Abstract
Purpose
To review the epidemiologic literature examining pesticide exposure and liver cancer incidence.
Methods
A search of the MEDLINE and Embase databases was conducted in October 2015. Eligibility criteria included examining hepatocellular carcinoma (HCC) or primary liver cancer, pesticides as an exposure of interest, and individual-level incidence. The review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Forty-eight papers were assessed for eligibility and 15 studies were included in the review. The majority of studies were conducted in China and Egypt (n = 8), used a case–control design (n = 14), and examined HCC (n = 14). Most studies showed no association between self-reported and/or occupational exposure to pesticides and liver cancer risk. Six studies demonstrated statistically significant positive associations, including three biomarker-based studies (two using pre-diagnostic sera) that reported higher serum levels of dichlorodiphenyltrichloroethane (DDT) were associated with increased HCC risk. Studies indirectly measuring pesticide exposure using self-reported exposure, occupation, job-exposure matrices, or geographic residence demonstrated inconsistent results. These studies were limited by exposure assessment methods, lack of confounder information, minimal case confirmation, selection bias, and/or over-adjustment.
Conclusions
There is mixed evidence suggesting a possible association between specific pesticides and HCC risk, with the strongest evidence observed in biomarker-based studies. In particular, organochlorine pesticides, including DDT, may increase HCC risk. Future research should focus on improved pesticide exposure assessment methods, potentially incorporating multiple approaches including biomonitoring while considering the chemicals of interest, historical exposure to address latency periods, and examining specific chemicals and exposure pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer is the sixth most common cancer in the world and the second leading cause of cancer-related death [1]. Approximately 70–85% of primary liver cancer cases are hepatocellular carcinoma (HCC) [2]. The second most common histology is intrahepatic cholangiocarcinoma [3]. Over 80% of HCC cases occur in East Asia and sub-Saharan Africa [4]. Age-adjusted liver cancer incidence in China in 2012 was 33.7 per 100,000 among males and 10.9 per 100,000 among females [1]. In the U.S., HCC is the most commonly occurring type of primary liver cancer and is increasing in incidence [5]. Between 2000 and 2005 and 2006–2012, age-adjusted HCC incidence in the US increased from 7.5 to 10.0 per 100,000 among males (33% increase; p < 0.00001), and from 2.1 to 2.7 per 100,000 among females (27% increase; p < 0.00001) [6]. The majority of HCC risk factors contribute to carcinogenesis by promoting the formation and progression of cirrhosis [7]. In parts of Asia and sub-Saharan Africa, predominant risk factors include chronic hepatitis B virus (HBV) infection and exposure to aflatoxin, a mycotoxin produced by the Aspergillus fungus forming on foods in damp conditions [8]. In Japan and Egypt, the major risk factor is chronic hepatitis C virus (HCV) infection [3]. Major risk factors in the U.S. and Europe include chronic HCV infection, heavy alcohol consumption (≥3 more drinks per day), and metabolic syndrome [3, 9, 10]. Other risk factors include obesity, diabetes, non-alcoholic fatty liver disease (and non-alcoholic steatohepatitis), and cigarette smoking; coffee and tea consumption may be protective [11–16]. However, between 15 and 50% of HCC cases have no established risk factors [4].
Pesticides and liver cancer
Pesticides are chemicals used to destroy, mitigate, prevent, or repel pests such as insects, mice, weeds, fungi, and microorganisms. Pesticides can be delineated into functional groups (e.g., insecticides) according to the organisms they control, or chemical classes (e.g., organochlorines) according to similar chemical structures and biological mechanisms of action [17]. Humans are exposed to pesticides via dermal contact, ingestion, and inhalation [18]. Occupational exposure to pesticides occurs among individuals employed in agriculture, pesticide manufacturing, pesticide application, and forestry; family members may be exposed if pesticides are introduced into the home (e.g., on clothing). Non-occupational exposure can also occur via residential use or dietary ingestion from contaminated drinking water and food [17, 19]. Residential proximity to agricultural pesticide applications is an important source of ambient environmental exposure, where pesticides applied from the air and ground may drift from intended sites [20, 21]. Pesticides are metabolized in the liver and are hypothesized to contribute to liver carcinogenesis through mechanisms of cell adhesion alterations, oxidative stress, genotoxicity, tumor promotion, immunotoxicity, and hormonal action [22–25]. Experimental studies have shown that exposure to dichlorodiphenyltrichloroethane (DDT), an organochlorine insecticide widely used in the mid-twentieth century, and its metabolite, dichlorodiphenyldichloroethylene (DDE), lead to the development of HCC and other liver tumors in rodents [26–28]. However, results from epidemiologic studies of pesticide exposure and liver cancer mortality in humans have been inconsistent [29–37].
Liver cancer is a significant and growing public health burden and a substantial number of cases are unexplained by known risk factors. A growing body of literature has examined pesticide exposure as a potential environmental factor related to liver cancer. However, to date, the literature has not been synthesized. Inconsistent results from epidemiologic studies may be due to methodological limitations such as selection bias. Importantly, it is difficult to reconcile results of studies that used different exposure assessment methods (e.g., biomarkers vs. self-report). The purpose of this review was to summarize the current epidemiologic literature examining the association between pesticide exposure and liver cancer and to interpret results in light of these challenges.
Methods
Search strategy
The review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [38]. The MEDLINE (January 1966 to October 2015) and Embase (1980 to October 2015) databases were searched for studies. In MEDLINE, the following terms were searched as exploded MeSH terms and in all fields (e.g., title and abstract): (liver neoplasms) and (agrochemicals OR environmental exposure OR rural population OR rural health) AND humans [MeSH] AND (risk OR epidemiologic studies OR incidence). In Embase, the following terms were searched as exploded Emtree terms: ‘liver cancer’ AND (‘environmental chemical’ OR ‘environmental exposure’ OR ‘rural area’ OR ‘rural population’) AND ‘human’ AND (‘risk’ OR ‘observational study’ OR ‘incidence’ OR ‘prospective study’ OR ‘controlled study’ OR ‘cohort analysis’). The Embase search was executed as broadly as possible (mapping to Emtree, searching free text in all fields, exploding using narrower Emtree terms). For both database searches, limits for humans, English language, and original research were applied.
Study selection
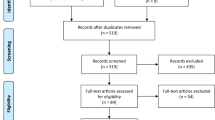
After combining studies from the MEDLINE and Embase searches, duplicates were removed and studies were screened by title and abstract for relevance. One review paper was removed during screening as it was not filtered during the database searches. Inclusion and exclusion criteria for this review were determined a priori by the authors. Full-text papers were evaluated according to the following eligibility criteria for inclusion into the review: the outcome of interest was HCC or primary liver cancer; an exposure of interest was pesticides (including proxies for exposure); and the study investigated individual-level risk of developing the outcome. Studies examining cancers other than liver were not considered due to internal validity concerns (i.e., inclusion of studies with adequate statistical power to detect an association and that ascertained liver cancer risk factors to assess potential confounding). All cited references in each evaluated paper were also examined for inclusion into the review. Among studies satisfying all eligibility criteria, the following information was extracted: study design, time period, sample size, source population, outcome, case confirmation, reference group, matching factors, exposure metric(s), measures of association, confounders, and effect modifiers. Potential sources of bias (e.g., selection) were evaluated for each study. Unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for five studies not reporting these results, but providing sufficient information for their calculation [39]. All exposure metrics used in each study are listed in Table 1. Results from each study included in the review are shown in Table 2. Among studies using multiple exposure metrics, one primary result is reported for each study in Table 2. Reporting of the primary result was performed according to the following order determined by quality and relevance of the exposure metric to pesticide exposure and/or scientific evidence supporting an association with liver cancer: DDT if measuring multiple organochlorine pesticides in biospecimens; self-reported pesticide exposure (overall); employment in agricultural occupation/job title (the occupation more relevant to pesticide exposure was reported, e.g., farmworker vs. farm manager); employment in agriculture industry. Results regarding specific pesticide chemical classes are reported in the text. Issues affecting internal validity (i.e., case confirmation, confounding, over-adjustment, and selection bias) as well as effect modification are reported.
Results
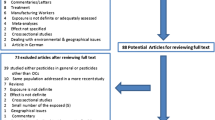
A total of 1,262 studies were screened (Fig. 1) and after exclusions, 48 full-text papers were assessed for eligibility. After the exclusions described above, 15 studies were included in the review (Table 1) [40–54]. Most studies were conducted in China (n = 4) and Egypt (n = 4). The majority of studies used a case–control design (n = 14 total; n = 12 retrospective; n = 2 prospective) and examined incident HCC as the outcome (n = 14). Most studies confirmed diagnoses, including via histology, imaging (e.g., ultrasonography), elevated alpha-fetoprotein (AFP) levels, and clinical examination. Most studies evaluated more than one measure of pesticide exposure such as job title in addition to self-reported pesticide exposure; results among these studies were inconsistent. While a majority of studies showed no association between pesticide exposure and liver cancer (n = 9; 60%), six studies (40%) reported statistically significant positive associations between pesticide exposure and liver cancer with ORs ranging between 2.19 and 4.07 (Table 2). These studies were conducted in China and Egypt. One study reported a statistically significant protective effect of employment in the agriculture industry, although there was no adjustment for major risk factors [44]. The strongest evidence for an association was observed among three biomarker-based studies (two of which were prospectively assessed) conducted in China directly measuring organochlorine pesticides in serum and demonstrating statistically significant linear trends with increasing DDT levels and increasing HCC risk [48, 49, 54].
DDT and other organochlorine pesticides
Biomonitoring
Three biomarker-based studies in China measured serum organochlorine pesticides and demonstrated statistically significant positive associations with HCC risk, with adjusted ORs ranging between 2.96 and 4.07 for DDT (Table 2), adjusting for variables including age, sex, hepatitis B surface antigen (HBsAg), and hepatitis C virus antibody (anti-HCV) [48, 49, 54]. Two of the three studies assessed exposure using sera preceding diagnosis [48, 49], while one study assessed exposure after diagnosis [54]. The time between blood draw and HCC diagnosis was between 0 and 17 years in McGlynn et al. [48], 1 and 7 years in Persson et al. [49], and not reported in Zhao et al. [54]. Zhao et al. [54] also showed statistically significant positive associations between both DDE and hexachlorocyclohexane (HCH), an organochlorine pesticide, and HCC. With the exception of Zhao et al. [54], all of these studies confirmed cases (e.g., via histology). Odds ratios for serum DDT and HCC risk increased between 39 and 65% after adjustment for confounders in two studies [48, 49]. Although Persson et al. [49] did not adjust for HCV, McGlynn et al. [48] showed that HCV was not a significant risk factor in a low-HCC rate region in China. McGlynn et al. [48] and Persson et al. [49] did not ascertain aflatoxin information, but cited previous research demonstrating low aflatoxin levels in the study area and no statistically significant association between corn consumption and HCC risk. Although two studies used population-based controls matched on major risk factors [48, 49], one study used hospital-based controls [54], representing a potential source of selection bias. A statistically significant interaction was observed between DDT and DDE in Zhao et al. [54] (interaction p = 0.001) and McGlynn et al. [48] (p = 0.042), where higher HCC risk was associated with increasing serum DDT and decreasing serum DDE. Zhao et al. [54] demonstrated higher serum DDT levels were associated with increased HCC risk among those with HBV (p = 0.001), not heavily consuming alcohol (p = 0.001), diabetes (p = 0.01), higher serum aflatoxin levels (p = 0.005), higher serum polyaromatic hydrocarbon levels (p = 0.0005), and higher serum HCH levels (beta isomer; p = 0.0096).
Self-reported pesticide exposure
Cordier et al. [43] interviewed participants from hospitals in Vietnam regarding occupational exposure to pesticides. Self-reported exposure to ≥30 L/year of organochlorine pesticides (adjusted OR 4.8, 95% CI 0.9, 25.1) compared to none is suggestive of an association with HCC among males in Vietnam, adjusting for age, hospital, place of residence, HBsAg, and alcohol consumption [43]. Over-adjustment may have occurred as Cordier et al. [43] matched on a variable correlated with pesticide exposure (place of residence), potentially biasing results towards the null. Usage of hospital-based controls represents a potential source of selection bias.
Residential history
VoPham et al. [53] assessed pesticide exposure by combining residential ZIP Codes with a pesticide exposure database in a geographic information system (GIS) in the U.S. ZIP Code-level organochlorine pesticide exposure ≥14.53 kg/km2 compared to <14.53 kg/km2 (adjusted OR 1.87, 95% CI 1.17, 2.99) was associated with a statistically significant increase in HCC risk among individuals residing in agriculturally intensive areas in the U.S., adjusting for liver disease and diabetes and stratifying by the matching factors of age, sex, race, duration of California residence, and year [53]. VoPham et al. [53] did not have access to individual-level occupation, a potential confounder. There was a statistically significant interaction between ZIP Code-level organochlorine pesticide exposure and sex (p = 0.0075), where pesticide exposure was associated with a statistically significant increase in HCC risk among males, but no association was observed among females [53].
Other pesticides
Self-reported pesticide exposure
Cordier et al. [43], a previously referenced study, found that self-reported exposure to ≥30 L/year of organophosphate pesticides (adjusted OR 4.7, 95% CI 1.1, 20.1) as well as other pesticides (adjusted OR 4.0, 95% CI 0.3, 47.0) compared to none suggested an association with HCC among males in Vietnam, although the sample size was small.
Job-exposure matrices
Ezzat et al. [45] estimated exposure using a job-exposure matrix (JEM), in addition to collecting self-reported information regarding occupational history, pesticide exposure, and agricultural activities in Egypt. Several selected pesticide chemical classes demonstrated statistically significant positive associations with HCC among males in rural Egypt, including carbamate pesticides (adjusted OR 2.9, 95% CI 1.4, 5.8) and organophosphate pesticides (adjusted OR 2.7, 95% CI 1.3, 5.3), adjusting for age, HCV RNA (ribonucleic acid), and HBsAg [45]. Regarding potential over-adjustment, Ezzat et al. [45] matched on rural/urban residence, although results were stratified by residence and sex. Controls were recruited from a hospital orthopedic department. Effect modification by sex was reported, where occupational exposure to agricultural pesticides was associated with a statistically significant increased risk of HCC among males in rural Egypt, but was not associated with HCC among females [45].
Residential history
VoPham et al. [53] (referenced earlier) reported no association between ZIP Code-level organophosphate pesticides or carbamate pesticides and HCC in the U.S.
Mixed exposures and/or unspecified pesticides
Self-reported pesticide exposure
Studies ascertaining self-reported pesticide exposure mostly showed no association between pesticide exposure and liver cancer [40, 41, 43, 45, 47]. Exposure assessment methods included interviews and questionnaires. However, Badawi et al. [41] reported pesticide exposure vs. no exposure was associated with a statistically significant increased risk of HCC in Egypt (adjusted OR 2.19, 95% CI 1.41, 3.43), adjusting for age, sex, occupation, smoking, family history of cancer, schistosomiasis, and HBV (Table 2). Ezzat et al. [45] reported agricultural pesticide exposure vs. never exposure was associated with a statistically significant increased risk of HCC among males in rural Egypt (adjusted OR 2.5, 95% CI 1.3, 5.0) (Table 2). Austin et al. [40] did not collect information regarding potential confounders including HCV. Badawi et al. [41] did not collect information regarding HCV, which is a major HCC risk factor in Egypt. Many studies used hospital-based controls, a potential source of selection bias [40, 41, 43, 45, 47].
Job-exposure matrices
Chang et al. [42] assessed exposure using a JEM, showing no association between liver cancer and ≥10 years of pesticide exposure compared to never exposure among females in China (adjusted OR 0.54, 95% CI 0.12, 2.32), adjusting for age at baseline, smoking, and alcohol consumption (Table 2). Metastatic liver cancer may have been included in the case group, as International Classification of Diseases, Ninth Revision (ICD-9) code 155.2 (malignant liver neoplasms not specified as primary or secondary) was used in the case definition [42]. Information on established liver cancer risk factors was not collected.
Occupation and industry
Several studies ascertained occupation and industry information from a national supplemental pension fund, interviews, and questionnaires [44, 46, 50–52]. Some studies coded occupational information using classification systems such as the International Standard Classification of Occupations and the International Standard Industrial Classification of All Economic Activities [44, 46, 50]. Job titles were typically defined as farmer, farm worker, and farm laborer, and industries as agriculture. Although farming occupation was generally not associated with liver cancer [46, 50, 52], Schiefelbein et al. [51] showed farming vs. never employed in farming was associated with a statistically significant increased risk of HCC in Egypt (adjusted OR 2.8, 95% CI 1.1, 7.2), adjusting for HBV, anti-HCV, schistosomiasis, cirrhosis, and blood transfusion (controls were matched to cases according to age and sex) (Table 2). Pesticide exposure defined based on working in the agriculture industry in Denmark was not associated with liver cancer (Table 2) [44].
Most of these studies confirmed diagnoses, although there is some potential evidence of inclusion of metastatic liver cancer in Dossing et al. [44]. Seven percent (n = 5) of randomly sampled liver cancer patients were considered metastatic by pathologists, but not by the Denmark Cancer Registry [44]. Two studies did not collect or collected minimal confounder information [44, 46]. Porru et al. [50] may have over-adjusted by controlling for area of residence. Several studies used hospital-based or healthy cancer center visitors/non-relatives accompanying patients, a potential source of selection bias [46, 50, 52]. Effect modification by HCV was reported in Soliman et al. [52], where farming occupation was associated with a statistically significant increased risk of HCC among individuals with HCV, but was not associated with HCC among those without HCV.
Residential history
VoPham et al. [53] (referenced earlier) reported that combined ZIP Code-level exposure to organochlorine, organophosphate, and carbamate pesticides was not associated with HCC risk in the U.S. after adjustment (Table 2).
Discussion
There is some evidence to suggest a positive association between exposure to particular pesticides and HCC in the published literature to date. To the best of our knowledge, this is the first review summarizing the epidemiologic literature on pesticide exposure and liver cancer. While most studies showed null results, several demonstrated statistically significant elevations in liver cancer risk associated with higher pesticide exposure ascertained directly via biomarkers or indirectly (e.g., self-report). Studies that indirectly measured pesticide exposure demonstrated inconsistent results, ranging from statistically significant positive associations to non-significant deficits in risk. The most convincing evidence was observed among three case–control studies directly measuring organochlorine pesticides such as DDT in serum (two of which were prospectively assessed). Aside from the possibility of chance, methodological issues likely contributed to inconsistent results, including pesticide exposure assessment, case confirmation, confounding, over-adjustment, and selection bias.
Pesticide usage in the U.S. has declined over the past 30 years [55], but remains a major issue in this country comprising 22% of the world pesticide market [56]. Worldwide pesticide production has continuously increased since 1940 [57]. China is the current global leader in usage [58] and developing countries, such as Vietnam, have experienced increasing use [59]. Specific pesticides demonstrated statistically significant associations with liver cancer among studies included in this review, including the organochlorine insecticide DDT. Broader pesticide chemical classes associated with increased risk of liver cancer include organochlorines, organophosphates, and carbamates, each comprised of pesticides showing carcinogenic potential [60–62]. Organochlorines are mostly insecticides, were widely used in the 1940s to 1960s, but have largely been banned in many countries due to adverse wildlife and human health effects and environmental persistence [18, 63]. DDT was banned in China in 1983, but use continues for malarial control and dicofol insecticide production [48]. Organophosphates and carbamates, predominantly insecticides, began to increase in use following the ban of organochlorines. One-third of the insecticides used in China are organophosphates [64]. Both chemical classes were widely used in the U.S. during the 1980s and 1990s, but have since declined in usage replaced by more environmentally friendly chemicals [65]. Many of the pesticides from these chemical classes (e.g., DDT) are persistent organic pollutants, remaining in the environment for long periods of time and accumulating and passing from one species to another through the food chain [66]. DDT bioaccumulates in human adipose tissue. Pesticides outside of these chemical classes may be relevant to liver carcinogenesis, but their effects may not have been documented due to a lack of environmental persistence.
The high quality of the two biomonitoring case–control studies using pre-diagnostic sera, in terms of adjustment for established risk factors and exposure assessment, bolster confidence in their findings linking specific pesticides to HCC as direct measurement captures exposure from all sources [48, 49]. These studies were able to establish a temporal relationship as blood samples were collected prior to disease diagnosis. The use of biomonitoring to objectively quantify exposure also minimizes particular biases (e.g., recall bias from self-report). Both studies demonstrated statistically significant positive associations with organochlorine pesticides, including DDT. Humans are exposed to DDT and DDE through oral, inhalation, and dermal routes [67]. Humans can directly ingest DDE present in foods containing animal fat, especially as DDE is relatively more persistent than DDT [28]. Oral DDT and DDE exposure results in absorption by the intestinal lymphatic system and into portal blood. DDT and DDE are distributed in the lymph and blood to all body tissues and are subsequently stored in fat. DDT is initially metabolized in the liver to DDE and DDD (dichlorodiphenyldichloroethane). DDE and DDD are subsequently converted to DDA (2,2-bis(4-chlorophenyl)acetic acid), the primary urinary metabolite of DDT, in the liver and kidney. Conversion of DDT to DDE is predicted to occur slowly (<20% over 23 years). DDE metabolism is purported to occur at a slower rate compared to DDT. DDT is excreted as its metabolites through urine, feces, semen, and breast milk [67]. McGlynn et al. [48] and Persson et al. [49] measured serum DDT at study baseline, which occurred between 0 and 17 years preceding HCC diagnosis. These studies showed a statistically significant dose–response relationship between DDT and HCC risk. Zhao et al. [54] also showed a statistically significant positive association, but assessed serum measurements at study recruitment, which may be susceptible to reverse causation, especially as DDE was not associated with HCC in the prospective case–control studies [48, 49], but was associated with a statistically significant increase in HCC risk in this retrospective case–control study [54]. Although these differing results may reflect variability in pesticide exposure across China, with more recent exposure in the Zhao et al. [54] study population, reverse causation cannot be ruled out as liver cancer is associated with weight loss [68], which has been linked with increases in DDT and DDE [69]. Since organochlorine compounds are stored in fat, loss of body fat may increase blood and organ concentrations [69]. It is not clear whether results from China, which has higher levels of pesticide exposure, would be generalizable to populations where pesticide exposure is relatively lower such as the U.S. For example, the highest serum DDT concentrations in the U.S. are lower than the 25th percentile in Chinese studies [3, 18, 49].
Studies demonstrating a statistically significant positive association between pesticide exposure and liver cancer risk were conducted in China and Egypt, while the majority of studies with null findings were conducted in Europe and the U.S. The major limitation of the evaluated studies was pesticide exposure assessment. Three studies used biomonitoring (two of which were prospectively assessed), considered the gold standard exposure assessment method that can assess long-term exposure to chemicals, particularly those with long biological half-lives whose concentrations are not affected by disease [17, 19]. Although informative, biomonitoring has limitations, including inability to determine the exact timing and amount at initial exposure (as levels change over time and may not reflect the magnitude of exposure), the source or route of exposure, a meaningful health benchmark, and difficulty in assessing exposure to chemicals with relatively shorter biological half-lives such as organophosphates and carbamates [70]. Most studies included in the literature review indirectly measured exposure. Proxy measures can be useful in capturing exposure to pesticides without known biomarkers or from residential use. Farmers and those involved in purchasing/using pesticides have been shown to provide reliable information [17]. GIS can integrate multiple exposure data sources to estimate ambient pesticide exposure based on location [53]. However, these measures are subject to exposure misclassification due to recall bias and uncertain geographic context. Most studies did not examine historical pesticide exposure, which would address a potential latency period of 20 years documented for some HCC risk factors [4]. Recent exposure may be irrelevant to hepatocarcinogenesis. Exposure misclassification was likely non-differential, particularly among the studies prospectively assessing exposure using biomonitoring [48, 49], using administrative data regarding geographic residence [53], occupational information from a pension fund [44], and studies blinding interviewers or occupational physicians to case–control status [43, 46]. However, as many studies relied on self-report, differential exposure misclassification due to more accurate recall of past occupations, exposures, and residential history among cases cannot be ruled out, which may bias results towards or away from the null, contributing to the observed inconsistent results.
The majority of studies confirmed cases by histology, clinical examinations, imaging, elevated AFP levels, or a combination of these. Histological confirmation through biopsy or surgical resection is considered the gold standard [71], thus other confirmation methods may introduce misclassification of cases and controls. Misclassification from including metastatic liver cancer or non-HCC primary liver cancers in the case group could bias results (e.g., towards the null if pesticides are specifically associated with HCC and not other histologies or primary tumors). Prevalent HCC cases may differ from incident cases in their pesticide exposure experiences, where those with prevalent HCC may have survived due to relatively less pesticide exposure compared to incident cases [47].
Many studies did not adjust for established risk factors of HCC. The effect of confounding will vary according to geographic region, source population, and pesticide exposure assessment, reflecting differences in relationships with confounders in addition to exposure misclassification. When comparing effect estimates between studies adjusting for confounders compared to those not/minimally adjusting, several variables appear to exhibit strong confounding, particularly liver disease (HBV, HCV, and/or cirrhosis), alcohol consumption, age, and sex.
Over-adjustment, or controlling for variables highly correlated with the exposure of interest, may have affected results. Pesticide exposure is a largely rural phenomenon, as agricultural activities are more common in less densely populated areas [19]. Adjustment for variables that are inherently geographic, such as rural or urban residence, may produce comparable cases and controls, but may consequently impact results from measures designed/intended to capture the effect of pesticide exposure. For example, over-adjustment may have biased some results towards the null as pesticide use is expected to be more prevalent in rural areas, manifest in some studies matching on/adjusting for area of residence [43, 50]. However, several studies showed statistically significant results despite adjustment for/matching on geography-related variables [43, 45, 48, 49]. Badawi et al. [41] adjusted for farming occupation and pesticide exposure in the same multivariable model. These wide-ranging results highlight differential impacts of potential over-adjustment depending on pesticide exposure assessment and the study area. For example, rural–urban demarcations used in some studies may not accurately reflect pesticide exposure practices, obscuring variability that may exist in pesticide use across particular study areas. The effect of over-adjustment should be considered in future research.
Many studies used hospital controls, representing a potential selection bias where conditions for admission and other cancers (e.g., bladder cancer [40]) may share pesticide exposure as a risk factor with HCC and bias results towards the null (assuming pesticides increase liver cancer risk). For example, Porru et al. [50] included hospital-based controls admitted for issues primarily related to the genitourinary, digestive, and circulatory systems. As bladder cancer, a genitourinary issue, has been linked with pesticide use [72], results may have been biased towards the null.
Future research should harness the advantages of multiple pesticide exposure assessment methods. Important considerations include historical exposure reconstruction to address latency periods, and evaluation of multiple and specific pesticides as well as multiple relevant sources and pathways (e.g., occupation, residential use, residential proximity to agricultural pesticide applications, and diet). Biomonitoring studies should consider pharmacokinetic variability in exposure assessment. Single biomarker measurements are subject to exposure misclassification, and including information regarding birth cohort, body mass index, and weight gain as surrogates for exposure onset and individual differences in absorption and excretion, respectively, in statistical analyses may reduce misclassification [73]. Additional information to consider includes diet, health conditions (e.g., thyroid disease), lactation, medications, metabolizing enzymes, occupation, and residence. Sequential longitudinal biomarkers can be obtained to determine secular trends affecting pharmacokinetic variability. Practical issues regarding biomonitoring should be considered, including invasiveness of collection, implementing quality assurance/control measures, and collection from sensitive populations such as children [74]. Given suggestive associations in some studies, future research should focus on specific pesticides, such as DDT, and pesticides from particular chemical classes including organochlorines, organophosphates, and carbamates, as well as others that may have similar biological mechanisms of action to any of these classes [89]. Focusing on individuals with an opportunity for exposure can allow for detecting health effects that would be rarer and more difficult to observe in the general population. For example, agricultural pesticide use is more common in rural areas. Closer examination of effect modification is warranted, including between pesticide exposure and HCC risk factors such as sex, alcohol consumption, diabetes, HCV, and HBV. Obesity and diabetes are continuing to increase in prevalence, particularly in developing countries, which has important implications especially if there is a true synergistic association between diabetes and pesticide exposure [3, 54]. Results from studies utilizing multiple pesticide exposure metrics and/or examining multiple chemicals that were not reported in Table 2 should be examined [40, 41, 43–50, 52–54]. For example, Ezzat et al. [45] examined dithiocarbamate fungicides and bridged diphenyl acaricides. Anticipated National Institutes of Health-funded research includes two nested case–control studies conducted in the U.S. and Norway measuring 11 organochlorine pesticides using blood samples collected between the 1960s and 1970s [75]. Although this review only included published studies, the overall null findings suggest the absence of a publication bias in favor of statistically significant positive associations. Studies that examined multiple cancer outcomes were excluded (n = 11). Excluding such studies may be viewed as a potential limitation as most were of prospective designs and thus less susceptible to certain biases compared to case–control studies (e.g., retrospective exposure assessment). However, as these studies were limited with respect to internal validity-related issues in statistical power (liver cancer is a rare outcome) and lack of adjustment for potential confounders critical to our association of interest, we a priori determined to exclude them from our review.
In summary, there is some evidence to suggest a positive association between particular pesticides and HCC. The most convincing evidence was observed in three studies directly measuring serum pesticide levels, although one of these studies used post-diagnostic sera and results may be subject to reverse causation. Specific pesticides, including the organochlorine DDT, demonstrated statistically significant positive associations with HCC. While many studies showed no association, these were largely limited by indirect pesticide exposure assessment methods likely resulting in exposure misclassification, minimal case confirmation, lack of adjustment for confounders, over-adjustment, and/or selection bias. Given the high prevalence of pesticide exposure in geographic areas with high HCC incidence and the high proportion of HCC cases in the U.S. that occur among those with no established risk factors, it is important to determine whether pesticides play a role in hepatocarcinogenesis. Future research should focus on improving pesticide exposure assessment, considering historical exposure, multiple pesticide exposures and exposure pathways, and the impact of specific organochlorine, organophosphate, and carbamate pesticides.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer. http://globocan.iarc.fr. Accessed 11 Aug 2015
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
McGlynn KA, Petrick JL, London WT (2015) Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 19(2):223–238. doi:10.1016/j.cld.2015.01.001
Carr BI (2010) Hepatocellular carcinoma: diagnosis and treatment, 2nd edn. Humana Press, Philadelphia
El-Serag HB (2007) Epidemiology of hepatocellular carcinoma in USA. Hepatology Res 37(Suppl 2):S88–S94. doi:10.1111/j.1872-034X.2007.00168.x
Surveillance, Epidemiology, End Results (SEER) Program, (http://www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (2000–2012) <Katrina/Rita Population Adjustment>—Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
El-Serag HB, Kanwal F (2014) Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 60(5):1767–1775. doi:10.1002/hep.27222
Yu MC, Yuan JM (2004) Environmental factors and risk for hepatocellular carcinoma. Gastroenterology 127(5 Suppl 1):S72–S78. doi:10.1016/j.gastro.2004.09.018
Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, Corrao G, Boffetta P, La Vecchia C (2014) Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Annu Oncol 25(8):1526–1535. doi:10.1093/annonc/mdu020
Jinjuvadia R, Patel S, Liangpunsakul S (2014) The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol 48(2):172–177. doi:10.1097/MCG.0b013e3182a030c4
Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C (2013) Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol 11(11):1413–1421. doi:10.1016/j.cgh.2013.04.039 (e1411)
Duan XY, Zhang L, Fan JG, Qiao L (2014) NAFLD leads to liver cancer: do we have sufficient evidence? Cancer Lett 345(2):230–234. doi:10.1016/j.canlet.2013.07.033
FonSing M, Yang WS, Gao S, Gao J, Xiang YB (2011) Epidemiological studies of the association between tea drinking and primary liver cancer: a meta-analysis. Eur J Cancer Prev 20(3):157–165. doi:10.1097/CEJ.0b013e3283447497
Larsson S, Wolk A (2007) Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 97(7):1005–1008. doi:10.1038/sj.bjc.6603932
Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K (2009) Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol 38(6):1497–1511. doi:10.1093/ije/dyp280
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L (2012) Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 130(7):1639–1648. doi:10.1002/ijc.26165
Alavanja MC, Hoppin JA, Kamel F (2004) Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health 25:155–197. doi:10.1146/annurev.publhealth.25.101802.123020
Centers for Disease Control and Prevention (2009) Fourth National Report on Human Exposure to Environmental Chemicals. http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf. Accessed 01 Oct 14
Franklin C, Worgan J (2005) Occupational and residential exposure assessment for pesticides. Wiley, Hoboken
Rull RP, Ritz B (2003) Historical pesticide exposure in California using pesticide use reports and land-use surveys: an assessment of misclassification error and bias. Environ Health Perspect 111(13):1582–1589. doi:10.1289/ehp.6118
Deziel NC, Friesen MC, Hoppin JA, Hines CJ, Thomas K, Freeman LE (2015) A review of nonoccupational pathways for pesticide exposure in women living in agricultural areas. Environ Health Perspect 123(6):515–524. doi:10.1289/ehp.1408273
Dich J, Zahm SH, Hanberg A, Adami HO (1997) Pesticides and cancer. Cancer Causes Control 8 (3):420–443. doi:10.1023/A:1018413522959
Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD (2008) Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 14(27):4300–4308. doi:10.3748/wjg.14.4300
Jin X, Chen M, Song L, Li H, Li Z (2014) The evaluation of p, p’-DDT exposure on cell adhesion of hepatocellular carcinoma. Toxicology 322:99–108. doi:10.1016/j.tox.2014.05.002
Jin XT, Song L, Zhao JY, Li ZY, Zhao MR, Liu WP (2014) Dichlorodiphenyltrichloroethane exposure induces the growth of hepatocellular carcinoma via Wnt/beta-catenin pathway. Toxicol Lett 225(1):158–166. doi:10.1016/j.toxlet.2013.12.006
Rossi L, Barbieri O, Sanguineti M, Cabral JR, Bruzzi P, Santi L (1983) Carcinogenicity study with technical-grade dichlorodiphenyltrichloroethane and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene in hamsters. Cancer Res 43(2):776–781
Turusov VS, Day NE, Tomatis L, Gati E, Charles RT (1973) Tumors in CF-1 mice exposed for six consecutive generations to DDT. J Natl Cancer Inst 51(3):983–997. doi:10.1093/jnci/51.3.983
Cohn BA, Wolff MS, Cirillo PM, Sholtz RI (2007) DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 115(10):1406–1414. doi:10.1289/ehp.10260
Cocco P, Blair A, Congia P, Saba G, Ecca AR, Palmas C (1997) Long-term health effects of the occupational exposure to DDT. A preliminary report. Ann NY Acad Sci 837:246–256. doi:10.1111/j.1749-6632.1997.tb56878.x
Cocco P, Fadda D, Billai B, D’Atri M, Melis M, Blair A (2005) Cancer mortality among men occupationally exposed to dichlorodiphenyltrichloroethane. Cancer Res 65(20):9588–9594. doi:10.1158/0008-5472.CAN-05-1487
Cocco P, Kazerouni N, Zahm SH (2000) Cancer mortality and environmental exposure to DDE in the United States. Environ Health Perspect 108(1):1–4. doi:10.1289/ehp.001081
Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT (2002) Eight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomark Prev 11(4):369–376
Hardell L, Bengtsson NO, Jonsson U, Eriksson S, Larsson LG (1984) Aetiological aspects on primary liver cancer with special regard to alcohol, organic solvents and acute intermittent porphyria–an epidemiological investigation. Br J Cancer 50(3):389–397. doi:10.1038/bjc.1984.188
Kauppinen T, Riala R, Seitsamo J, Hernberg S (1992) Primary liver cancer and occupational exposure. Scand J Work Environ Health 18(1):18–25. doi:10.5271/sjweh0.1616
London WT, Evans AA, McGlynn K, Buetow K, An P, Gao L, Lustbader E, Ross E, Chen G, Shen F (1995) Viral, host and environmental risk factors for hepatocellular carcinoma: a prospective study in Haimen City, China. Intervirology 38(3–4):155–161
Stemhagen A, Slade J, Altman R, Bill J (1983) Occupational risk factors and liver cancer. A retrospective case–control study of primary liver cancer in New Jersey. Am J Epidemiol 117(4):443–454
Suarez L, Weiss NS, Martin J (1989) Primary liver cancer death and occupation in Texas. Am J Ind Med 15(2):167–175. doi:10.1002/ajim.4700150205
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed.1000097
Szklo M, Nieto FJ (2007) Epidemiology Beyond the Basics, 2nd edn. Jones and Bartlett, Sudbury
Austin H, Delzell E, Grufferman S, Levine R, Morrison AS, Stolley PD, Cole P (1987) Case–control study of hepatocellular carcinoma, occupation, and chemical exposures. J Occup Med 29(8):665–669
Badawi AF, Michael MS (1999) Risk factors for hepatocellular carcinoma in Egypt: the role of hepatitis-B viral infection and schistosomiasis. Anticancer research 19(5C):4565–4569
Chang CK, Astrakianakis G, Thomas DB, Seixas NS, Ray RM, Gao DL, Wernli KJ, Fitzgibbons ED, Vaughan TL, Checkoway H (2006) Occupational exposures and risks of liver cancer among Shanghai female textile workers–a case-cohort study. Int J Epidemiol 35(2):361–369. doi:10.1093/ije/dyi282
Cordier S, Le TB, Verger P, Bard D, Le CD, Larouze B, Dazza MC, Hoang TQ, Abenhaim L (1993) Viral infections and chemical exposures as risk factors for hepatocellular carcinoma in Vietnam. International journal of cancer Journal international du cancer 55(2):196–201. doi:10.1002/ijc.2910550205
Dossing M, Petersen KT, Vyberg M, Olsen JH (1997) Liver cancer among employees in Denmark. Am J Ind Med 32(3):248–254. doi:10.1002/(SICI)1097-0274(199709)32:33.0.CO;2-V
Ezzat S, Abdel-Hamid M, Eissa SA, Mokhtar N, Labib NA, El-Ghorory L, Mikhail NN, Abdel-Hamid A, Hifnawy T, Strickland GT, Loffredo CA (2005) Associations of pesticides, HCV, HBV, and hepatocellular carcinoma in Egypt. Int J Hyg Environ Health 208(5):329–339. doi:10.1016/j.ijheh.2005.04.003
Ferrand JF, Cenee S, Laurent-Puig P, Loriot MA, Trinchet JC, Degos F, Bronovicky JP, Pelletier G, Stucker I (2008) Hepatocellular carcinoma and occupation in men: a case–control study. J Occup Environ Med 50(2):212–220. doi:10.1097/JOM.0b013e31815d88e2
Heinemann K, Willich SN, Heinemann LA, DoMinh T, Mohner M, Heuchert GE (2000) Occupational exposure and liver cancer in women: results of the Multicentre International Liver Tumour Study (MILTS). Occup Med (Lond) 50(6):422–429. doi:10.1093/occmed/50.6.422
McGlynn KA, Abnet CC, Zhang M, Sun XD, Fan JH, O’Brien TR, Wei WQ, Ortiz-Conde BA, Dawsey SM, Weber JP, Taylor PR, Katki H, Mark SD, Qiao YL (2006) Serum concentrations of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and risk of primary liver cancer. J Natl Cancer Inst 98(14):1005–1010. doi:10.1093/jnci/djj266
Persson EC, Graubard BI, Evans AA, London WT, Weber JP, Leblanc A, Chen G, Lin W, McGlynn KA (2012) Dichlorodiphenyltrichloroethane and risk of hepatocellular carcinoma. Int J Cancer J int du Cancer 131(9):2078–2084. doi:10.1002/ijc.27459
Porru S, Placidi D, Carta A, Gelatti U, Ribero ML, Tagger A, Boffetta P, Donato F (2001) Primary liver cancer and occupation in men: a case–control study in a high-incidence area in Northern Italy. Int J Cancer J Int du Cancer 94(6):878–883. doi:10.1002/ijc.1538
Schiefelbein E, Zekri A-R, Newton D, Soliman G, Banerjee M, Hung C-W, Seifeldin I, Lo A-C, Soliman A (2012) Hepatitis C virus and other risk factors in hepatocellular carcinoma. Acta Virol 56(3):235
Soliman AS, Hung CW, Tsodikov A, Seifeldin IA, Ramadan M, Al-Gamal D, Schiefelbein EL, Thummalapally P, Dey S, Ismail K (2010) Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Hepatol Int 4(4):681–690. doi:10.1007/s12072-010-9187-1
VoPham T, Brooks MM, Yuan JM, Talbott EO, Ruddell D, Hart JE, Chang CC, Weissfeld JL (2015) Pesticide exposure and hepatocellular carcinoma risk: a case–control study using a geographic information system (GIS) to link SEER-Medicare and California pesticide data. Environ Res 143(Pt A):68–82. doi:10.1016/j.envres.2015.09.027
Zhao B, Shen H, Liu F, Liu S, Niu J, Guo F, Sun X (2012) Exposure to organochlorine pesticides is an independent risk factor of hepatocellular carcinoma: a case–control study. J Expo Sci Environ Epidemiol 22(6):541–548. doi:10.1038/jes.2011.29
Fernandez-Cornejo J, Nehring R, Osteen C, Wechsler S, Martin A, Vialou A (2014) Pesticide use in US agriculture: 21 selected crops, 1960–2008, EIB-124. US Department of Agriculture, Economic Research Service
Environmental Protection Agency (2011) Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. http://www.epa.gov/sites/production/files/2015-10/documents/market_estimates2007.pdf. Accessed 12 Jan 15
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671–677. doi:10.1038/nature01014
Li Y, Zhang C, Yin Y, Cui F, Cai J, Chen Z, Jin Y, Robson MG, Li M, Ren Y, Huang X, Hu R (2014) Neurological effects of pesticide use among farmers in China. Int J Environ Res Public Health 11(4):3995–4006. doi:10.3390/ijerph110403995
Dasgupta S, Meisner C, Wheeler D, Xuyen K, Thi Lam N (2007) Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int J Hyg Environ Health 210(2):121–132. doi:10.1016/j.ijheh.2006.08.006
Peyre L, Zucchini-Pascal N, de Sousa G, Rahmani R (2012) Effects of endosulfan on hepatoma cell adhesion: epithelial-mesenchymal transition and anoikis resistance. Toxicology 300(1–2):19–30. doi:10.1016/j.tox.2012.05.008
Galloway T, Handy R (2003) Immunotoxicity of organophosphorous pesticides. Ecotoxicology 12(1–4):345–363. doi:10.1023/A:1022579416322
Voccia I, Blakley B, Brousseau P, Fournier M (1999) Immunotoxicity of pesticides: a review. Toxicol Ind Health 15(1–2):119–132. doi:10.1177/074823379901500110
Longnecker MP, Rogan WJ, Lucier G (1997) The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health 18:211–244. doi:10.1146/annurev.publhealth.18.1.211
Zhang Y, Han S, Liang D, Shi X, Wang F, Liu W, Zhang L, Chen L, Gu Y, Tian Y (2014) Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: a birth cohort study in Shenyang, China. PloS one 9(2):e88491. doi:10.1371/journal.pone.0088491
Wells J (2011) Pesticide Use Trends in California Agriculture. Environmental Solutions Group
Environmental Protection Agency (2015) Persistent Organic Pollutants: A Global Issue, A Global Response. http://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response. Accessed 21 Dec 2015
Faroon O, Harris MO, Llados F, Swarts S, Sage G, Citra M, Gefell D (2002) Toxicological profile for DDT, DDE, and DDD. ATSDR (Agency for Toxic Substances and Disease Registry)
Befeler AS, Di Bisceglie AM (2002) Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 122(6):1609–1619
Chevrier J, Dewailly E, Ayotte P, Mauriege P, Despres J, Tremblay A (2000) Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. International journal of obesity 24(10):1272–1278
Angerer J, Bird MG, Burke TA, Doerrer NG, Needham L, Robison SH, Sheldon L, Zenick H (2006) Strategic biomonitoring initiatives: moving the science forward. Toxicol Sci 93(1):3–10
Nasca PC, Pastides H (2008) Fundamentals of cancer epidemiology, 2nd edn. Jones and Bartlett Publishers, Sudbury
Koutros S, Silverman DT, Alavanja MC, Andreotti G, Lerro CC, Heltshe S, Lynch CF, Sandler DP, Blair A, Freeman LEB (2015) Occupational exposure to pesticides and bladder cancer risk. Int J Epidemiol:dyv195
Wolff MS, Anderson HA, Britton JA, Rothman N (2007) Pharmacokinetic variability and modern epidemiology—the example of dichlorodiphenyltrichloroethane, body mass index, and birth cohort. Cancer Epidemiol Biomark Prev 16 (10):1925–1930
Sobus J, Morgan M, Pleil J, Barr D (2010) Biomonitoring: uses and considerations for assessing non-occupational human exposure to pesticides. In: Krieger R (ed) Hayes’ Handbook of Pesticide Toxicology. Oxford, pp 1021–1036
NIH Research Portfolio Online Reporting Tools (2015) 5R01ES014662-03 Serum organochlorine levels and primary liver cancer: a nested case–control study. https://projectreporter.nih.gov/. Accessed 12 Jan 2015
Acknowledgments
The authors declare no conflicts of interest. Dr. VoPham is supported by the National Institutes of Health (NIH) National Cancer Institute (NCI) Training Program in Cancer Epidemiology (T32 CA009001). Dr. Hart is supported by the NIH National Institute of Environmental Health Sciences (NIEHS) P30 ES000002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
VoPham, T., Bertrand, K.A., Hart, J.E. et al. Pesticide exposure and liver cancer: a review. Cancer Causes Control 28, 177–190 (2017). https://doi.org/10.1007/s10552-017-0854-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-017-0854-6