Abstract
Nickel-aluminum layered double hydroxide with aluminum ions partially substituted by samarium ones was successfully synthesized via coprecipitation followed by hydrothermal treatment. X-ray diffraction data showed that the obtained sample is single-phase material with hydrotalcite-like structure. The presence of samarium in the sample was confirmed by elemental analysis. Electron microscopy demonstrated that the compound consists of very small plate-like particles with a shape similar to hexagonal. The study of thermal transformations of the material revealed that it decomposed upon heating above 300 °C with the formation of mixed oxide, and spinel-type oxide was formed while the heating temperature was increased up to 1000 °C. The rehydration ability of the sample was rather limited: no reconstruction of layered structure took place after mixed oxide was formed. The “memory effect” was observed only after heating the hydroxide at a temperature not higher than 300 °C. The thermal properties of samarium-containing samples resemble closely those of nickel-containing hydrotalcites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
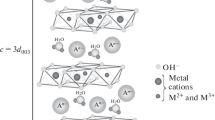
Layered double hydroxides (LDHs), also called hydrotalcite-like compounds or anionic clays, are inorganic compounds consisting of positively charged metal hydroxide layers and charge-balancing interlamellar anions. LDHs can be described by the general formula [M(II)1−x M(III)x (OH)2]x+ [An−x/n·yH2O]x−, where M(II) and M(III) are divalent and trivalent metal cations, respectively, and An− is n-charged anion. One of the main features of LDHs is a variation of cationic and anionic composition, which makes it possible to synthesize compounds with predetermined properties. Calcined forms of LDHs possess other unique property — “memory effect” — the ability to reconstruct damaged layers upon contact with water solutions containing anions [1]. The fields of application of layered double hydroxides can be rather extensive due to remarkable properties of LDHs. LDHs have potential use in catalysis [2], energy storage [3], drug delivery [4], waste treatment [5], etc.
Transition metals in LDHs have been studied for decades, while the majority of the studies devoted to hydrotalcites containing rare earth metal cations have appeared in recent years. These compounds and their derived materials may demonstrate some specific properties, e.g., optical, electrical, and magnetic ones. For example, nickel-containing LDHs possess photochromic [6], catalytic [7, 8], and magnetic properties [9,10,11], they can be used to obtain electrode materials [12,13,14]. In [15] cerium-containing Ni/Al layered double hydroxide was used as a matrix to obtain material with enhanced luminescence properties. At the same time, incorporation of samarium provided an enhancement of properties in various compounds. Bellardita et al. [16] prepared samarium loaded titanium dioxides — rutile, brookite, and anatase. Photocatalytic activity for all loaded powders was higher than that of pristine TiO2. The improvement of photoactivity was attributed to an increased charge separation of the photogenerated electron–hole pairs. The introduction of Sm3+ cations allowed to enhance luminescence properties of phosphors based on phosphates [17] and sulfides [18]. Singh et al. [19] successfully synthesized samarium substituted spinel nanoferrites MSmxFe2-xO4 (M = Ni, Co; x = 0, 0.02, 0.06, and 0.1), which were used as recoverable photocatalyst for the removal of organic pollutants from wastewater. The presence of samarium was monitored by energy-dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy. Higher surface area and reduction in the band gap values were demonstrated to improve significantly the catalytic activity of samarium-doped materials as compared to original ferrites. It is worth noting that spinel-like compounds can be formed as a result of calcination of layered double hydroxides. Thus, hypothetically, LDHs can be precursors for similar materials.
Currently, samarium-containing LDHs are characterized insufficiently. Only a few publications are devoted to them, some of which have appeared quite recently. Typically, these publications comprise 3–4 different rare earth dopants. In [20] Mg/AlSm hydrotalcites with 1, 5, 7.5, and 10 mol% were synthesized using the sol–gel method and examined by X-ray diffraction. Samples containing 1 and 5 mol% Sm are reported to be single phase ones. However, an investigation of luminescence properties of samarium-containing LDH showed the absence of light emission. Other studies describe catalytic properties of Ln-Mg–Al-O (Ln = Ce, Sm, Dy, Yb) mixed oxides obtained by calcinations of corresponding LDHs, which contain 5 mol% Ln in case of samarium. Lanthanide content was confirmed by energy-dispersive X-ray spectroscopy. These oxides were used for propane dehydrogenation [21] and oxidation of methane [22]. It was shown that rare earth additives had a positive impact on catalytic performance. However, the most of the original LDH precursors are not single phase, and the samarium-containing one is among them. Taherian et al. [23] report the synthesis of hydrotalcite-like NiMgAlSm (3 wt% Sm) catalyst for dry and steam reforming of methane with significant coke-resistance properties. The authors state that the presence of samarium ions, which was proved by energy-dispersive X-ray spectroscopy, resulted in greater CO2 adsorption and high thermal and time stability without nickel aggregation due to carbon deletion from the catalyst surface. Shen et al. [24] successfully synthesized single phase Sm-doped NiAl LDHs via hydrothermal-assisted coprecipitation method. The electrochemical effect of samarium doping was investigated. The predetermined molar ratios of Sm/Al were 0.05, 0.1, 0.2, and 0.3. Energy-dispersive X-ray spectroscopy confirmed the presence of samarium, although in smaller quantities: in the best case, it reached up to 21.5%. Such content of lanthanide is an achievement, since the significant difference in ionic radii between M(II) and M(III) is a problem for synthesis of monophase LDHs [1]. The data on lanthanide-containing LDHs are incomplete and the limit of lanthanide quantities is also not fully defined. It may be individual for each element. Also, it is worth to mention the study of sorption of radionuclides onto binary MgLn (Ln = Ce, Pr, Sm, Gd) layered double hydroxides [25]. Presented diffractograms do not look quite similar to hydrotalcite-like compounds, although authors inform about the formation of LDHs.
In our previous studies, we have already obtained cerium-containing nickel-aluminum [26] and cobalt-aluminum [27] layered double hydroxides by coprecipitation followed by hydrothermal treatment. Cerium appeared to be incorporated into the crystal lattice despite its rather large ionic radius, though the amount of incorporated cerium was limited. The objective of the current study was the synthesis and characterization of well crystallized monophase samarium-containing LDH and to track its thermal transformations in detail.
Experimental
The Ni/AlSm hydrotalcite-like compound was synthesized via coprecipitation method followed by hydrothermal treatment. Ni(NO3)2•6H2O (NevaReaktiv), Al(NO3)3•9H2O (NevaReaktiv), and Sm(NO3)3•6H2O (ZRM) were used as metal precursors, and aqueous solution of sodium hydroxide NaOH (NevaReaktiv) was applied as precipitating agent. Molar ratio M2+/M3+ and trivalent cations molar ratio Sm3+/(Al3+ + Sm3+) were predetermined as 3 and 0.05, respectively. These values were selected on the base of our previous successful synthesis of Ni/AlCe LDH [26]. The aqueous solution of nitrates with a total cationic concentration of 1 mol.l−1 was mixed under vigorous stirring with 2 mol.l−1 precipitant solution. The resulting mixture was transferred into 50 ml autoclave reactor (Parker autoclave Engineers) for 30 h at 120 °C. The synthesized material was separated from the mother liquor (pH≈8) by centrifugation, then washed with distilled water and dried at 110 °C for 8 h.
The phase composition of the sample was verified by powder X-ray diffraction (PXRD). PXRD patterns were recorded on Rigaku Ultima IV diffractometer using CuKα radiation (λ = 1.54056 Å) with 2 deg/min from 2θ = 5° to 75° and steps of 0.02. The identification of peaks was made using the PDF database. Lattice parameters were determined by PDXL program. The elemental composition of the obtained material was investigated by the energy-dispersive X-ray spectrometry (EDX) using the QUANTA 200 3D scanning electron microscope equipped with an energy-dispersive analyzer at the operating voltage of 20 kV. The morphology of the sample was examined by transmission electron microscopy (TEM) using JEM-2100 at 200 kV with 0.2 nm resolution and scanning electron microscopy (SEM). For TEM research, the single-layer non self-supporting sample was prepared. Copper grid with a sprayed thin carbon film was covered with a drop of a suspension of the sample in anhydrous isopropyl alcohol, pretreated in an ultrasonic bath for 30 min. The prepared sample was air-dried to remove isopropyl alcohol for 30 min. For SEM analysis, powder material was fixed to a specialized object stage using conductive double-sided carbon tape. To avoid the surface charge, the study was carried out in low vacuum mode.
The behavior of the obtained samples upon heating was studied using SDT Q 600 combined thermal analyzer able to carry out simultaneously thermogravimetric (TGA) and differential scanning calorimetric (DSC) analyses. The investigation was performed in an inert atmosphere in a range of temperatures of 25–1000 °C at a rate of heating of 10 °C/min. Weighted amounts of hydrotalcite-like material were calcined in air in the microwave muffle furnace Phoenix (CEM) during 1 h at 200, 300, 400, 500, 600, 700, 800, and 1000 °C and their decomposition products were analyzed by PXRD. Annealed samples were rehydrated to estimate the “memory effect” of obtained material. Rehydration procedure consisted in adding 25 ml 0.05 mol.l−1 sodium nitrate NaNO3 aqueous solution to calcined forms (m≈0.1 g) and storing for 5 days at ambient temperature. After that, they were washed, dried, and also investigated by PXRD.
Results and discussion
The powder X-ray diffraction pattern of the obtained material looks absolutely typical for hydrotalcite-like compounds (Fig. 1). Basal reflections (003) and (006) as well as reflections (012), (015), (018) and a doublet with reflections (110) and (113) are observed as it is usually for the structures of this type [1]. Calculated by Scherrer equation, crystallite diameter is approximately 5 nm. The broad peaks give evidence to the moderate crystallinity of the sample. It could either be related to the lack of ageing time or to result from the incorporation of samarium into the lattice since the ionic radius of samarium is much larger than aluminum one (0.097 ± 0.005 nm for Sm3+ versus 0.051 ± 0.003 nm for Al3+ with coordination number of 6 [28]). No evident reflections of impurity phases are observed, but their presence cannot be completely excluded. Another possible reason for broadening and asymmetry of the reflections can be polytypism, which is common for layered double hydroxides, as well as structural disorder. The simulations of diffractograms affected by interstratifications, turbostraticity, and stacking faults were carried out in [29,30,31]. The resulting patterns demonstrate the features, which are quite similar to those observed for our Sm LDH.
Determined lattice parameters are the following: a = 3.065 Å and c = 23.865 Å. Parameter a depends on ionic radii of cations and corresponds to cation-cation distance in brucite-like layers. Parameter c mainly depends on electrostatic interaction between brucite-like sheets and interlamellar anions and corresponds to the thickness of the layers. According to [32], lattice parameters of nitrate form of Ni/Al LDH with M2+/M3+ = 3 are: a = 3.01 Å and c = 24.91 Å. Mahjoubi et al. [33] for the compound with the same M2+/M3+ ratio received values 3.02 Å and 23.47 Å for a and c, respectively. One can notice that in these cases, parameter a is less than that for our sample. This is in good agreement with the assumption of isomorphic substitution of aluminum ions by samarium ones, since Sm3+ radius is almost twice as much as Al3+ radius. Samarium-containing Ni/Al LDH with almost the same exchange degree for samarium (Sm/Al = 0.057) obtained by Shen et al. has a = 3.045 Å and c = 23.595 Å [24]. As for rare earth containing Mg/Al LDHs presented in [20], it is reported that lattice parameters increase from about 3.065 to 3.076 Å (a parameter) and from about 23.699 to 23.899 (c parameter) with an increase of the amount of lanthanide elements though without any comments. Considering that ionic radii of Nd, Sm, and Eu are quite close as well as ionic radius of Mg2+ is close to Ni2+ [28], these parameters correspond well to our values.
The presence of nickel, aluminum, and samarium is clearly visible on the EDX spectrum of the sample (Fig. 2). Contents of metals in the precipitated material are as follows: Ni — 23.70 at%, Al — 7.52 at%, and Sm — 0.37 at%. Thereby, cations molar ratio M2+/M3+ equals to 3, which is the same as predetermined value. The molar ratio of triply charged cations Sm3+/(Al3+ + Sm3+) amounts to 0.047 which is very close to a predetermined value of 0.05. Generally, the set of PXRD and EDX data allows to assert that the synthesized material is samarium-containing single phase layered double hydroxide. The assumed ideal formula for this compound is [Ni0.75Al0.238Sm0.012(OH)2]0.25+[(NO3)−0.25*yH2O]0.25−. However, the synthesis was not carried out in an inert atmosphere; therefore, carbonate anions are definitely present in the sample.
The study of morphology with transmission electron microscopy (Fig. 3) showed that the sample consisted of very small (about 8 nm in diameter) plate-like particles with a shape similar to hexagonal. The average particle size was determined using Digimizer software. This morphology is generally typical for hydrotalcite-like compounds and was observed for LDHs of other composition [10, 13, 34, 35]. SEM image (Fig. 4) shows that these particles form agglomerates of different shape and size. This pattern is quite similar to the one obtained in the previous work [24].
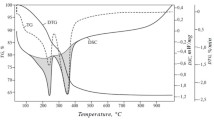
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were used to study the thermal behavior of Ni/AlSm layered double hydroxide (Fig. 5). It can be seen on the TGA curve that weight loss occurs in two main stages, which correspond to two endothermic effects. This picture is quite typical for hydrotalcite-like structures [36, 37]. Removal of physically absorbed and crystallization water matches the first stage (100–250 °C). The calculation based on the weight loss at this stage gives the value for y in the formula of the compound, it is equal to 0.62. The second process (300–500 °C) can be related to the destruction of brucite-like layers and loss of interlamellar anions.
For the study of thermal transformations of Ni/AlSm LDH portions of the material were calcined in microwave muffle furnace at different temperatures and then decomposition products were analyzed by PXRD (Fig. 6). At temperatures up to 300 °C, the sample keeps layered structure, although its crystallinity significantly reduced. After heating above 300 °C, brucite-like layers entirely collapse with the formation of NaCl-type mixed metal oxide with the assumed composition of Ni(Al,Sm)Ox. Analysis of TG data, namely weight loss at the second stage, allows to suppose that the mixed oxide is oxygen-deficient and in its general formula, x is close to 1. Further increase in calcination temperature to 1000 °C leads to crystallization of spinel-type oxide NiAl2O4. These data correlate well with results of TGA–DSC analysis. It was expected that samarium should crystallize in a separate oxide phase, but it was not registered on the PXRD pattern. The sample calcined at 600 °C was examined via EDX analysis, which confirms the presence of samarium. Thus, samarium is most likely incorporated into mixed oxide structure after calcinations, or it can be contained in some amorphous phase. The absence of differentiated lanthanide-containing phases after annealing of layered double hydroxides was observed earlier in [38]. It is worth to note that the color of decomposition products changes with calcination temperatures. Original green powder turns black when it is heated up to 400–500 °C, but at a higher calcination temperature, it starts to turn green again. Such color variations can be explained as follows. Nickel (II) oxide NiO, which has NaCl-type structure, changes its color depending on stoichiometry: non-stoichiometric NiO is black, while stoichiometric is green [39]. Moreover, NiAl2O4, which crystallizes at high temperatures, is also green.
The thermal behavior of the sample is comparable with that for other Ni/Al hydrotalcites. According to [9, 40], annealing of nickel-aluminum LDH at 400 °C and above leads to the formation of NaCl-type mixed metal oxide. Below 400 °C, the layered brucite-like structure still exists. As the temperature increases to 1000 °C, spinel-like nickel aluminate NiAl2O4 starts to crystallize [40].
Samples calcined at 200–600 °C were placed in sodium nitrate water solution to test their rehydration ability (Fig. 7). It was found that the procedure led to a slight improvement in crystallinity for samples annealed at 200 °C and 300 °C. At higher calcination temperatures when the mixed oxide is formed, layer reconstruction does not occur expectedly, only aluminum hydroxide appears as a separate phase. Apparently, samarium remains incorporated in oxide structure or in amorphous phase. Thus, the rehydration ability of Ni/AlSm LDH is moderate. The fact is in substantial agreement with the results obtained by Sato et al. [40] for nickel-containing hydrotalcites. The authors reported that it was possible to reconstruct the layered structure from mixed oxide only under hydrothermal conditions at 250 °C and 4 MPa for 12 h. This complexity is associated with thermodynamic difficulty of the hydration reaction of metal oxide in the matrix.
Conclusion
A successful attempt to incorporate triply charged samarium cation into the lattice of hydrotalcite-like layered double hydroxide is reported. Based on PXRD and EDX data, we claim that we have obtained samarium-containing nickel-aluminum layered double hydroxide with molar ratio Sm3+/(Al3+ + Sm3+) = 0.047 by coprecipitation followed by hydrothermal treatment. Electron microscopy shows that it consists of very small (about 8 nm) plate-like particles with a shape similar to hexagonal. The compound withstands heating up to 300 °C. Thermal decomposition proceeds with the formation of NaCl-type mixed metal oxide. When this mixed oxide is formed (400 °C and above), no subsequent reconstruction of layers is observed. The obtained information on thermal properties and “memory effect” are in a substantial agreement with previously received data for nickel-containing hydrotalcites.
References
Cavani, F., Trifirò, F., Vaccari, A.: Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 11, 173–301 (1991). https://doi.org/10.1016/0920-5861(91)80068-K
Xu, Z.P., Zhang, J., Adebajo, M.O., Zhang, H., Zhou, C.: Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 53, 139–150 (2011). https://doi.org/10.1016/j.clay.2011.02.007
Sarfraz, M., Shakir, I.: Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 13, 103–122 (2017). https://doi.org/10.1016/j.est.2017.06.011
Mishra, G., Dash, B., Pandey, S.: Layered double hydroxides: a brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 153, 172–186 (2018). https://doi.org/10.1016/j.clay.2017.12.021
Zümreoglu-Karan, B., Ay, A.N.: Layered double hydroxides—multifunctional nanomaterials. Chem. Pap. 66, 1–10 (2012). https://doi.org/10.2478/s11696-011-0100-8
Wei, M., Xu, X., Wang, X., Li, F., Zhang, H., Lu, Y., Pu, M., Evans, D.G., Duan, X.: Study on the photochromism of Ni–Al layered double hydroxides containing nitrate anions. Eur. J. Inorg. Chem. 2006, 2831–2838 (2006). https://doi.org/10.1002/ejic.200600058
Takei, T., Miura, A., Kumada, N.: Soft-chemical synthesis and catalytic activity of Ni-Al and Co-Al layered double hydroxides (LDHs) intercalated with anions with different charge density. J. Asian. Ceram. Soc. 2, 289–296 (2014). https://doi.org/10.1016/j.jascer.2014.06.002
Deng, X., Huang, J., Wan, H., Chen, F., Lin, Y., Xu, X., Ma, R., Sasaki, T.: Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation. J. Energy Chem. 32, 93–104 (2019). https://doi.org/10.1016/j.jechem.2018.07.007
Pérez-Ramírez, J., Ribera, A., Kapteijn, F., Coronado, E., Gómez-García, C.J.: Magnetic properties of Co–Al, Ni–Al, and Mg–Al hydrotalcites and the oxides formed upon their thermal decomposition. J. Mater. Chem. 12, 2370–2375 (2002). https://doi.org/10.1039/B110314H
Abellán, G., Coronado, E., Martí-Gastaldo, C., Waerenborgh, J., Ribera, A.: Interplay between chemical composition and cation ordering in the magnetism of Ni/Fe layered double hydroxides. Inorg. Chem. 52, 10147–10157 (2013). https://doi.org/10.1021/ic401576q
Coronado, E., Galán-Mascarós, J.R., Martí-Gastaldo, C., Ribera, A., Palacios, E., Castro, M., Burreil, M.: Spontaneous magnetization in Ni-Al and Ni-Fe layered double hydroxides. Inorg. Chem. 47, 9103–9110 (2008). https://doi.org/10.1021/ic801123v
Liu, X.-M., Zhang, Y.-H., Zhang, X.-G., Fu, S.-Y.: Studies on Me/Al-layered double hydroxides (Me = Ni and Co) as electrode materials for electrochemical capacitors. Electrochim. Acta 49, 3137–3141 (2004). https://doi.org/10.1016/j.electacta.2004.02.028
Wang, J., Song, Y., Li, Z., Liu, Q., Zhou, J., Jing, X., Zhang, M., Jiang, Z.: In situ Ni/Al layered double hydroxide and its electrochemical capacitance performance. Energy Fuels 24, 6463–6467 (2010). https://doi.org/10.1021/ef101150b
Wang, W., Zhang, N., Shi, Z., Ye, Z., Gao, Q., Zhi, M., Hong, Z.: of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chem. Eng. J. 338, 55–61 (2018). https://doi.org/10.1016/j.cej.2018.01.024
Sanati, S., Rezvani, Z.: Co-intercalation of acid Red-27/sodium dodecyl sulfate in a Ce-containing Ni-Al-layered double hydroxide matrix and characterization of its luminescent properties. J. Mol. Liq. 249, 318–325 (2018). https://doi.org/10.1016/j.molliq.2017.10.145
Bellardita, M., Di Paola, A., Palmisano, L., Parrino, F., Buscarino, G., Amadelli, R.: Preparation and photoactivity of samarium loaded anatase, brookite and rutile catalysts. Appl. Catal. B 104, 291–299 (2011). https://doi.org/10.1016/j.apcatb.2011.03.016
Dillip, G.R., Kumar, P.M., Raju, B.D.P., Dhoble, S.J.: Synthesis and luminescence properties of a novel Na6CaP2O9:Sm3+ phosphor. J. Lumin. 134, 333–338 (2013). https://doi.org/10.1016/j.jlumin.2012.08.025
Ashwini, K., Pandurangappa, C., Avinash, K., Srinivasan, S., Stefanakos, E.: Synthesis, characterization and photoluminescence studies of samarium doped zinc sulfide nanophosphors. J. Lumin. 221, 117097 (2020). https://doi.org/10.1016/j.jlumin.2020.117097
Singh, S., Kaur, P., Kumar, V., Tikoo, K.B., Singhal, S.: Traversing the advantageous role of samarium doped spinel nanoferrites for photocatalytic removal of organic pollutants. J. Rare Earths 39, 781–789 (2021). https://doi.org/10.1016/j.jre.2020.12.008
Smalenskaite, A., Şen, S., Salak, A.N., Ferreira, M.G.S., Beganskiene, A., Kareiva, A.: Sol–gel derived lanthanide-substituted layered double hydroxides Mg3/Al1−xLnx. Acta Phys. Pol. A 133, 884–886 (2018). https://doi.org/10.12693/APhysPolA.133.884
Mitran, G., Urda, A., Tanchoux, N., Fajula, F., Marcu, I.-C.: Propane oxidative dehydrogenation over Ln-Mg-Al-O catalysts (Ln = Ce, Sm, Dy, Yb). Catal. Lett. 131, 250–257 (2009). https://doi.org/10.1007/s10562-009-0057-1
Urdă, A., Popescu, I., Cacciaguerra, T., Tanchoux, N., Tichit, D., Marcu, I.-C.: Total oxidation of methane over rare earth cation-containing missed oxides derived from LDH precursors. Appl. Catal., A. 464–465, 20–27 (2013). https://doi.org/10.1016/j.apcata.2013.05.012
Taherian, Z., Gharahshiran, V.S., Khataee, A., Orooji, Y.: Anti-coking freeze-dried NiMgAl catalysts for dry and steam reforming of methane. J. Ind. Eng. Chem. 103, 187–194 (2021). https://doi.org/10.1016/j.jiec.2021.07.032
Shen, S., Guo, W., Zhuang, W., Yang, W., Qin, L., Liu, X., Yue, Z.: Effect of Sm-doped Ni-Al layered double hydroxide on electrochemical performance for supercapacitors. J. Phys. Conf. Ser. 2009, 012008 (2021). https://doi.org/10.1088/1742-6596/2009/1/012008
Kulyukhin, S.A., Krasavina, E.P., Rumer, I.A.: Sorption of 60Co, 90Sr, 90Y and 137Cs from aqueous solutions onto Mg-Ln layered double hydroxides (Ln = Ce, Pr, Sm, Gd). Radiochemistry 55, 569–600 (2013). https://doi.org/10.1134/S1066362213060052
Golovin, S.N., Yapryntsev, M.N., Ryltsova, I.G., Veligzhanin, A.A., Lebedeva, O.E.: Novel cerium-containing layered double hydroxide. Chem. Pap. 74, 367–370 (2020). https://doi.org/10.1007/s11696-019-00877-9
Golovin, S. N., Yapryntsev, M. N., Ryl’tsova, I. G., Savilov, S. V., Maslakov, K. I., Lebedeva, O. E.: Synthesis and thermal behavior of Co AlCe layered double hydroxide. Solid State Sci. 111:106498 (2021). https://doi.org/10.1016/j.solidstatesciences.2020.106498
Bugaenko, L.T., Ryabykh, S.M., Bugaenko, A.L.: A nearly complete system of average crystallographic ionic radii and its use for determining ionization potentials. Moscow Univ. Chem. Bull. 63, 303–317 (2008). https://doi.org/10.3103/S0027131408060011
Ramesh, T.N., Jayashree, R.S., Kamath, P.V.: Disorder in layered hydroxides: DIFFaX simulation of the X-ray powder diffraction patterns of nickel hydroxide. Clays Clay Miner. 51, 570–576 (2003). https://doi.org/10.1346/CCMN.2003.0510511
Shivaramaiah, R., Navrotsky, A.: Energetics of order-disorder in layered magnesium aluminum double hydroxides with interlayer carbonate. Inorg. Chem. 54, 3253–3259 (2015). https://doi.org/10.1021/ic502820q
Sławińsky, W.A., Sjåstad, A.O., Fjellvåg, H.: Stacking faults and polytypes for layered double hydroxides: what can we learn from simulated and experimental X-ray powder diffraction data? Inorg. Chem. 55, 12881–12889 (2016). https://doi.org/10.1021/acs.inorgchem.6b02247
Wang, L., Lü, Z., Li, F., Duan, X.: Study on the mechanism and kinetics of the thermal decomposition of Ni/Al layered double hydroxide nitrate. Ind. Eng. Chem. Res. 47, 7211–7218 (2008). https://doi.org/10.1021/ie800609c
Mahjoubi, F.Z., Elhalil, A., Elmoubarki, R., Sadiq, M., Khalidi, A., Cherkaoui, O., Barka, N.: Performance of of Zn-, Mg- and Ni-Al layered double hydroxides in treating an industrial textile wastewater. J. Appl. Surf. Interfaces. 2, 1–11 (2017). https://doi.org/10.48442/IMIST.PRSM/jasi-v2i1-3.10033
Herrero, M., Benito, P., Labajos, F.M., Rives, V.: Stabilization of Co2+ in layered double hydroxides (LDHs) by microwave-assisted ageing. J. Solid State Chem. 180, 873–884 (2007). https://doi.org/10.1016/j.jssc.2006.12.011
Zhang, Y., Liu, J., Li, Y., Yu, M., Yin, X., Li, S.: Enhancement of active anticorrosion via Ce-doped Zn-Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy. J. Wuhan. Univ. Technol. Mater. Sci. Ed. 32, 1199–1204 (2017). https://doi.org/10.1007/s11595-017-1731-6
Rey, F., Fornés, V., Rojo, J.M.: Thermal decomposition of hydrotalcites An infrared and nuclear magnetic resonance spectroscopic study. J Chem Soc Faraday Trans. 88, 2233–2238 (1992). https://doi.org/10.1039/FT9928802233
Prevot, V., Caperaa, N., Taviot-Guého, C., Forano, C.: Glycine-assisted hydrothermal synthesis of NiAl-layered double hydroxide nanostructures. Cryst. Growth Des. 9, 3646–3654 (2009). https://doi.org/10.1021/cg900384n
Vicente, P., Pérez-Bernal, M.E., Ruano-Casero, R.J., Duarte, A., Almeida Paz, F.A., Rocha, J., Rives, V.: Luminescence properties of lanthanide-containing layered double hydroxides. Microporous Mesoporous Mater. 226, 209–220 (2016). https://doi.org/10.1016/j.micromeso.2015.12.036
Dubey, P., Kaurav, N.: Stoichiometric and nonstoichiometric compounds. In: Tanasescu, S. (ed.) Structure Processing Properties Relationships in Stoichiometric and Nonstoichiometric Oxides. IntechOpen, London (2019). https://doi.org/10.5772/intechopen.89402
Sato, T., Fujita, H., Endo, T., Shimada, M., Tsunashima, A.: Synthesis of hydrotalcite-like compounds and their physico-chemical properties. React. Solids 5, 216–228 (1988). https://doi.org/10.1016/0168-7336(88)80089-5
Funding
The reported study was funded by Russian Foundation for Basic Research according to the research project no. 20–33-90178. The work was carried out using the equipment of the Joint Research Center of Belgorod State National Research University «Technology and Materials».
Author information
Authors and Affiliations
Contributions
Conceptualization: OEL; methodology: OEL; investigation: SNG, MNY; writing — original draft: SNG; writing — review and editing: OEL; funding acquisition: OEL; resources: MNY; supervision: OEL; visualization: SNG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golovin, S.N., Yapryntsev, M.N. & Lebedeva, O.E. Synthesis and thermal transformations of layered double hydroxide containing samarium. J Aust Ceram Soc 58, 1615–1622 (2022). https://doi.org/10.1007/s41779-022-00798-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00798-z