This paper reviews the structural and group compositions of three winter base diesel fuels and their influence on the low-temperature and lubricating properties of the fuels. It is shown that a high content of saturated hydrocarbons, primarily medium-molecular-mass n-alkanes, and arenes with a higher proportion of substitution worsens the low-temperature properties. A decrease in the proportion of medium-molecular-mass alkanes and even a slight increase in the content of bi- and polycyclic aromatic hydrocarbons degrades the lubricating properties of the fuel. The influence of the component composition of the diesel fuels on the effectiveness of anti-wear and depressor-dispersing additives was noted. A study of the compatibility of additives of different functionalities revealed that an anti-wear additive based on tallow-oil fatty acids did not affect the activity of a depressant-dispersing additive while the combined use of these additives slightly worsened the lubricating properties but did not shift this indicator beyond the established standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Current quality requirements for automobile fuel in the RF are defined by a Technical Regulation of the Customs Union (TR CU 013/2011 of Oct. 18, 2011 No. 826) [1]. Compliance with such strict requirements is not possible today without using functional additives recommended by manufacturers such as cetane-booster (ignition-promoter), anti-wear (or lubricating), depressor-dispersing, and other additives [2,3,4,5,6]. The efficiency of functional additives depends mainly on the hydrocarbon composition of the diesel fuel [5], the chemical nature of the additives themselves [7], and the compatibility of the additives of various functionalities in the base fuel [8].
Depressor-dispersing additives are the most common type of additive used in diesel fuel. The purpose of this type of additive is to improve the low-temperature characteristics of diesel fuels and to increase the production volume of winter diesel fuels [9,10,11,12,13]. The main requirement applied to this type of additive is good mixing and dissolution in the fuel. Currently, polymeric additives are most common [14,15,16,17,18,19,20,21,22,23,24]. Practically all depressor-dispersing additives permitted for use in Russia are imported [2, 6].
Anti-wear additives represent another type of additive for modem diesel fuels with ultralow total sulfur contents [3, 6, 25,26,27,28]. Two brands of anti-wear additives that are permitted for use in diesel fuel and are produced from domestic feedstocks [tallow-oil fatty acids (TOFAs)] are now available in Russia. These are Baikat manufactured by JSCǀ Angarsk Catalyst and Organic Synthesis Plant (ACOSP) and Kompleksal Eko “D” manufactured by LLL Novokuibyshevsk Oils and Additives Plant. Many refineries use imported additives to manufacture diesel fuel.
A composition including additives of various functionalities should be selected considering the structures of intermolecular complexes formed by interaction of various additive components and diesel fuels of various chemical group compositions because additives recommended for use in fuel are based on compounds belonging to various chemical classes [5, 29, 30].
The goal of the present work was to compare the efficiencies of anti-wear and depressor-dispersing additives in winter diesel fuels of various hydrocarbon group compositions. This research area is crucial because science-based principles for selecting compositions of functional additives and their optimal concentrations depending on the specific hydrocarbon composition of the diesel fuel must be developed.
Winter base diesel fuels from three large refineries using different crude oils were used in the research. Table 1 presents the physicochemical and operating properties of the fuels. All samples were taken after the hydrofining unit, did not contain additives, and belonged to diesel fuel class 5 (Euro-5) according to wt% sulfur and wt.% polycyclic aromatic hydrocarbons.
Industrially manufactured depressor-dispersing additives from various manufacturers were tested in the work. They included additives “A” and “B”, the main component of which was a copolymer of ethylene and vinylacetate, and additive “D”, which was produced from TOFAs.
The structural group composition of the diesel fuels was determined using gas chromatography (GC) and PMR spectroscopy. The molecular-mass distribution of the n-alkanes in the diesel fuel was determined using an Agilent 7890A gas chromatograph. The content of n-alkanes in the fuel was calculated by a research method based on an internal standard method. The contents of hydrocarbon groups were determined using high-performance liquid chromatography (HPLC) on a Waters 1525 instrument according to GOST EN 12916-2012. The relative contents of various structural groups in the hydrocarbons contained in the diesel fuels were calculated from PMR spectra obtained on a Broker Avance 400 instrument. The lubricity was determined on a PCS Instruments High Frequency Reciprocating Rig [31] according to GOST RISO 12156-1-2012. Cloud point and pour point temperatures were determined on a LAZ-Ml instrument according to GOST 5066-91 and GOST 20287-91, respectively. The cold filter plugging point (CFP) temperature was measured on an LOIP CFP-LAB-11 instrument according to GOST 22254-92.
Table 1 presents the physicochemical and operating characteristics of the studied fuel samples.
Table 1 shows that the main characteristics of the studied diesel fuels manufactured by the various refineries differed noticeably. The CFP temperatures varied from —19°C (fuel 1) to —36°C (fuel 3); pour point temperatures, from-25°C (fuel 3) to —37°C (fuel 1); despite all fuels being winter blends. Differences were also observed in the cloud points, densities, and kinematic viscosities of the tested fuels.
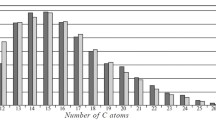
n-Alkanes are known to have the main influence on the low-temperature properties of fuels. The quantitative contents of n-alkanes in the tested fuels were calculated using their molecular-mass distributions (ISINIDs) in the chromatograms obtained using a published method [32]. Table 2 presents the results for the hydrocarbon group compositions of the tested fuels using HPLC, mass contents, and group distributions of the n-alkanes from GC data.
Table 2 shows that fuel 1 had the greatest total content of n-alkanes. High contents of n-alkanes are known to worsen the fuel parameters at low temperatures. However, fuel 1 had the best low-temperature properties of all three samples. These results could be explained by the high content (25 wt.%) in it of low-molecular-mass n-alkanes, which was 89.3% of the total n-alkane content in the fuel. This hydrocarbon group could be considered a solvent for high-molecular-mass hydrocarbons, including high-molecular-mass n-alkanes. The MIADs and total n-alkane contents in fuels 2 and 3 were similar although even the slight difference in the NSIDs of the n-alkanes in these fuels affected their low-temperature characteristics, in particular, the pour point temperatures. Hence. it was concluded that the mutual group distribution of low-molecular-mass, medium-molecular-mass, and high-molecular-mass n-alkanes was more important than their total contents for the low-temperature properties of diesel fuels, especially winter blends.
The effects of other hydrocarbon groups on the low-temperature properties of diesel fuels should be considered while giving priority to n-alkanes.
PMR spectroscopy was used to study in more detail the structural group compositions of the fuels. The obtained MIR spectra were divided into seven ranges in intervals from 0.3 to 8 ppm. It is noteworthy that resonances in the range 4.4-6.2 ppm that were characteristic of olefins were observed for all fuels.
The region of 6 ppm, which is characteristic of protons of monocyclic aromatic hydrocarbons, was taken as a standard for quantitative evaluation of the proton contents in various aliphatic groups in both saturated and aromatic structures. Its integrated intensity was set equal to 1. Integrated intensities of other regions were obtained by difference. Table 3 presents the analytical results.
Integrated areas of resonances in regions 1-2 decreased in the order fuel 3 → fuel 2 → fuel 1. This indicated that fuel 3 had the greatest content of saturated hydrocarbons; fuel 1, the lowest. The greatest fraction in fuel 3 was CH3,-groups, which was indicative of relatively high branching of the paraffin chains. It should be noted that resonances of CH—, CH2–, and CH3– groups in β- and γ-positions on aromatic rings were located in this same range. However, it was difficult to isolate them from other resonances.
Integrated intensities in ranges 3-5, which were characteristic of primary, secondary, and tertiary C atoms in the aromatic a-position, were approximately equal for all three fuels. However, this parameter was slightly greater in fuel 3, which could indicate a greater amount of disubstituted monocyclic aromatic hydrocarbons, i.e., cracking products of the corresponding tetralins. The areas of resonances in ranges 3 and 5 were less for fuel 2 than for fuel 3; in range 4, approximately the same. This could indicate that the contents of the corresponding tetralins were greater than those of their cracking products. Fuel 1 had the lowest integrated intensity in this range. A relatively stronger resonance in range 7 was observed for fuel 1 and was characteristic of bi- and polycyclic arenes. This agreed with the HPLC data given in Table 2.
Thus, fuel 3, which exhibited the worst low-temperature properties, was characterized by greater fractions of saturated hydrocarbons and had greater substitution in its arene composition. Also, the data in Table 3 led to a conclusion about the reasons for the differences in the low-temperature properties of fuels 1 and 2. These fuels had similar total contents of aliphatic structures but differed qualitatively. The aliphatic chains were shorter in fuel 1 (fewer CH2 and CH— groups), which was entirely consistent because the end of boiling of fuel 1 was 40°C less (Table 1). This conclusion also agreed with the NIMD of the n-alkanes (Table 2) and explained the very low pour point and CFP temperatures of this fuel. Furthermore, this hydrocarbon composition of fuel 1 was the basis for its poor lubricating properties as compared with the other two fuels because of the high relative content of low-melting allcanes, lower fraction of substituted armies, and slightly elevated contents of bi- and polycyclic aromatic hydrocarbons.
The effects of two industrially manufactured depressor-dispersing additives and an anti-wear additive on the low-temperature and lubricating properties of the studied diesel fuels with different distributions of separate hydrocarbon groups were compared.
Depressor-dispersing additives A and B were added to the tested fuels at concentrations of 200, 400, and 600 ppm. Figures 1 and 2 illustrate the low-temperature characteristics of the fuels in the presence of these additives.
The results showed that the overall natures of the dependences of pour point temperature on concentration of additive B in the tested fuels were similar (Fig. lb). The pour point temperature of the fuels decreased by 10-16°C with increasing concentration of additive B. The largest depression of the pour point temperature of 7-9°C was observed for the minimum concentration of 200 ppm. Increasing the concentration of the additive further to 600 ppm increased the depression of the pour point temperature by 3-5°C. The practically parallel path of the curve in Fig. 1b indicated that the mutual distribution of the separate hydrocarbon groups and subgroups in the fuels did not have a significant effect on the efficiency of additive B and that the overall contents of medium- and high-molecular-mass n-alkanes played a role.
Conversely, the efficiency of additive A depended more on the fuel hydrocarbon group composition. The smallest depression (3°C) of the pour point temperature was observed in fuel 1, which was characterized by high contents of low-molecular-mass n-alkanes (up to C15) and low contents of medium-molecular-mass n-alkanes (C16-C21). However. the pour point temperature depression in the presence of this additive was 16°C in fuel 3, where the fraction of medium-molecular-mass (C16-C21) branched alkanes was greater. The largest depression of pour point temperature (12°C) was attained in fuel 2 at the minimum concentration of 200 ppm. Increasing the additive concentration in the fuel to 600 ppm had an insignificant effect on the reduction of the pour point temperature of the fuel.
The largest depression of the CFP temperature was observed in fuels 2 and 3 for a concentration of 400 ppm for both additive A(10 and 9T, respectively) and additive B (7 and 8°C, respectively). The depressor effect decreased slightly in fuel 3 if the additive concentration was increased from 400 to 600 ppm. The effect from adding depressor-dispersing additives to fuel 1, which had an initially low CFP, was insignificant. Thus, the effect from adding depressor additives was evident in fuels with a relatively high fraction of high-molecular-mass n-alkanes because they crystallized out first from the fuel bulk and formed a disperse phase that plugged a standard filter. Depressor additives affect crystal growth by promoting the formation of smaller crystalline particles, which reduces the fuel CFP.
It is noteworthy that additives that efficiently lower the pour point temperature are not always efficient for lowering the CFP. The differences in the performance of depressor-dispersing additives could also be related to their compositions, namely the chemical structure and contents of the main component and the solvent used to prepare commercial forms of these additives.
Figure 3 compares anti-wear test results for additive Data concentration of 200 ppm in the tested fuels.
Figure 3 shows that winter base diesel fuels with ultralow sulfur contents (<10 mg/kg) had poor lubricities. The diameter of the wear scar exceeded the regulatory limits 460 μm according to GOSTR32511-2013). However, this parameter improved to the standard requirements if the anti-wear additive was added to the fuel. Fuel 1 showed the greatest effect from using anti-wear additive D and had the lowest initial lubricity of the tested fuels.
Samples containing simultaneously depressor-dispersing additive B (600 ppm) and anti-wear additive D (200 ppm) were prepared to analyze the compatibility of the additives in diesel fuel.
Table 4 presents the determined low-temperature properties of fuel with combined use of these additives.
Table 4 shows that the low-temperature parameters of the tested fuels were practically unchanged after adding the anti-wear additive, which indicated that the anti-wear additive components did not reduce the efficiency of the depressor-dispersing additive.
Table 5 compares test results for the lubricating properties of diesel fuels with combined use of depressor-dispersing (B) and anti-wear additives (D).
Table 5 shows that combined use of anti-wear (D) and depressor-dispersing additives (B) worsened the lubricating properties of the fuels, which was evident from the increased corrected diameter of the wear scar. This was suggestive of a negative effect of depressor-dispersing additive B on the operation of anti-wear additive D. Nevertheless. the results for the corrected wear scar remained within the standard limits.
References
Decision of the Customs Union Commission of Oct. 18, 2011, No. 826 “On the adoption of technical regulations of the customs union ‘On requirements for automobile and aviation gasoline, diesel and marine fuel, jet fuel and heating oil’.”
A. M. Danilov. Use of Fuel Additives. Handbook [in Russian], Khimizdat, St. Petersburg, 2010, 368 pp.
A. M. Danilov, Neftekhimiya, 60, No. 2, 163-171(2020).
T. N. Mitusova and M. V. Kalinina, “On diesel fuel additives,” in: Proceedings of the 6th International Conference Fuel Additives 2017 [in Russian], Moscow, Sept. 2017.
E. A. Burov, L. V. Lava, V. N. Koshelev, et al., Khim. Tekhnol Topl. Masel, No. 2, 16-20 (2020).
D. F. Grishin, Neftekhimiya, 57, No. 5, 489-502 (2017).
T. N. Mitusova, E. V. Polina, and M. V. Kalinina, Modem Diesel Fuels and Their Additives [in Russian], Tekhmla, Moscow, 2002, 64 pp.
T. N. Mitusova, V. A. Lyubimenko, and A. S. Nedaiborshch, Neftepererab. Neftekhim., No. 5, 23-26 (2015).
T. N. Mitusova et al., “On diesel fuel additives,” in: Proceedings of the 6th International Conference Fuel Additives 2017 [in Russian], CREON Energy, Moscow, 2017.
J. Denis and J.-P. Durand, Rev. Inst. Fr. Pet., 46, No. 5, 637 (1991).
N. Sh. Mukhtorov, S. A. Karpov, and V. M. Kapustin, Neftepererab. Neftekhim., No. 10, 46-48 (2012).
R. A. Terteryan, Depressor Additives for Oil, Fuel and Lubricants [in Russian], Khimiya, Moscow, 1990,226 pp.
T. N. Mitusova, V. A. Khavkin, L. A. Gulyaeva, et al., Mir Nefteprod., No. 2, 6-8 (2012).
D. W. Jennings and J. Breitigam, Energy Fuels, 24, No. 4, 2337-2349 (2010).
B. Wei, J. Pet. Explor. Prod. Technol., 5, No. 4, 391-401(2015).
P. V. Ivcbenko and I. E. Nifant2ev, Vysokomol. Soedin., Ser. A, 60, No. 5, 384-401 (2018).
B. Wei, J. Pet. Explor. Prod. Technol, 5, No. 4, 391-401 (2015).
D W. Jennings and J. Breitigam, Energy Fuels, 24, No. 4, 2337-2349 (2010).
S. Yi and J. Zhang, Energy Fuels, 25, No. 12, 5660-5671(2011).
K. B. Rndyak, K. B. Polyanskii, et al., RU Pat. 2,715,896, Mar. 4, 2020.
K. B. Polyanskii, D. B. Zemtsov, et al., RU Pat. 2,684,412, Apr. 9, 2019.
T. V. Rasknlova, I. M. Prokhorchenko, et al., RU Pat. 2,599,778, Jul. 20, 2016.
S. V. Grobov, A. I. Dudko, et al., RU Pat. 2,685,550, Apr. 22, 2019.
A. V. Batyreva, P. I. Fedotov, et al., RU Pat. Appl. 2009126336, Nov. 20, 2013.
T. N. Mitasova, Mir Nefteprod., No. 9-10,10-16 (2009).
T. N. Mitusova, M. V. Kalinin, and E. E. Safonova, Mir Nefteprod., No. 5, 51-53 (2010).
E. B. Shevehenko, A. I. Sukhanberliev, M. M. Abbasov, et al., Zh. Prikl. Khim.,92, No. 1,133-136 (2019).
A. M. Saifullin, M. M. Abbasov, et al.. RU Pat 2.641.736, Jan. 23, 2018.
S. T. Bashkatova and V. A. Vinalcurov, Tr. Ross. Gos. Univ. Nefti Gaza im.I. M. Gubkina, No. 2, 45-56 (2009).
V. A. Lyubimenko, Tr. Ross. Gos. Univ. Nefti Gaza im. I. M. Gubkina, No. 3, 88-96 (2014).
Standard EN 590 (BS EN 590:2004/DIN EN 590-2004), Automotive fuels. Diesel. Requirements and test methods.
L. V. Ivanova, G. N. Gordadze, and V. N. Koshelev, Tr. Ross. Gos. Univ. Nefti Gaza im. I M. Gubkina, No. 3 (264), 61-68 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 1, pp. 11 — 16, January —February, 2021.
Rights and permissions
About this article
Cite this article
Burov, E.A., Ivanova, L.V., Koshelev, V.N. et al. Evaluation of the Efficiency of Functional Additives to Winter Diesel Fuels of Various Hydrocarbon Group Compositions. Chem Technol Fuels Oils 57, 16–25 (2021). https://doi.org/10.1007/s10553-021-01223-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-021-01223-0







 ).
).