In this work, we studied the phase composition and structure of the mixed oxide obtained from a layered Ni-Al double hydroxide (Ni:Al = 3:1). The hydroxide obtained by precipitation from a solution of nickel and aluminum nitrates has a hexagonal crystal lattice with crystallite diameter 7 nm. The crystallite diameter was reduced to 4 nm after roasting of this hydroxide at 500°C. The crystallites consist of octahedral layers and a slight amount spine/ structure. The products of the cracking of normal alkanes in the presence of this mixed Ni-Al oxide feature an increased amount of their low-boiling homologs and branched alkanes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Layered double hydroxides are commonly used in heterogeneous catalysis, mainly, as precursors in the preparation of oxide catalysts. The catalytic activity and selectivity of these catalysts are a function of their chemical composition (the cations and cation-to-anion ratios) and activation processes such as drying, roasting, oxidation/reduction, and/or reconstruction as well as the reaction conditions (temperature, pressure, and coprecipitant). Layered double hydroxides (LDH), also known as hydrotalcite-like compounds, consist of brucite-like layers held together by hydrogen bonding, in which the excess positive charge of a layer formed by isomorphic substitution of the M2+ ion by a trivalent M3+ cation is compensated by the charge of the anions in the interlayer space and water molecules [1,2,3,4].
Altering the compatibility and metal-base interaction, which further expands the catalytic applications of layered hydroxides, can be achieved using various transition metals [5, 6]. Upon heating layered double hydroxides above 250°C, water within the interlayer space is liberated with subsequent dihydroxylation of the hydroxide layers and formation of nonstoichiometric mixed-phase metal oxides with characteristic properties, which have been studied as active catalysts in many important catalytic reactions [7, 8].
The advantages of the basic and oxidation-reduction properties of layered double hydroxides are used in catalysis because the dispersion of the cations in the hydroxylated layers remains after roasting, which leads to the formation of well-stabilized metallic species [9]. Various roasted, rehydrated, and functionalized layered double hydroxides have been studied in catalytic reactions such as the partial oxidation and steam reforming of methane in the production of hydrogen, one-step liquid-phase heterogeneous synthesis, combined oxidation and dry (CO2) reforming of methane to give synthesis gas, alkylation, isomerization-aromatization of olefins, selective oxidation of phenol to give catechol and hydroquinone, oxidation of benzene to give phenol, hydroxylation of benzene, and liquid-phase oxidation of aromatic hydrocarbons [10,11,12,13,14,15,16,17].
The study of methods for the preparation of mixed double oxides with the required properties for catalytic systems in the conversion of hydrocarbons is presently in the stage of experimental data accumulation. In order to obtain the mixed Ni-Al oxide, we synthesized the Ni-Al mixed layered hydroxide by the controlled coprecipitation of nickel and aluminum nitrates in aqueous NaOH [18,19,20]:
A 1 M aqueous solution of the nitrates with M2+/3+ cation ratio equal to 0.33 was brought to an identical volume with the alkali solution and poured with 2 M NaOH using a peristaltic pump into distilled water. The resultant solution was stirred for 6 h at 70°C using a constant-velocity stirrer at constant pH (9-11). The precipitate was washed by centrifugation and the product was dried in a desiccator at 105°C.
Differential thermal analysis of our prepared sample of layered Ni-Al hydroxide was carried out using a Z-1500D MOM derivatograph in an air stream at from 20° to 1000°C. The oven heating rate was 10 deg/min.
A study of the phase composition of these samples was carried out with a Bruker Axs powder x-ray diffractometer in Bragg-Brentano geometry in the 2θ range from 5° to 70°. The phase composition was determined using the DIFFRAC.EVA software package.
The microstructure of the surface of the layered Ni-Al double hydroxide and Ni-Al oxide was obtained using a Hitachi TM 1000 microscope.
The measurement of the specific surface of the Ni-Al oxide was carried out by nitrogen thermal desorption on a Quantachrom NOVA 2200e surface area analyzer. The specific surface of our sample of the Ni-Al mixed oxide calculated by the BET method was approximately158 m2/g.
A study of the selectivity of our samples of Ni-Al oxide in the cracking of normal alkanes was carried in a laboratory flow-type catalytic apparatus with a fixed bed consisting of the mixed oxide particles. The temperature in the reactor was 500°C and the temperature in the injector was 400°C. The pressure was 0.5 MPa. The establishment of these conditions was carried out in a nitrogen stream. Then, the nitrogen stream was stopped and the introduction of the starting mixture of normal alkanes was carried out at a rate of 0.5 ml/min. The volumetric ratio of the normal alkanes to the Ni-Al oxide charge was 9:1. Normal alkanes characteristic for the diesel fuel fraction made up the starting mixture: 16 wt. % undecane, 15 wt. % dodecane, 15 mass % pentadecane, 15 wt.% hexadecane, 15 wt. % icosane, and 12 wt. % docosane.
The gaseous products of the cracking of the normal alkanes were analyzed by gas chromatography on a Shimadzu GC-2010 Plus chromatograph equipped with an injector for capillary columns with electronic automatic gas flow control, a 30-m x 0.53-mm x 40 μm HP-PLOT-Q capillary column, and a thermal conductivity detector.
The composition of the liquid cracking products of the normal alkanes was analyzed by gas-liquid chromatography on a Chromatec Crystal 2000M chromatograph with a flame ionization detector, DB-1 capillary column, and a Perkin Elmer Clarus 680 chromatograph equipped with an SQ8 MS mass spectrometric detector.
The thermogravimetric (TG) curves for our sample of Ni-Al layered double hydroxide showed a nonlinear temperature dependence of mass loss (Fig. 1). The removal of physically adsorbed water occurs at from 200 to 250°C. Desorption of the water trapped in the interlayer space of the layered double hydroxide occurs at 250-400°C followed by dihydroxylation of the brucite-like layers at 400-500°C.
The mass loss segments in the TG analysis due to the removal of physically adsorbed water and water from the interlayer space are accompanied by endothermal effects observed on the differential scanning calorimetry (DSC) curves (Fig. 1).
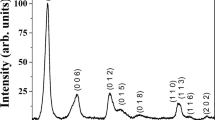
X-ray phase analysis indicated that the structure of our sample of Ni-Al oxide was identical to the natural analog, namely, hydrotalcite, which possesses a hexagonal crystal lattice. The diameter of the Ni-Al hydroxide crystallites was 7 nm. Some of the Ni2+ cations are replaced by Al3+ cations in the structure of the mixed oxide formed upon roasting of Ni-Al layered double hydroxide at atmospheric pressure and 500°C in a muffle furnace with the appearance of a nickel oxide phase. The replacement of Ni2+- by Al3+ at the nickel positions was 16% and led to a reduction of the unit cell dimensions of the crystallites (4 nm) consisting of bunsenite-like octahedral layers with some amount of spinel structures. Thus, the product of the roasting of the Ni-Al layered double hydroxide is a mixed oxide of variable composition containing an amorphous phase.
The microscopy study showed that the surface of the Ni-Al layered double hydroxide shown in Fig. 2a) consists of aggregates of rod-like shapes with length —245 nm and diameter —50-80 nm. Such aggregates form agglomerates with length greater than 1 μm.
Roasting of the Ni-Al layered double hydroxide to give mixed oxides is accompanied by transformation of the surface and the appearance of rod-like nickel oxide particles with length about 600 nm and spherical particles of the mixed oxides with mean diameter approximately 131 nm.
Cracking of the normal alkanes in the presence of the Ni-Al oxide with mixed phase composition led to the redistribution of the gaseous product components (Fig. 3). Thus, methane (32%) predominated among the gaseous products in a control experiment (heating without oxide catalyst) along with ethane (21%) and propane (17.3%).
On the other hand, the fraction of ethane is increased to 24% and the amount of propane is increased to 18.6% in the gaseous products of the cracking of our mixture of normal alkanes using the Ni-Al mixed oxide. Isoalkanes, namely, isobutane, isopentane, and neopentane, also appear among the lower alkanes.
The effect of the Ni-Al mixed oxide on the amount and composition of the hydrocarbons formed in the cracking of normal alkanes is shown in Fig. 4.
The conversion in the cracking of normal alkanes on the Ni-Al mixed oxide catalyst (13.8%) was enhanced by a factor of more than 1.3 relative to the conversion in the thermal cracking (10.9%). The content of unsaturated and aromatic hydrocarbons increased along with the increased conversion. A greater than six-fold increase in the selectivity relative to the formation of normal alkanes was also observed from 0.7 to 4.4%. Greater selectivity was also found in this case for the formation of isoalkanes. The appearance of isoalkanes in the final product of the cracking of the starting normal alkanes on the Ni-Al mixed oxide indicates that reactions involving a carbocation mechanism proceed along with radical chain reactions leading to unsaturated compounds.
References
J. Ma et al., Journal of Materials Research and Technology, 8, No. 1, 292-298 (2018).
P. S. Braterman, Z. Xu, and F. Yarberry, "Layered Double Hydroxides," in: S. M. Auerbach, K. A. Carrado, and P. K. Dutta (editors), Handbook of Layered Materials, Marcel Dekker, New York (2004), pp. 373-474.
V. Rives (editor), Layered Double Hydroxides: Present and Future, Nova Publishers, New York (2001).
X. Duan, D. G. Evans (editors), Layered Double Hydroxides, Springer, Berlin-Heidelberg (2006).
S. Otvos, et al., Applied Catalysis A: General, 501, 63-73 (2015).
S. Kaman, Catalysis Surveys from Asia, 10, Nos. 3-4, 117-137 (2006).
German Patent No. 2029282.
S. Miyata, Clays Clay Miner, 28, No. 1, 50-56 (1980).
F. Basile, et al., Applied Clay Science, 28, No. 1, 50-56 (2010).
H. Liu, et al., Kinetics and Catalysis, 51, No. 1, 81-87 (2010).
D. Sachdev, et al., Catalysis Communications, 11, 1063-1067 (2010).
R. J. Chimentao, et al., Journal of Catalysis, 252, 249-257 (2007).
A. I. Tsyganok, et al., Applied Catalysis A: General, 275, 149-155 (2004).
S. Velu and C. S. Swamy, Applied Catalysis A: General, 119, 241-252 (1994).
W. T. Reichle, Journal of Catalysis, 94, 547-557 (1985).
E. M. Serwicka, Catalysis Today, 90, 85-92 (2004).
J. S. Valente, et al., Applied Catalysis B: Environmental, 90, No. 3, 330-338 (2009).
F. Kooli, et al., Clays and Clay Materials, 45, No.1, 92-98 (1997).
Z. Xu and H. C. Zeng, Chem. Mater, 11, 67-74 (1999).
E. O. Butenko and A. E. Kapustin, Pershiy Nezalezhny Naukoviy Visnik, No. 4, 94-99 (2015).
This study was carried out with a grant from the Russian Science Fund (Project No. 18-77-10023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 2, pp. 3 — 6, March —April, 2019.
Rights and permissions
About this article
Cite this article
Lakhova, A.I., Valieva, G.R., Nocova, A.A. et al. Synthesis of Mixed Nickel-Aluminum Oxides and a Study of Their Catalytic Activity. Chem Technol Fuels Oils 55, 119–124 (2019). https://doi.org/10.1007/s10553-019-01011-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01011-x







 ) and catalytic cracking (■) of normal alkanes.
) and catalytic cracking (■) of normal alkanes.
 ) and catalytic cracking (■) processes.
) and catalytic cracking (■) processes.