Abstract
Purpose
To compare the diagnostic performance (detection, assessment of correct disease extent and multifocality/centricity) of Contrast-Enhanced Mammography (CEM) Versus Breast Magnetic Resonance (MRI) in the study of lobular neoplasms.
Methods
We retrospectively selected all the patients who underwent surgery for a lobular breast neoplasm, either an in situ or an invasive tumor, and had undergone both breast CEM and MRI examinations during the pre-surgical planning. Wilcoxon Signed Rank test was performed to assess the differences between size measurements using the different methods and the post-surgical pathological measurements, considered the gold standard. The agreement in identifying multifocality/multicentricity among the different methods and the pathology was assessed using the Kappa statistics.
Results
We selected 19 patients, of which one presented a bilateral neoplasm. Then, the images of these 19 patients were analyzed, for a total of 52 malignant breast lesions. We found no significant differences between the post-surgical pathological size of the lesions and the calculated size with CEM and MRI (p-value of the difference respectively 0.71 and 0.47). In all 20 cases, neoplasm detection was possible both with CEM and MRI. CEM and MRI showed an excellent ability to identify multifocal and multicentric cases (K statistic equal to 0.93 for both the procedures), while K statistic was 0.11 and 0.59 for FFDM and US, respectively.
Conclusion
The findings of this study suggest that CEM is a reliable imaging technique in the preoperative setting of patients with lobular neoplasm, with comparable results to breast MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast neoplasm remains, to this day, the most common neoplasm in women, with more than 2.3 million new cases diagnosed globally each year [1]. Prompt and appropriate diagnosis is essential to properly manage patients with this condition, reducing mortality and comorbidities. Invasive lobular carcinoma (ILC) and lobular neoplasia (LN) are two different conditions in the large plethora of breast malignancies, whose diagnosis and management are still challenging [2, 3].
ILC is the second most common type of breast cancer (BC), accounting for 10–15% of all invasive breast tumors [4].
During the last two decades, an increase in ILC detection has been observed, mostly thanks to the improvements in BC diagnosis and due to the increasing use of hormone replacement therapy in post-menopausal women [5].
ILC is more frequently diagnosed in older women, with a higher incidence in Western countries [5] and, it may be associated with certain cancer-predisposing genetic alterations [6,7,8].
ILC typically presents as a large tumor, often multifocal or multicentric, with bilateral manifestation and with nodal involvement already at the time of diagnosis, having a high reintervention rate [9].
Furthermore, due to an insidious proliferative pattern with a lack of desmoplastic and fibrotic reactions [10], in many cases, lobular carcinoma remains clinically and radiologically elusive, with a high rate of tumor size underestimation and a high prevalence of missed synchronous lesions. For these reasons, detecting ILC with conventional imaging techniques, such as full field digital mammography or ultrasound, represents a real radiological challenge [11], eventually rendering the role of MRI essential.
Indeed, it is well known that Breast Magnetic Resonance Imaging (MRI) improves the management of patients diagnosed with breast lobular neoplasia: according to the most critical studies in literature, MRI imaging helps in the identification of new ipsilateral and contralateral lesions in up to 39% of cases and influences surgical management in about the 25% of cases investigated [12,13,14].
While the role of breast MRI in the study of lobular neoplasms is well established, there are very few published data, with few patients, on the diagnostic performance of Contrast Enhanced Spectral Mammography (CEM) in the evaluation of patients with lobular neoplasia [15, 16]: CEM is a relatively new method showing huge potential in the study of breast malignancies, although its role applied specifically to lobular neoplasms has been little investigated [17].
With this study, we aimed to compare the diagnostic performance of CEM versus Full Field Digital Mammography (FFDM), Breast Ultrasound (US), and Breast MRI in the study of lobular neoplasms. It is particularly intended to compare the ability of CEM and breast MRI in the assessment of correct disease extent and multifocality/centricity of the disease.
As CEM has been shown to be faster and cheaper than MRI and generally better tolerated by patients [18], demonstrating its utility in the study of lobular neoplasms, could provide a new personalized and effective approach in the assessment of those neoplasms without recurring to MRI.
Methods
This retrospective study was notified to the Ethics Committee and approved by the Institutional Review Board.
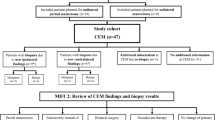
We retrospectively selected from our institution's datasets all operated patients with lobular breast neoplasm, either invasive or in situ, who underwent pre-surgical breast CEM and MRI. It was also assessed whether the same patients had performed breast ultrasounds and/or mammography.
Therefore, the inclusion criteria of the study were as follows:
-
Patients diagnosed with lobular neoplasm of the breast.
-
Patients underwent both CEM and MRI examinations before surgery.
-
Patients treated in our institution.
The study exclusion criteria were:
-
Patients with other type of breast malignancies (non-lobular neoplasms) or benign lesions.
-
Patients who did not undergo both CEM and MRI before surgery (to be enrolled in the study, patients had to have performed both CEM and MRI before surgery.)
-
Patients in whom the quality of the image stored in the PACS was not of sufficient quality for reevaluation. (Low-quality images that did not allow review by radiologists were excluded.)
The images were evaluated, retrospectively, by two expert radiologists with more than 5 years of experience in breast imaging: images were evaluated in consensus by the two radiologists included in the study. These radiologists first evaluated the low-energy CEM image, interpreting it as a conventional full field digital mammography. They then evaluated the recombined CEM image by assessing any additional information. This reevaluation of images also included evaluation of MRI, FFDM, and US performed in the same group of patients (in patients with images stored in institution PACs). In the re-evaluation, we assessed whether the lesion was visible with the method, the extent of the lesion, and the presence of multifocality and multicentricity for each exam. We then compared, among the various methods (CEM; MRI; US, and FFDM), the ability to detect the lesion(s), identify multicentricity/multifocality, and define the extent of the lesion correctly. Images were analyzed following the latest version of BIRADS for MRI, CEM, US and FFDM [19,20,21].
The post-surgical pathological report was used as the gold standard to define the extent of the lesion(s) and to evaluate the multicentricity/multifocality. The term “multifocality” denoted the presence of multiple lesions in the same quadrant, while “multicentricity” the presence of multiple lesions in different quadrants.
CEM protocol
We used GE® Healthcare, Senographe Pristina®, Chalfont St. Giles, UK, mammograph for CEM examination: after the intravenous injection of an iodinated contrast agent (Ioexolo) (300 mg/mL, 1.5 mL/kg, Omnipaque®, GE Healthcare, Chalfont St. Giles, UK) two bilateral Cranio Caudal (CC) and mediolateral oblique (MLO) projection views were acquired. Low-energy (26–32 kVp) and high-energy (45–49 kVp) exposures were acquired and then recombine to highlight the uptake of the contrast agent.
MRI protocol
All patients underwent breast MRI with a 1.5-Tesla scanner (Optima MR450w, General Electric Medical Systems, Milwaukee, WI, USA) equipped with a 34 mT/m gradient and a dedicated 8-channel breast coil. A standardized MRI protocol was performed consisting of an axial FSE T2-weighted image, axial DWI with the relative apparent diffusion coefficient (ADC) maps, and dynamic series performed once before and four times after intravenous administration of 0.1 mmol/kg of a gadolinium-chelate at 90 s, post-processing subtraction, and maximal intensity projection (MIP) images.
Statistical analysis
Continuous data were reported as median and ranges, or median and interquartile ranges (IQR). Categorical data were reported as counts and percentages.
Wilcoxon Signed Rank test was performed to assess the differences between size measurements using the different methods and the post-surgical pathological size of the lesion, considered as the gold standard.
The agreement in identifying multifocality/multicentricity among the different methods and the post-surgical pathology was assessed using the Kappa statistic, with 95% CI.
All reported p-values were two-sided, with a p-value less than 0.05 considered as statistically significant.
All analyses were performed with the statistical software SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
We selected 19 patients, of which one presented a bilateral neoplasm. Then, the images of these 19 patients were analysed for a total of 52 malignant breast lesions. The median age at surgery was 49 years (range 28–84). In 90% of the cases, the histology was that of an infiltrating lobular carcinoma. In the remaining cases, we had lobular carcinomas in situ. Table 1 summarizes the main descriptive characteristics of patients at surgery. Most patients (85%) had dense glandular breasts (ACR classification C or D). All patients were examined with CEM, MRI, and FFDM, while preoperative ultrasound was not performed in one case.
In 15% of cases, the neoplasm presented as multifocal; in 15% of cases as multicentric; in 25% of cases, both multifocal and multicentric. All cases were hormone-receptor-positive; Ki-67 was ≤ 20% in 68% (n = 13) of cases, and Her-2 negative in 85% of cases.
We considered the diameter at surgery as the gold standard.
We found no statistically significant differences between the lesion diameter measured at surgery and the lesion diameter measured by CEM and MRI (p-value of the difference 0.71 and 0.47, respectively).
In contrast, we found statistically significant differences between the lesion diameter measured at surgery and the lesion diameter measured with FFDM and US (p-value of the difference 0.041 and 0.014, respectively).
These results are schematized in Table 2 and Fig. 1.
In all 20 cases, neoplasm detection was possible by CEM and MRI. Detection of neoplasia was possible in 18/20 cases by ultrasound and in 10/20 cases by mammography (Table 3).
Considering post-surgical pathological report as the gold standard in multifocality/multicentricity assessment, we obtained: 9 cases (45%) without multifocality and multicentricity, 3 cases (15%) with only multifocality, and 3 cases (15%) with only multicentricity. In 5 cases (25%), we had both multifocality and multicentricity.
The agreement in identifying multifocality/multicentricity (considering surgery as the gold standard) was excellent for CEM and MRI [Kappa statistic 95% CI 0.93 (0.79–1.00)]; moderate for ultrasound [Kappa Statistic 95% CI 0.59 (0.30—0.88)] and very bad for mammography [Kappa statistic 95% CI 0.11 (− 0.09–0.30)]. (See Table 4).
In all cases, the lesions demonstrated enhancement in both CEM and MRI. In most cases (65% for CEM and 60% for MRI), the neoplasm showed non-mass enhancement (see Fig. 2).
Forty-six-year-old woman with bloody discharge from the nipple. The patient performs both MRI (a) and CEM (b). In the first post-contrast subtracted (a), we can appreciate a pathologic enhancement (arrow) consisting of multiple confluent nodules (multifocal and pluricentric pathology). One of the satellite pathological findings also shows up, separated from the central lesion (arrowhead). The exact appearance is appreciated in the recombined image of the CEM in the Mediolateral oblique projection. Again, the pathologic enhancement (arrow) is appreciated, with the satellite lesion evident (arrowhead). In this glandular breast, conventional mammography is not so clear in identifying the pathologic lesion. The histopathological examination of the surgical sample confirmed the presence of moderately differentiated invasive lobular carcinoma. The neoplastic cells exhibited expression of both estrogen (90%) and progesterone (60%) receptors, whereas there was no membrane immunoreactivity for Her-1/neu (score 0); the Ki-67 labeling index was determined to be 10%
Discussion
The diagnosis of lobular carcinoma of the breast represents a challenge both from the clinical and radiological standpoint, in particular with conventional imaging methods that may miss early signs of the neoplasm. In fact, lobular carcinomas have a peculiar growth pattern, with initial subtle changes involving the stroma, with only minor alterations of the surrounding architecture. This feature signifies that, in many cases, the diagnosis is delayed, occurring in more advanced stages, as the lesion becomes more evident [22].
Mammography has a low sensitivity in detecting this type of pathology: more than 30% of cases of lobular neoplasia can be missed by this method [23]. The sensitivity of breast ultrasound in detection seems to be better but still suboptimal and operator dependent [24, 25]. The sensitivity of breast MRI is superior in detecting lobular neoplasms, reaching a sensitivity of more than 90% [26].
Moreover, breast MRI allows us to cope with two additional aspects related to lobular neoplasms: the definition of the correct extent of pathology and the problem of multifocality/multicentricity, a tendency frequently displayed by lobular carcinoma, as also confirmed by our case series [27, 28]. In turn, these features are essential to plan the best surgical approach.
While the role of MRI in the pre-surgical assessment of lobular neoplasms is well established, there are very few studies [15, 29, 30] that have evaluated the performance of CEM in the assessment of lobular neoplasms. In our work, we sought to compare the performance of CEM to MR in the detection, assessment of lesion extent, and identification of multifocality and/or pluricentric lobular neoplasms. The results, although preliminary and obtained on a limited set of patients, are highly encouraging: CEM is faster and generally better tolerated by patients [31, 32] and it might stand as a reliable alternative to MRI in the preoperative assessment of patients with lobular neoplasm [29, 30]. Some typical examples of CEM and MRI appearance of breast lobular neoplasm are shown in Figs. 2 and 3.
Sixty-four-year-old patient with recent mammographic findings of newly appeared left breast lesion. An asymmetric mammographic thickening is appreciated in the mediolateral oblique projection of conventional mammography (a). At this thickening, a non-mass-like enhancement (arrow) with suspicious characters is appreciated in the recombined EMC image in mediolateral oblique projection (b). The focal non-mass enhancement (arrow) is also appreciated in the first post-contrast subtracted image (c). The histopathological examination of the surgical sample confirmed the presence of invasive lobular carcinoma. The neoplastic cells exhibited expression of both estrogen (95%) and progesterone (80%) receptors, whereas there was no membrane immunoreactivity for Her-1/neu (score 0); the Ki-67 labelling index was determined to be 13%
Based on these preliminary results, we think CEM could be proposed to patients at higher risk of developing lobular neoplasm. (e.g., patients with previous lobular neoplasm) in preventive and follow-up examinations. In study protocols, CEM could also be proposed as a replacement or complement to MRI in patients with a biopsy diagnosis of lobular neoplasm for proper preoperative assessment: our study presents auspicious results in this direction. In particular, CEM could be very promising in a setting where MRI may not be readily available or in cases where MRI is not tolerated or performable.
The main limitation of our work is the low number of patients involved and its retrospective nature. This work can be considered as a basis for prospective study protocols with a more significant number of patients that can evaluate the potential replacement role of CEM compared with MRI in the proper preoperative management of the patient with lobular neoplasia.
Conclusion
The findings of this study suggest that CEM is a reliable imaging technique in the preoperative setting of patients with lobular neoplasm, with comparable results to breast MRI for what concerns the lesion measurement and assessment of its extent and distribution (multicentric/multifocal).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A (2021) Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel) 13(17):4287. https://doi.org/10.3390/cancers13174287
Oliveira TM, Elias J Jr, Melo AF, Teixeira SR, Filho SC, Gonçalves LM, Faria FM, Tiezzi DG, Andrade JM, Muglia V (2014) Evolving concepts in breast lobular neoplasia and invasive lobular carcinoma, and their impact on imaging methods. Insights Imaging 5(2):183–194. https://doi.org/10.1007/s13244-014-0324-6
Li CI, Anderson BO, Daling JR, Moe RE (2003) Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 289:1421–1424
Van Baelen K, Geukens T, Maetens M, Tjan-Heijnen V, Lord CJ, Linn S, Bidard FC, Richard F, Yang WW, Steele RE, Pettitt SJ, Van Ongeval C, De Schepper M, Isnaldi E, Nevelsteen I, Smeets A, Punie K, Voorwerk L, Wildiers H, Floris G, Vincent Salomon A, Derksen PWB, Neven P, Senkus E, Sawyer E, Kok M, Desmedt C (2022) Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol 33(8):769–785. https://doi.org/10.1016/j.annonc.2022.11.010
Li CI, Malone KE, Daling JR (2002) Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev 11(7):601–607
Girardi A, Magnoni F, Vicini E, Kouloura A, La Vecchia C, Veronesi P, Corso G (2022) CDH1 germline mutations in families with hereditary lobular breast cancer. Eur J Cancer Prev 31(3):274–278. https://doi.org/10.1097/CEJ.0000000000000688
Magnoni F, Corso G (2022) Progress in breast cancer surgical management. Eur J Cancer Prev 31(6):551–553. https://doi.org/10.1097/CEJ.0000000000000741
Yeatman TJ, Cantor AB, Smith TJ et al (1995) Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg 222(4):549–559
Waljee JF, Hu ES, Newman LA, Alderman AK (2008) Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol 15(5):1297–1303
Thomas M, Kelly ED, Abraham J, Kruse M (2019) Invasive lobular breast cancer: a review of pathogenesis, diagnosis, management, and future directions of early-stage disease. Semin Oncol 46:121–132
Mann RM, Loo CE, Wobbes T et al (2010) The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat 119(2):415–422
Heil J, Buehler A, Golatta M et al (2012) Do patients with invasive lobular breast cancer benefit in terms of adequate change in surgical therapy from a supplementary preoperative breast MRI? Ann Oncol 23(1):98–104
Ha SM, Chae EY, Cha JH, Kim HH, Shin HJ, Choi WJ (2018) Breast MR imaging before surgery: outcomes in patients with invasive lobular carcinoma by using propensity score matching. Radiology 287(3):771–777. https://doi.org/10.1148/radiol.2018171472
Costantini M, Montella RA, Fadda MP, Tondolo V, Franceschini G, Bove S, Garganese G, Rinaldi PM (2022) Diagnostic challenge of invasive lobular carcinoma of the breast: what is the news? Breast Magnetic Resonance Imaging and emerging role of contrast-enhanced spectral mammography. J Pers Med 12(6):867. https://doi.org/10.3390/jpm12060867
Lobbes MBI, Neeter LMFH, Raat F, Turk K, Wildberger JE, van Nijnatten TJA, Nelemans PJ (2023) The performance of Contrast-Enhanced Mammography and breast MRI in local preoperative staging of invasive lobular breast cancer. Eur J Radiol 164:110881. https://doi.org/10.1016/j.ejrad.2023.110881
Zamora K, Allen E, Hermecz B (2021) Contrast mammography in clinical practice: current uses and potential diagnostic dilemmas. Clin Imaging 71:126–135
Ferranti FR, Vasselli F, Barba M, Sperati F, Terrenato I, Graziano F, Vici P, Botti C, Vidiri A (2022) Diagnostic accuracy of contrast-enhanced, spectral mammography (CESM) and 3T magnetic resonance compared to full-field digital mammography plus ultrasound in breast lesions: results of a (Pilot) open-label, single-centre prospective study. Cancers (Basel) 14(5):1351. https://doi.org/10.3390/cancers14051351
Neeter LMFH, Robbe MMQ, van Nijnatten TJA, Jochelson MS, Raat HPJ, Wildberger JE, Smidt ML, Nelemans PJ, Lobbes MBI (2023) Comparing the diagnostic performance of Contrast-Enhanced Mammography and breast MRI: a systematic review and meta-analysis. J Cancer 14(1):174–182. https://doi.org/10.7150/jca.79747
Lee CH, Phillips J, Sung JS, Lewin JM, Newell MS (2022) Contrast enhanced mammography (CEM) (a supplement to ACR BI-RADS® mammography 2013) atlas, breast imaging reporting and data system. American College of Radiology, Reston
Nicosia L, Bozzini AC, Palma S, Pesapane F, Meneghetti L, Pizzamiglio M, Abbate F, Latronico A, Bagnardi V, Frassoni S, Sangalli C, Cassano E (2023) Breast imaging reporting and data system and contrast enhancement mammography: lesion conspicuity likelihood of malignancy and relationship with breast tumor receptor status. Acad Radiol. https://doi.org/10.1016/j.acra.2023.02.008
Morris EA, Comstock CE, Lee CH, et al. (2013) ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston
McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT (2015) Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res 17(1):12. https://doi.org/10.1186/s13058-015-0519-x
Porter AJ, Evans EB, Foxcroft LM, Simpson PT, Lakhani SR (2014) Mammographic and ultrasound features of invasive lobular carcinoma of the breast. J Med Imaging Radiat Oncol 58(1):1–10. https://doi.org/10.1111/1754-9485.12080
Selinko VL, Middleton LP, Dempsey PJ (2004) Role of sonography in diagnosing and staging invasive lobular carcinoma. J Clin Ultrasound 32(7):323–332. https://doi.org/10.1002/jcu.20052
Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, Ioffe OB (2004) Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 233(3):830–849. https://doi.org/10.1148/radiol.2333031484
Mann RM, Hoogeveen YL, Blickman JG, Boetes C (2008) MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat 107(1):1–14. https://doi.org/10.1007/s10549-007-9528-5
Cocco D, ElSherif A, Wright MD, Dempster MS, Kruse ML, Li H, Valente SA (2021) Invasive lobular breast cancer: data to support surgical decision making. Ann Surg Oncol 28(10):5723–5729. https://doi.org/10.1245/s10434-021-10455-7
Chen Z, Yang J, Li S, Lv M, Shen Y, Wang B, Li P, Yi M, Zhao X, Zhang L et al (2017) Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS ONE 12:e0182397
Hogan MP, Amir T, Sevilimedu V, Sung J, Morris EA, Jochelson MS (2021) Contrast-enhanced digital mammography screening for intermediate-risk women with a history of lobular neoplasia. AJR Am J Roentgenol 216(6):1486–1491. https://doi.org/10.2214/AJR.20.23480
Amato F, Bicchierai G, Cirone D, Depretto C, Di Naro F, Vanzi E, Scaperrotta G, Bartolotta TV, Miele V, Nori J (2019) Preoperative loco-regional staging of invasive lobular carcinoma with contrast-enhanced digital mammography (CEDM). Radiol Med 124(12):1229–1237. https://doi.org/10.1007/s11547-019-01116-7
Jochelson MS, Lobbes MBI (2021) Contrast-Enhanced Mammography: state of the art. Radiology 299(1):36–48. https://doi.org/10.1148/radiol.2021201948
Cozzi A, Magni V, Zanardo M, Schiaffino S, Sardanelli F (2022) Contrast-Enhanced Mammography: a systematic review and meta-analysis of diagnostic performance. Radiology 302(3):568–581. https://doi.org/10.1148/radiol.211412
Acknowledgements
This work was partially supported by the Italian Ministry of Health Ricerca Corrente 5x1000 funds.
Funding
This study did not receive funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: LN, OB; AR; methodology: FP and ACB; validation: ACB and GP; resources: NF; EC; writing—original draft preparation: LN and OB; writing—review and editing: all authors. Statystical analysis: SF; VB; data managment: CS; supervision, GC and EC. All authors read and approved the Final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the local Institution (European Institute of Oncology, 20141, Milano).
Informed consent
All patients have signed a written informed consent (Protocol number IEO 960).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nicosia, L., Rotili, A., Pesapane, F. et al. Contrast-Enhanced Mammography (CEM) compared to Breast Magnetic Resonance (MRI) in the evaluation of breast lobular neoplasia. Breast Cancer Res Treat 203, 135–143 (2024). https://doi.org/10.1007/s10549-023-07096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07096-7