An adsorbent obtained by applying orthophosphoric acid to a peat surface was investigated during the refining of used mineral lubricating oil. The degree of regeneration (clarification) of the used oil depended on the adsorbent modification method, i.e., the acid/peat ratio, adsorbent/oil residue mass ratio, and mixing time of the phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Organized collection and reprocessing of used oils remain problematical in many instances [4, 5], including on a global scale in terms of carrying out economical and environmental policy [6,7,8,9] on recycling of secondary hydrocarbon feedstock. Used oils are environmentally hazardous. Therefore, theoretical studies of lubricant aging and used oil regeneration are critical. An electrokinetic model of the state of oil in an internal combustion engine was based on results of colloidal chemical studies [1]. Studies of oil aging supported the theory of interphase mass and energy exchange in disperse systems [2, 3].
Extraction technology for regenerating used industrial oils and fuels by dispersing (spraying) orthophosphoric acid in bulk used oil products was developed at the Institute of Petroleum Chemistry, SB, RAS [10, 11]. However, the emulsion of orthophosphoric acid in the used oil was kinetically unstable because of the large difference in the densities of the acid and the oil products. The extent of refinement (97-98%) of the hydrocarbon base of oil wastes for this method depended greatly on the rate (numbers) of heterophase shifts of products or on the contact surface area of the extractant—oil-waste phases. This increased the energy demand of the industrial process. Peat and wood chips modified by orthophosphoric acid as adsorbents for regenerating the hydrocarbon base of metal-working industrial oil wastes improved the method technology [12. 13].
Adsorbents for refining oil wastes were prepared by treating wood chips and upper and lower peats from a deposit in Tomsk Oblast with orthophosphoric acid. Peat and wood chips (500-700 μm) were dried to constant mass. mixed with acid in a certain mass ratio. and stored for 20 min to 72 h without heating. The
resulting modified adsorbents were suspended in oil wastes in a certain adsorbent/oil-waste mass ratio for 30-200 min. The clarified raffinate was separated from the adsorbent by centrifugation or filtration. The degree of refinement (clarification) of used oil was determined using photocolorimetry (KFK-2) and the change of optical density at 540 and 750 nm, which were isolated by light filters, and using an SX-300 Octanometr. Unmodified natural peat and wood chips clarified oil wastes by only 1-2%. Upper peat was a significantly more effective adsorbent for regenerating the oil-waste hydrocarbons than modified lower peat because of the more branched surface after acid treatment.
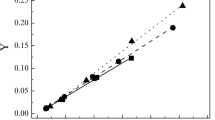
Studies of the refinement process parameters found that the degree of clarification of oil wastes depended on the starting solid adsorbent modification conditions, e.g., on the acid/peat mass ratio (Fig. 1); on the peat modification time (Fig. 2); on the modified peat content in the oil waste, and on the oil-waste refining time (Fig. 3).
Figure 1 shows the degree of clarification (refinement) of used oils as functions of acid/peat ratio and confirmed that these process parameters needed to be optimized. The maximum degree of clarification of the used oils by modified upper peat depended on not only the acid/peat ratio but also the adsorbent content in the hydrocarbon feedstock. Also, these functions changed practically the same. Thus, the first maximum in the degree of clarification at 5 wt.% adsorbent in the used oil corresponded to an orthophosphoric-acid/peat ratio of 3.3:1.0. The acid/peat ratio shifted to 5:1 with 1 wt.% adsorbent. The difference in the degree of clarification of the oil wastes diminished as the acid in the peat increased (with adsorbent contents of S and 1 wt.%). This result enabled the process parameter set to be selected, i.e., an increase of the acid/peat ratio or the adsorbent content in oil wastes at the minimal acid/peat ratio.
The degree of clarification of oil wastes increased with increasing acid/peat ratio for adsorbent concentrations 1 and 5 wt.% (Fig.1). The degree of clarification of hydrocarbon feedstock decreased asymptotically with increasing peat modification time for both adsorbent concentrations and became the same if the peat was modified for 72 h (Fig.2).
The degree of oil-waste clarification reached a maximum for adsorbent concentrations 1-1.5 wt.% in the feedstock, peat modification time 1-24 h, and acid/peat ratio 6.2:1 and was practically unchanged with increasing adsorbent content in the used oil (Fig.3, curves 1 and 2). The degree of oil clarification was sensitive to a change of adsorbent content in the oil wastes of 0.5-1.5 wt.% with peat treatment time by orthophosphoric acid of 1-24 h. Acid-modification of upper peat at an acid/peat ratio of 6.2:1 for 3 d caused a sharp drop in the degree of regeneration of the oil waste hydrocarbon base that was practically independent of the adsorbent content in the feedstock (Fig.3, curve 3). Thus, the degree of refinement of the used technical oils depended strongly on the peat modification time and acid/peat ratio and least of all on the adsorbent content in the oil residues.
References
R. V. Bartko and V. A. Zolotov, Khim. Tekhnol. Topl. Masel, No. 5, 35-38 (2014).
A. L. Chudinovskikh, Khim. Tekhnol. Topl. Masel, No. 3, 3-11 (2015).
Z. T. Dmitrieva, Khim. Tekhnol. Topl. Masel, No. 5, 24-27 (2015).
S. E. Maiboroda, Mir Nefteprod., No. 10, 4-10 (2014).
O. Middleton, T. Habicht, et al., Pat. WO 2013049918, Apr. 11, 2013.
F. I. Samedova, R. Z. Gasanova, and S. B. Logmanova, Mir. Nefteprod., No. 10, 1-14 (2014).
L. Stankovski, D. A. Chumakov, and V. A. Dorogochinskaya, Tekhnol. Nefti Gaza, No. 1, 16-19 (2014).
G. N. Razina and O. O. Tsekov, Khim. Prom-st. Segodnya, No. 12, 37-44 (2012).
D. D. Fazullin, G. V. Mavrin, and M. P. Sokolov, Khim. Tekhnol. Topl. Masel, No. 1, 54-56 (2015).
Z. T. Dmitrieva, S. I. Kashtykin, et al., RU Pat. 2,094,450, Oct. 27, 1997.
Z. T. Dmitrieva, Khim. Tekhnol. Topl. Masel, No. 4, 50-52 (2017).
Z. T. Dmitrieva, N. V. Judina, et al., RU Pat. 2,188,851, Sept. 10, 2002.
Z. T. Dmitrieva, N. V. Judina, et al., RU Pat. 2,213,129, Sept. 27, 2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Mosel, No. 5, pp. 19 — 21, September — October, 2019.
Rights and permissions
About this article
Cite this article
Dmitri, Z.T. Extraction-Adsorption Methods for Regenerating Spent Oils. Chem Technol Fuels Oils 55, 536–539 (2019). https://doi.org/10.1007/s10553-019-01063-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01063-z