We have experimentally investigated a number of properties of polyglycol in deepwater drilling fluids. Polyglycol retards pressure transmission from the fluid to the formation, and improves the shale cuttings recovery rate. Measurements of the electrokinetic potential (the zeta potential) and the clay particle size showed that the inhibiting effect of polyglycol involves adsorption on clay particles and plugging pores and cracks. Polyglycol inhibits gas hydrate formation, has good lubricity and low toxicity, and does not have a significant impact on the rheological properties of drilling fluids at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The deepwater environment makes additional demands on drilling fluid. The major challenges of deepwater drilling include instability of the unconsolidated formation, gas hydrate formation, problems ensuring the required rheological properties of the drilling fluids at low temperatures, stringent environmental requirements, etc. [1]. Polyglycols can effectively inhibit shales in deep water, and are widely used in water-based drilling fluids. Currently, the inhibiting properties of polyglycols have mainly been studied [2–4], even though polyglycols have other properties that are important for deepwater drilling. In this paper, we study a number of properties of the polyglycol SD-301, aimed at arguing for its use in deepwater drilling.
Polyglycol SD-301 is a commercial product obtained by copolymerization of ethylene oxide and propylene oxide. The density of the polyglycol is 1020 kg/m3 and its cloud point is 27 °C-29 °C. The shales used in this work, consisting mainly of clay minerals and quartz, were obtained from wells in the South China Sea.
A description of the equipment and the procedure for studying pressure transmission are given in [5,6]. The axial pressure and the confining pressure were 5 MPa. We studied solutions of several inhibitors: 4 % SD-301, 3 % KCl, 3 % polyamine, and 5 % sodium silicate. The adsorption test (for adsorption of the polyglycol on the rock) and the shale cuttings dispersion test were conducted according to the procedures in [7,8].
The zeta potential and the shale particle size were measured on a Zetasizer 3000 (Malvern Instruments Ltd., UK); the measurement procedure is described in [9].
An experimental unit was designed to evaluate inhibition of gas hydrate formation. After injection of the fluid into the cell, the cell was evacuated and then methane was injected until a certain pressure was achieved. The cell was cooled down to the required temperature. Inhibition of hydrate formation by solutions with different polyglycol concentrations were studied, simulating conditions at a depth of 3000 m (3 °C, 20 MPa) with stirring rate 200 rpm.
The lubricant properties of the drilling fluid containing polyglycol or other additives were determined on an EP (extreme pressure) lubrication tester. The rheological properties of the drilling fluids were tested using a 6-speed rotational viscometer at temperatures of 2 °C and 20 °C. The apparent viscosity, the plastic viscosity, and the yield point were calculated according to API specifications. The toxicity of the polyglycol relative to luminescent bacteria was evaluated using the median effective concentration EC50.
Pressure transmission test: The primary reason for borehole instability involves transmission of pressure and filtrate from the drilling fluid to the formation. Since polyglycols cannot retard pressure transmission at a temperature below the cloud point, the experiments were conducted at a temperature of 30 °C. As we see from Fig. 1, in the absence of an inhibitor, the downstream pressure is equal to the upstream pressure. In the presence of KCl or polyamine, the downstream pressure increases up to 1.2 MPa in less than 5 hours, while in the presence of SD-301 the downstream pressure after 20 hours was only 0.88 MPa, i.e., the polyglycol effectively retards pressure transmission. The mechanism for this phenomenon is determined by the cloud point of the polyglycol, and involves plugging pore throats and microcracks with its particles, which retards pressure and filtrate transmission. In this test, sodium silicate exhibited the best ability to retard pressure transmission; silicates and aluminates have the highest efficiency in water-based drilling fluids [10].
Shale cuttings recovery rate. Table 1 presents the data on the shale cuttings recovery rate for different polyglycol concentrations in water. As we see, the polyglycol allows us to significant improve the shale cuttings recovery rate. The recovery rate is maximum for a 3 % concentration of the polyglycol solution. The mechanism for shale inhibition by polyglycol includes reducing the activity of the water, plugging pores with insoluble particles, adsorption of polyglycol on clay particles, and increasing the viscosity of the filtrate.
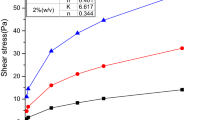
Adsorption of polyglycol on a clay surface. At a temperature below the cloud point (25 °C), the polyglycol can be adsorbed on clay as a result of hydrogen bond formation, where the amount of adsorbed polyglycol increases as its concentration in the aqueous solution increases (Fig. 2). At a temperature above the cloud point (80 °C), the amount of adsorbed polyglycol is less than for adsorption at a temperature of 25 °C.
Immediately after the borehole is drilled, a significant amount of polyglycol can be adsorbed on clay in a short time, which will inhibit shale dispersion and improve wellbore stability.
Effect of the polyglycol on particle size and electrical properties of shale. Table 2 presents data on particle size of shales sampled at different depths and their zeta potential in the presence of and in the absence of the polyglycol. The size of the hydrated shale particles is larger when polyglycol is present in the system, which indicates it is adsorbed on the clay particles. The larger size of the particles results in an increase in the shale cuttings recovery rate. The zeta potential of hydrated clay particles decreases in the presence of polyglycol, since the degree of ionization of the hydroxyl groups on the surface of clay particles decreases due to adsorption of polyglycol.
The polyglycol competes with water molecules for adsorption sites on the shale surface, thus reducing the degree of hydration of the clay. Adsorption of polyglycol also can reduce the negative charge on the clay particles, thus reducing swelling of the shale.
Inhibition of hydrate formation. The ability of 1 %, 3 %, and 5 % aqueous solutions of polyglycol to inhibit hydrate formation was studied in 4 % bentonite mud. Gas hydrate formation leads to a rise in temperature in the system due to the exothermicity of this process [11] and a sharp drop in pressure as a result of methane absorption. Furthermore, we observe an increase in drill string torque due to friction caused by deposition of solid particles (hydrates).
The kinetics of the hydrate formation process in the presence of polyglycol were studied for the example of a 1 % solution (Fig. 3). An appreciable pressure drop during the experiment (the start of hydrate formation) was detected after 1.05 hours (2.65 °C, 19.02 MPa). First the hydrates are formed around the stirring shaft, then a thin layer is formed on the surface. By 3.43 hours (2.35 °C, 17.85 MPa), a small amount of hydrates were formed, as indicated by a gradual pressure drop and only a slight change in torque. In this stage, hydrate formation moves from the gas–liquid interface into the mud volume, but hydrates continue to form at a slow rate. This may suggest that polyglycol molecules are adsorbed on the hydrate crystals, preventing their fast growth. Catastrophic hydrate growth is observed 3.43 hours from the start of the experiment, as indicated by a sharp pressure drop and an abrupt increase in temperature and torque.
In the system with 4 % bentonite mud but without polyglycol, hydrate formation begins by 0.12 hours. Formation of a large amount of hydrates in the drilling fluid is a risk for deepwater operations and causes plugging of the near-wellbore area and fluid flow problems.
For comparison, the indicated experiment was conducted with sodium chloride and ethylene glycol, which are widely used hydrate inhibitors. Table 3 shows the temperature, pressure, and time from the beginning of the experiment at which hydrate formation starts. Increasing the concentration of the polyglycol solution from 1 % to 5 % results in an increase in the time that hydrate formation starts, from 1.06 hours to 3.2 hours. A polyglycol solution of concentration greater than 3 % is more effective than the 20 % solution of ethylene glycol and the 10 % NaCl solution. Consequently, the polyglycol solution with concentration that is optimal for shale inhibition also appreciably inhibits hydrate formation.
Based on the results of the experiments and previous studies of the mechanism for hydrate inhibition [12], we can conclude that polyglycols with hydroxyl groups can retard formation of hydrate crystals by disrupting their structure. After hydrate crystals form, the inhibitor interacts with the surface of the crystal, preventing its growth.
In bentonite mud, a large number of water molecules are adsorbed on clay particles, which creates conditions for water molecules to form structures involving hydrogen bonds. Dissolved methane molecules tend to adsorb on a clay surface, which leads to their encapsulation in cavities of the water lattice to form hydrate cages. Therefore the presence of clay particles results in more sites for hydrate crystallization. Polyglycol, competing with water in the adsorption process on clay particles, reduces the number of crystallization sites.
Lubricity of polyglycol. Thick salt formations exist in almost all deepwater locations. A higher coefficient of friction when rotating in salt formations leads to significant energy losses. Accordingly, the drilling fluid should have good lubricity. The coefficient of friction is reduced from 0.196 to 0.117 by adding 3 % polyglycol to the drilling fluid studied in our previous paper [13]: 4 % bentonite mud + 0.1 % xanthan gum (SDX) + 0.4 % polyanionic cellulose (PAC-LV) + 3 % sulfonated phenol formaldehyde resin (SD-102) + 25 % NaCl. For comparison, the coefficient of friction is reduced down to 0.129 and 0.122 when 2 % graphite or 3 % EP lubricant L-2 is added to the indicated fluid. This confirms the good lubricity of the polyglycol.
Effect of polyglycol on the rheological properties of drilling fluids at low temperatures. The drilling fluids used in this series of experiments had the following composition:
-
I:
water + 0.3 % SDX + 3 % SDN-1 (deformable polymer for preventing invasion of drilling fluid) + 25 % NaCl;
-
II:
4 % bentonite mud + 0.1 % SDX + 0.4 % PAC-LV + 3 % SD-102 + 25 % NaCl.
Table 4 shows the effect of adding polyglycol on the rheological properties of drilling fluids. We see that polyglycol causes an increase in the plastic viscosity and the yield point. From the ratio of the plastic viscosities at 2 °C and 20 °C, we evaluated the increase in the viscosity of the drilling fluid as the temperature is lowered. For drilling fluid I, with and without polyglycol, this ratio is 1.33 and 1.27; for fluid II, 1.36 and 1.33 respectively. Obviously adding polyglycol to drilling fluid does not have an appreciable impact on its rheological properties at low temperature.
Toxicity. Polyglycols are biodegradable and generally have low toxicity. Tests have shown that the polyglycol SD-301 has ultralow toxicity (EC50 > 110 000 mg/L) and meets environmental requirements for drilling fluid.
Therefore, in addition to shale inhibition, the polyglycol SD-301 inhibits hydrate formation, has lubricating action, has almost no impact on the rheological properties of drilling fluids, and has low toxicity.
References
M. Zamora, P. N. Broussard, and M. P. Stephens, “The top 10 mud-related concerns in deepwater drilling operations,” in: SPE Drilling Conference, Villahermosa, 1-3 February 2000; SPE 59019.
M. E. Brady, B. Craster, J. M. Getliff, and P. I. Reid, “Highly inhibitive, low-salinity glycol water-base drilling fluid for shale drilling in environmentally sensitive locations,” in: SPE Drilling Conference, Caracas, 7-10 June 1998; SPE 46618.
S. Eirik, A. Eva, N. Hydro, G. Fimreite, A. Dzialowski, and G. S. Svanes, “Design of water based drilling fluid system for deepwater Norway,” in: SPE/IADC Drilling Conference. Amsterdam, 27 February - 1 March 2001; SPE/IADC 67834.
M. Khodja, J. P. Canselier, F. Bergaya, K. Fourar, M. Khodja, N. Cohaut, and A. Benmounah, “Shale problems and water-based drilling fluid optimisation in the Hassi Messaoud Algerian oil field,” Appl. Clay Sci., 49, 383-393 (2010).
E. Van Oort, A. H. Hale, F. K. Mody, and S. Roy, “Transport in shales and the design of improved water-based shale drilling fluids,” SPE Drilling and Completion, 3, 137-146 (1996).
J. F. Xu, Z. S. Qiu, and K. H. Lu, “Pressure transmission testing technology and simulation equipment for hydra-mechanics coupling of shale,” Acta Petrolei Sinica, 6, 115-118 (2005).
S. Erkekol, I. H. Gucuyener, and M. V. Kok, “An experimental investigation on the chemical stability of selected formation and determination of the proper type of water-base drilling fluids. Part 1. Descriptive Tests,” Energy sources, Part A, 28, 875-883 (2006).
Z. S. Qiu, W. A. Huang, J. F. Xu, K. H. Lu, and Z. M. Wang. “Development of modified polyalcohol anti-sloughing agent and its anti-sloughing mechanisms,” Journal of China University of Petroleum, 6, 51-58 (2006).
H. Y. Zhong, Z. S. Qiu, W. A. Huang, and J. Cao, “Shale inhibitive properties of polyether diamine in water-based drilling fluid,” Journal of Petroleum Science and Engineering, 78, 510-515 (2011).
M. A. Ramirez, S. Benaissa, G. Ragnes, and A. Almaraz, “Aluminum-based HPWBM successfully replaces oil-based mud to drill exploratory well in the Magellan Strait, Argentina,” in: SPE/IADC Drilling Conference, Cairo, 22-24 October 2007; SPE/IADC 108213.
J. H. Yang and B. Tohidi, “Characterization of inhibition mechanisms of kinetic hydrate inhibitors using ultrasonic test technique,” Chemical Engineering Science, 66, 278-283 (2011).
B. J. Anderson, J. W. Tester, G. P. Borghi, and B. L. Trout, “Properties of inhibitors of methane hydrate formation via molecular dynamics simulations,” J. Am. Chem. Soc., 127, 17852-17862 (2005).
J. Sheng, “Study on inhibiting technology of natural gas hydrate in deepwater drilling fluid,” Master’s Dissertation, China University of Petroleum (2009).
This work was financially supported by the National Science & Technology Project (2011ZX05030-005-07), the Research Fund for the Doctoral Program of Higher Education of China (20110133110008), and a Project of the Innovation Team of the Ministry of Education (IRT1086).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 36 – 40, May – June, 2014.
Rights and permissions
About this article
Cite this article
Zhao, X., Qiu, Z., Huang, W. et al. Multifunctional Properties of Polyglycol in Deepwater Drilling Fluids. Chem Technol Fuels Oils 50, 233–239 (2014). https://doi.org/10.1007/s10553-014-0515-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-014-0515-3