et al. (2008) Effect of left ventricular lead concordance to the delayed contraction segment on echocardiographic and clinical outcomes after cardiac resynchronization therapy. J Cardiovasc Electrophysiol 5:536–538
Bleeker GB, Kaandorp TAM, Lamb HJ, Boersma E, Steendijk P, De Roos A et al (2006) Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 113:969–976
Hummel JP, Lindner JR, Belcik JT, Ferguson J, Mangrum M, Bergin J et al (2005) Extent of myocardial viability predicts response to biventricular pacing in ischemic cardiomyopathy. Heart Rhythm 2:1211–1217
Setser R, Bexell D, O’Donnell T, Stillman A, Lieber M, Schoenhagen P et al (2003) Quantitative assessment of myocardial scar in delayed enhancement magnetic resonance imaging. J Magn Reson Imaging 18:434–441
Ypenburg C, Roes SD, Bleeker GB, Kaandorp TA, De Roos A, Schalij MJ et al (2007) Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol 5:657–660
Jongbloed MR, Lamb HJ, Bax JJ, Schuijf J, De Roos A, Van der Wall E et al (2005) Noninvasive evaluation of the cardiac venous system using multislice computed tomography. J Am Coll Cardiol 45:749–753
Koos R, Sinha AM, Markus K, Breithardt O, Mischke K, Zarse M et al (2004) Comparison of left ventricular lead placement via the coronary venous approach versus lateral thoracotomy in patients receiving cardiac resynchronization therapy. Am J Cardiol 94:59–63
Porciani MC, Dondina C, Macioce R, Demarchi G, Pieragnoli P, Musilli N et al (2005) Echocardiographic examination of atrioventricular and interventricular delay optimization in cardiac resynchronization therapy. Am J Cardiol 95:1108–1110
Zhang Q, Fung JW, Chan YS, Chan HC, Lin H, Chan S et al (2008) The role of repeating optimization of atrioventricular interval during interim and long-term follow-up after cardiac resynchronization therapy. Int J Cardiol 2:211–217
Sogaard P, Eglebad H, Pedersen AK, Kim W, Kristensen B, Hansen P et al (2002) Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation 106:2078–2084
Vanderheyden M, De Backer T, Rivero-Ayzera M, Geelen P, Bartunek J, Verstreken S et al (2005) Tailored echocardiographic interventricular delay programming further optimizes left ventricular performance after cardiac resynchronization therapy. Heart Rhythm 2:1066–1072
Leon AR, Abraham WT, Brozena S, Daubert J, Fisher W, Gurley J et al (2005) Cardiac resynchronization therapy with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol 46:2298–2304
Abraham WT, Fisher WG, Smith AL, Delurgio D, Leon A, Loh E et al (2002) Multicenter InSync randomized clinical evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 346:1845–1853
Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier A et al (2003) Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol 42:2109–2116
Young JB, Abraham WT, Smith AL, Leon A, Lieberman R, Wilkoff B et al (2003) Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA 289:2685–2694
Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus K, Franke A et al (2003) Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol 41:765–770
Yu CM, Chau E, Sanderson JE, Fan K, Tang M, Fung W et al (2002) Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 105:438–445
Yu CM, Fung J, Zhang Q, Sanderson J (2005) Understanding nonresponders of cardiac resynchronization therapy—current and future perspectives. J Cardiovasc Electrophysiol 16:1117–1124
Fung JW, Chan JY, Kum LC, Chan HC, Yip GW, Zhang Q et al (2007) Suboptimal medical therapy in patients with systolic heart failure is associated with less improvement by cardiac resynchronization therapy. Int J Cardiol 2:214–219
Ypenburg C, Sieders A, Bleeker GB, Holman ER, Van der Wall EE, Schalij MJ et al (2007) Myocardial contractile reserve predicts improvement in left ventricular function after cardiac resynchronization therapy. Am Heart J 6:1160–1165
Byrne M, Helm R, Daya S, Osman N, Halperin H, Berger R et al (2007) Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol 15:1484–1490
Garrigue S, Reuter S, Labeque JN, Jais P, Hocini M, Shah DC et al (2001) Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol 88:1436–1441
Ghio S, Constantin C, Klersy C, Serio A, Fontana A, Campana C et al (2004) Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J 25:571–578
Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, De Spirito S, Guerra R, Patruno N, Serra F (2003) Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol 42:2117–2124
Turner MS, Bleasdale RA, Vinereanu D, Mumford CE, Paul V, Fraser AG et al (2004) Electrical and mechanical components of Dyssynchrony in heart failure patients with normal QRS duration and left bundle-branch block: impact of left and biventricular pacing. Circulation 109:2544–2549
Beshai JF, Grimm RA, Nagueh SF, Baker J, Beau S, Greenberg S et al (2007) Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 357:2461–2471
Chan CP, Zhang Q, Yip GW, Fung JW, Lam YY, Lee PW et al (2008) Relation of left ventricular systolic dyssynchrony in patients with heart failure to left ventricular ejection fraction and to QRS duration. Am J Cardiol 5:602–605
Maisel W, Stevenson L (2003) Atrial fibrillation in heart failure: epidemiology, pathophysiology and rationale for therapy. Am J Cardiol 91:2D–8D
Hay I, Melenosky V, Fetics B, Judge D, Kramer A, Spinelli J et al (2004) Short-term effects of right-left heart sequential cardiac resynchronization in patients with heart failure, chronic atrial fibrillation and atrioventricular nodal block. Circulation 110:3404–3410
Leclercq C, Walker S, Linde C, Clementy J, Marshalle A, Ritter P et al (2002) Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J 23:1780–1787
Linde C, Leclercq C, Rex S et al (2002) Long-term benefits of biventricular pacing in congestive heart failure: results from the MUSTIC study. J Am Coll Cardiol 40:111–118
Molhoek SG, Bax JJ, Bleeker GB, Boersma E, Van Erven L, Steendijk P et al (2004) Comparison of response to cardiac resynchronization therapy in patients with sinus rhythm versus chronic atrial fibrillation. Am J Cardiol 94:1506–1509
Fung JW, Yip GW, Zhang Q, Fang F, Chan JY, Li CM et al (2008) Improvement of left atrial function is associated with lower incidence of atrial fibrillation and mortality after cardiac resynchronization therapy. Heart Rhythm 6:780–786
Zhang Q, Fung J, Yip G, Chan J, Lee A, Lam Y et al (2008) Improvement of left ventricular myocardial short axis, but not long-axis function or torsion after cardiac resynchronisation therapy: an assessment by two-dimensional speckle tracking. Heart 94:1464–1471
Delgado V, Ypenburg C, Van Bommel RJ, Tops LF, Mollema SA, Marsan NA et al (2008) Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol 20:1944–1952
St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR et al (2003) Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 15:1985–1990
Duncan A, Wait D, Gibson D, Daubert JC, MUSTIC (Multisite Stimulationin Cardiomyopathies) Trial (2003) Left ventricular remodelling and haemodynamic effects of multisite biventricular pacing in patients with left ventricular systolic dysfunction and activation disturbances in sinus rhythm: sub-study of the MUSTIC (Multisite Stimulationin Cardiomyopathies) trial. Eur Heart J 5:430–441
Molhoek SG, Bax JJ, Van Erven L, Bootsma M, Boersma E, Steendijk P et al (2004) Comparison of benefits from cardiac resynchronization therapy in patients with ischemic cardiomyopathy versus idiopathic dilated cardiomyopathy. Am J Cardiol 7:860–863
Breithardt OA, Stellbrink C, Franke A, Balta O, Diem BH, Bakker P et al (2002) Acute effects of cardiac resynchronization therapy on left ventricular Doppler indices in patients with congestive heart failure. Am Heart J 1:34–44
Zhang Q, Fung JW, Chan JY, Yip GW, Lam YY, Liang YJ et al. (2009) Difference in long-term clinical outcome after cardiac resynchronization therapy between ischemic and non-ischemic etiologies of heart failure. Heart 2:113–118
Ypenburg C, Van Bommel RJ, Marsan NA, Delgado V, Bleeker GB, Van der Wall EE et al (2008) Effects of interruption of long-term cardiac resynchronization therapy on left ventricular function and dyssynchrony. Am J Cardiol 6:718–721
Zagzebski JA (1996) Essentials of ultrasound physics. Mosby Inc., vol 3, pp 57–58, ISBN: 0-8151-9852-3
Pavlopoulos H, Nihoyannopoulos P (2008) Strain and strain rate deformation parameters: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging 24:479–491
D’hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F (2000) Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 1:154–170
Koos R, Neizel M, Schummers G, Krombach GA, Stanzel S, Günther RW (2008) Feasibility and initial experience of assessment of mechanical dyssynchrony using cardiovascular magnetic resonance and semi-automatic border detection. J Cardiovasc Magn Reson 10:49
Chung E, Leon A, Tavazzi L, Sun J, Nihoyannopoulos P, Merlino J (2008) Results of the predictors of response to CRT(PROSPECT) trial. Circulation 117:2608–2616
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlopoulos, H., Nihoyannopoulos, P. Recent advances in cardiac resynchronization therapy: echocardiographic modalities, patient selection, optimization, non-responders—all you need to know for more efficient CRT. Int J Cardiovasc Imaging 26, 177–191 (2010). https://doi.org/10.1007/s10554-009-9523-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-009-9523-5