Abstract
Purpose
To determine whether tumor uptake of 18F-fluorodeoxyglucose (18F-FDG) is associated with invasive disease-free survival (IDFS) in patients with hormone receptor (HR)-positive ERBB2-negative early-stage breast cancer treated with adjuvant chemotherapy.
Methods
This is a single-center cohort study of women with breast cancer who underwent surgery between 2008 and 2015 at Asan Medical Center, Seoul, Korea. Patients were enrolled if they were diagnosed with HR-positive ERBB2-negative breast cancer with histology of invasive ductal carcinoma, had an American Joint Committee on Cancer pathologic tumor stage of T2N1 with 1–3 positive axillary nodes, underwent preoperative 18F-FDG positron emission tomography/computed tomography (PET/CT), and underwent breast cancer surgery followed by anthracycline- or taxane-based adjuvant chemotherapy. The primary outcome measure was IDFS. The maximum standardized uptake value (SUVmax) was dichotomized using a predefined cut-off of 4.14.
Results
A total of 129 patients were included. The median follow-up period for IDFS in those without recurrence was 82 months (interquartile range, 65–106). Multivariable Cox analysis showed that SUVmax was independently associated with IDFS [adjusted hazard ratio 2.49; 95% confidence interval (CI), 1.06–5.84]. Ten-year IDFS estimates via the Kaplan–Meier method were 0.60 (95% CI, 0.42–0.74) and 0.82 (95% CI, 0.65–0.91) for high and low SUVmax groups, respectively. The overall association between SUVmax and IDFS appeared to be consistent across subgroups divided according to age, progesterone receptor status, histologic grade, or presence of lymphovascular invasion.
Conclusion
High SUVmax on preoperative 18F-FDG PET/CT was independently associated with reduced long-term IDFS in T2N1 HR-positive ERBB2-negative breast cancer patients who underwent adjuvant chemotherapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hormone receptor (HR)-positive and ERBB2-negative breast cancer comprises about 70% of breast cancer [1]. Although this hormonal subtype shows a favorable short-term outcome, relapse can occur at any time in the 10–15-year post-operation, with a 15-year mortality rate of over 20% [2]. Adding chemotherapy to adjuvant endocrine therapy is generally associated with a 30% reduction in disease recurrence. However, the absolute benefit from adjuvant chemotherapy depends on the individual risk of recurrence [3]. The decision to use systemic adjuvant therapy requires consideration and balancing of the risk of disease recurrence with local therapy alone, the magnitude of benefit from applying adjuvant therapy, the predicted short- and long-term toxicities of the therapy, general health status, and comorbidities [4, 5].
In cases where the indications for adjuvant chemotherapy are uncertain, multigene assays such as the 21-gene expression assay (Oncotype-Dx), 70-gene signature (MammaPrint), 50-gene assay (Prosigna), 12-gene assay (EndoPredict), and Breast Cancer Index are recommended for assessing the risk of recurrence and appropriateness of systemic adjuvant chemotherapy [4, 5]. These gene assays are mainly based on estrogen receptor (ER)-signaling and proliferation-related pathway gene members [6] and are applicable to prognosis assessment in various therapeutic settings, including the receipt of adjuvant chemotherapy and patients with 1–3 positive lymph nodes [7, 8]. However, intratumoral genomic heterogeneity [9], frequent disagreement between multiple genomic assays [10, 11], and menstrual cycle- and menopause-associated changes in gene expression [12, 13] may potentially limit the clinical significance of prognostic gene assays. In addition, the cost-effectiveness of the 21-gene assay is still under debate [14].
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is an imaging modality frequently used for the preoperative staging of breast cancer [15]. It visualizes the enhanced glycolytic activity that is a metabolic hallmark of cancer and that provides the energy, molecules for biosynthesis, and reducing power required to maintain rapid proliferation [16]. The maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT shows strong associations with estrogen and progesterone receptor (PR) status, histologic grade, nodal metastasis, and recurrence score on the 21-gene assay for breast cancer [17,18,19]. Previous prognostic studies of HR-positive primary breast cancer showed that baseline 18F-FDG PET parameters were independently associated with recurrence or event-free survival [20,21,22]. However, the patient populations studied were heterogeneous and included patients with advanced stage or HER2-positive disease. In addition, optimum cut-off values were determined on the basis of patient outcome data and were not subsequently validated in an independent dataset. Evidence for the long-term prognostic value of SUVmax in early-stage HR-positive ERBB2-negative breast cancer should therefore still be considered to be exploratory.
Our previous research on patients with ER-positive ERBB2-negative breast cancer who underwent neoadjuvant chemotherapy demonstrated that SUVmax is an independent predictor of long-term clinical outcomes in terms of distant metastasis and death [23]. Although 18F-FDG PET/CT was performed in the neoadjuvant setting in this previous study, 18F-FDG metabolism reflected baseline prognostic features. The purpose of this study was to validate the prognostic value of SUVmax using a separate cohort of HR-positive ERBB2-negative patients who were treated with adjuvant chemotherapy. The primary objective of this study was to determine whether tumor SUVmax categorized as high or low according to a cut-off determined in our previous study can contribute independent prognostic information on invasive disease-free survival (IDFS) in patients with breast cancer. The studied population included women diagnosed with early-stage HR-positive ERBB2-negative breast cancer with one to three positive lymph nodes, in whom gene expression assays are usually indicated to assess prognosis [5]. The prespecified hypothesis tested was that high SUVmax levels in the tumor at diagnosis are associated with shorter IDFS. The secondary objective was to examine whether SUVmax is associated with distant relapse-free survival (DRFS) and overall survival (OS).
Methods
Study design, setting, and patients
This is a single-center cohort study of women with breast cancer who underwent surgery between January 2008 and December 2015 at Asan Medical Center, Seoul, Republic of Korea. During this period, 18F-FDG PET/CT was performed preoperatively, in addition to the standard staging studies. Patients were identified from the local database of the Department of Breast Surgery. Electronic medical records and PET/CT images were reviewed by the authors, who have more than 5 years of experience in breast cancer surgery or PET/CT imaging. Risk factors were assessed in relation to outcomes that had already occurred at the start of the study. Follow-up ended on January 13, 2021. Our local institutional review board approved the study protocol and waived the need for informed patient consent (2020-1648). This study was conducted in accordance with the Declaration of Helsinki and our institutional guidelines.
All the female patients of the study cohort were evaluated for study eligibility. Patients were enrolled if they were diagnosed with HR-positive ERBB2-negative breast cancer with invasive ductal carcinoma histology, had an American Joint Committee on Cancer pathologic tumor stage of T2N1 with 1–3 positive axillary nodes, underwent preoperative 18F-FDG PET/CT, and had breast cancer surgery followed by anthracycline- or taxane-based adjuvant chemotherapy. Patients were excluded if they had double primary malignancy or bilateral breast cancer. The number of patients enrolled during the study period determined the sample size of this study.
PET/CT image acquisition and analysis
Patients fasted for at least 6 h before the PET/CT scanning and had a venous blood glucose level of less than 150 mg/dl. PET imaging was performed from the skull base to the mid-thigh at 50–70 min after intravenous injection of 5.2–7.4 MBq/kg of 18F-FDG using one of the following scanners: Discovery STe 8, Discovery 690, Discovery 690 Elite, Discovery 710 (GE Healthcare, Waukesha, WI, USA), Biograph Sensation 16, or Biograph TruePoint 40 (Siemens Healthineers, Erlangen, Germany). PET/CT images were reconstructed using an ordered-subset expectation-maximization algorithm with attenuation correction using CT maps.
A volume of interest was manually drawn on either the primary breast cancer or metastatic lymph nodes to assess the SUVmax of the tumor. This volume of interest was drawn by a board-certified nuclear medicine physician in a blinded manner using our in-house software ANTIQUE (Asan Medical Center Nuclear Medicine Image Quantification Toolkit of Excellence). The SUVmax was harmonized across different PET/CT scanners using a previously described technique [23, 24]. In brief, the recovery coefficient profiles of variable hot cylinders of American College of Radiology-approved PET phantoms (Data Spectrum, Hillsborough, NC, USA) were compared between PET scanners [25, 26]. By resampling and smoothing with Gaussian kernels, PET images from the higher-resolution scanners were matched to those of the lower-resolution scanners. The spatial resolution of the harmonized PET images was approximately 8-mm full-width-half-maximum.
Variables
The primary outcome measure of this study was IDFS [8, 27, 28]. The secondary outcomes included DRFS and OS. All survival measures used in this study adhere to the Standardized Definitions for Efficacy End Points (STEEP) system [29]. IDFS was defined as the interval from the date of surgery to locoregional recurrence, distant metastasis, death from any cause, or secondary primary invasive cancer. DRFS was measured until the date of occurrence of distant metastasis or death from any cause. OS was defined as the time between surgery and death from any cause. Patients without events were censored on the date of the last follow-up.
Potential predictors prespecified in the study protocol included age, histologic grade, ER/PR status, and the presence of lymphovascular invasion [30,31,32,33]. The prognostic significance of the type of breast surgery, chemotherapy regimen, and radiation treatment was also explored. SUVmax values were dichotomized using a predefined cut-off value of 4.14 determined in our previous study [23]. Patients were also dichotomized according to age and histologic grade using commonly used cut-off values relevant for prognosis: age of 20–50 vs. > 50 years [4, 30, 31] and histologic grade of 1–2 vs. 3 [34, 35]. According to the National Comprehensive Cancer Network guideline, ER and PR were considered positive if more than or equal to 1% of cancer cells were positive on immunohistochemical HR testing [5]. Among the ER-positive tumors, those with 1–10% positive cells were regarded as ER low positive. ERBB2 was considered negative when a result of 0 or 1+ was obtained on immunohistochemistry or a result of 2+ on immunohistochemistry with negative on subsequent fluorescence or silver-enhanced in situ hybridization testing [36].
Statistical analysis
Continuous variables are described as median and interquartile range (IQR) and categorical variables as number (%). Two-sided P values of less than 0.05 were considered statistically significant. The Wilcoxon rank-sum test or Kruskal–Wallis test was used to compare continuous variables across groups. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test. The Spearman rank correlation test was used to evaluate associations between two variables.
Survival analyses were predetermined for the primary objectives in the study protocol. Survival curves were estimated using the Kaplan–Meier method and were compared using the log-rank test. Univariable and multivariable Cox proportional hazards regression analyses were performed. The multivariable Cox regression analysis used stepwise model selection based on the Akaike information criterion. Crude and adjusted hazard ratios and 95% confidence intervals (CIs) were derived. The proportional hazards assumption was checked using the log-minus-log plot and Schoenfeld’s residual test. The possibility for influential observations was examined using deviance residuals and dfbeta values. Post hoc extended Cox proportional hazards analyses were performed to explore whether overall associations appeared consistent across all subgroups according to the aforementioned potential predictors of survival. Statistical tests were performed using R software (version 4.0.5, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
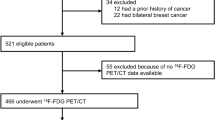
Of 524 initially identified patients, 129 who underwent preoperative 18F-FDG PET/CT were included in our analysis (Fig. 1). The patient characteristics are described in Table 1. The demographics of the included patients and those without 18F-FDG PET/CT were comparable (Supplementary table 1). The median age was 47 years (IQR 40–55). The median time between 18F-FDG PET/CT and surgery was 9 days (IQR, 4–18). The 21-gene assay was performed in 17 patients. Patients received anthracycline- and taxane-based (n = 104), anthracycline-based (n = 11) or taxane-based (n = 10) adjuvant chemotherapy, followed by hormonal therapy with selective ER modulator and/or aromatase inhibitor, except for one patient planning for pregnancy. The remaining four patients received chemotherapy with unknown regimens at outside hospitals.
18F-FDG PET/CT and harmonized SUVmax
The median blood glucose level before 18F-FDG injection was 101 mg/dL (IQR, 92–111). The administered dose of 18F-FDG was 363 MBq (IQR, 289–444). PET/CT imaging was performed a median of 59 min (IQR 55–62) after 18F-FDG injection. The SUVmax was measured in primary breast tumors in 118 patients and axillary lymph nodes in 11 patients. There were no clinical and pathologic difference between the two groups (Supplementary table 2). The median harmonized SUVmax was 4.58, with IQR ranging from 3.08 to 6.82. The harmonized SUVmax was significantly higher in tumors with histologic grade 3 than in those with grades 1–2 [median 5.68 (IQR 4.30–7.46) vs. 4.22 (IQR 2.75–6.40), P = 0.009], but was not associated with primary tumor size (rho = 0.09, P = 0.311), number of positive lymph nodes (P = 0.516), PR status (P = 0.991), or lymphovascular invasion (P = 0.553). It was also not associated with recurrence score on the 21-gene assay (rho = 0.22, P = 0.399).
Survival analysis
The median follow-up periods for patients without relevant events were 82 months (IQR 65–106) for IDFS, 83 months (IQR 65–104) for DRFS, and 95 months (IQR 74–117) for OS. There were a total of 29 events for IDFS, 18 for DRFS, and 11 for OS during the follow-up period.
Univariable Cox proportional hazards regression analyses showed that a high SUVmax above 4.14 was associated with worse IDFS [Table 2, crude hazard ratio 2.51 (95% CI, 1.07–5.87)], whereas the location of SUVmax, age, histologic grade, PR status, lymphovascular invasion, type of breast surgery, chemotherapy regimen and radiation treatment were not. In the stepwise multivariable Cox analysis, SUVmax was independently associated with IDFS [adjusted hazard ratio 2.49 (95% CI, 1.06–5.84)]. Survival curves for IDFS stratified by the SUVmax cut-off of 4.14 are shown in Fig. 2. Ten-year IDFS estimates via the Kaplan–Meier method for high and low SUVmax groups were 0.60 (95% CI, 0.42–0.74) and 0.82 (95% CI, 0.65–0.91), respectively. The overall association between SUVmax and IDFS appeared to be consistent across subgroups divided according to age, PR status, histologic grade, and the presence of lymphovascular invasion (Fig. 3).
Regarding DRFS and OS, patients with low SUVmax tended to have longer DRFS or OS, but the differences were not statistically significant (Supplementary Fig.). The 10-year survival rates of high and low SUVmax groups were 0.73 (95% CI, 0.59–0.90) and 0.88 (95% CI, 0.79–0.99), respectively, for DRFS and 0.87 (95% CI, 0.79–0.97) and 0.94 (95% CI, 0.89–1.00) for OS. In the univariable Cox regression analyses, no other variables were significantly associated with DRFS or OS (Supplementary table 3).
Discussion
The present study evaluated the prognostic value of 18F-FDG PET/CT in patients with early-stage HR-positive ERBB2-negative breast cancer. Considering the spatial resolution of the PET scanners and the prognostic relevance of SUVmax, we studied patients with T2N1 breast cancer. Using a predetermined cut-off value identified in a previous neoadjuvant study, we demonstrated that the SUVmax of 18F-FDG PET/CT was of independent prognostic value in IDFS. To the best of our knowledge, this is the first study to confirm the long-term prognostic value of 18F-FDG PET/CT for early breast cancer of the HR-positive ERBB2-negative subtype. Patients with high-tumor 18F-FDG metabolism should be advised to strictly adhere to their ongoing screening and medication.
Unlike our previous study in a neoadjuvant setting, this study included a cohort of patients who received adjuvant chemotherapy. Although randomized trials demonstrated a similar long-term prognosis when patients were given the same treatment preoperatively compared with postoperatively [5, 37], there were no patients with advanced stages in this study. However, gene expression studies revealed that primary tumor and metastatic node samples from the same patient are usually more similar than those between patients, indicating that the primary tumor’s molecular program is retained in advanced tumors [38]. In addition, multigene assays provide the same prognostic information even in patients with lymph node metastasis [39, 40]. Therefore, it is likely that prognostic information provided by 18F-FDG metabolism may be applied regardless of tumor stage. Furthermore, the population enrolled in this study had similar ER and ERBB2 characteristics to the population in our previous neoadjuvant study and the patients were treated in a similar manner, which indicates that the validation obtained in this study should be legitimate. Therefore, our validation of SUVmax in this separate patient population suggests that 18F-FDG PET/CT is reliable and that SUVmax is likely to be of prognostic value in HR-positive ERBB2-negative patients.
An important question is whether our results on the prognostic value of SUVmax in patients who underwent adjuvant chemotherapy can be applied to those without adjuvant chemotherapy. The prognostic value of SUVmax would be more clinically relevant if it allows determination of those patients who would benefit or not from adjuvant chemotherapy. Previous studies investigating multigene prognostic studies in HR-positive breast cancer after chemotherapy have shown that survival is influenced by baseline biological features and sensitivity to endocrine therapy [41,42,43,44]. Sensitivity to chemotherapy does not fully compensate for a poor prognosis and low endocrine sensitivity. Therefore, although 18F-FDG PET/CT was performed in patients who received adjuvant chemotherapy, the 18F-FDG metabolism measured in this study might reflect baseline prognostic features. Our results suggest the complementary use of SUVmax to identify a high-risk population in the adjuvant setting if prognostic gene assays are not available. Prognostication based on SUVmax can be simply performed without additional cost in patients who undergo pretreatment 18F-FDG PET/CT for staging purposes, with SUVmax being the most simple and widely used PET parameter in clinical practice. Further studies are warranted to establish the prognostic role of 18F-FDG PET/CT in patients who undergo adjuvant endocrine therapy.
Our study is subjected to several limitations. First, it is retrospective in nature. However, the baseline characteristics of the patients who underwent 18F-FDG PET/CT were not significantly different from those who did not undergo 18F-FDG PET/CT. We enrolled a consecutive series of eligible patients and used predetermined statistical methods for analysis of the primary objectives to minimize possible selection or information bias. Second, we did not show statistical significance in the analysis of DRFS and OS, with there being rather low numbers of events for these secondary endpoints. Third, caution is required when applying our harmonized SUVmax cut-off of 4.14 to other PET centers using different PET scanners. SUVmax is a single-voxel value that shows inter-scanner variability with different resolution, acquisition, and reconstruction parameters [45]. Our harmonization method might be suitable for overcoming the generalizability issue surrounding the use of SUVmax as a prognostic biomarker.
Conclusion
High SUVmax on preoperative 18F-FDG PET/CT was independently associated with reduced long-term IDFS in patients with T2N1 HR-positive ERBB2-negative breast cancer who underwent adjuvant chemotherapy. Therefore, patients with high-tumor 18F-FDG metabolism should be advised to strictly adhere to their ongoing screening and medication.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P (2020) Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US from 2010 to 2016. JAMA Netw Open 3(8):e2013226. https://doi.org/10.1001/jamanetworkopen.2020.13226
Davies C, Godwin J, Gray R et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Peto R, Davies C, Godwin J et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444. https://doi.org/10.1016/S0140-6736(11)61625-5
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E (2019) Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194–1220. https://doi.org/10.1093/annonc/mdz189
Gradishar WJ, Moran MS, Abraham J et al Breast Cancer, Version 5.2021, NCCN Clinical Practice Guidelines in Oncology
Wirapati P, Sotiriou C, Kunkel S et al (2008) Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 10(4):R65. https://doi.org/10.1186/bcr2124
Piccart M, van ’t Veer LJ, Poncet C, et al (2021) 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol 22(4):476–488. https://doi.org/10.1016/s1470-2045(21)00007-3
Kalinsky K, Barlow WE, Meric-Bernstam F et al (2021) Abstract GS3-00: First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/- chemotherapy (CT) in patients (pts) with 1-3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer (BC) with recurrence score (RS)< 25: SWOG S1007 (RxPonder). Cancer Research 81(4):3. https://doi.org/10.1158/1538-7445.SABCS20-GS3-00
Karthik GM, Rantalainen M, Stålhammar G et al (2017) Intra-tumor heterogeneity in breast cancer has limited impact on transcriptomic-based molecular profiling. BMC Cancer 17(1):802. https://doi.org/10.1186/s12885-017-3815-2
Sestak I, Buus R, Cuzick J et al (2018) Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 4(4):545–553. https://doi.org/10.1001/jamaoncol.2017.5524
Vallon-Christersson J, Häkkinen J, Hegardt C et al (2019) Cross comparison and prognostic assessment of breast cancer multigene signatures in a large population-based contemporary clinical series. Sci Rep 9(1):12184. https://doi.org/10.1038/s41598-019-48570-x
Haynes BP, Viale G, Galimberti V, Rotmensz N, Gibelli B, A’Hern R, Smith IE, Dowsett M (2013) Expression of key oestrogen-regulated genes differs substantially across the menstrual cycle in oestrogen receptor-positive primary breast cancer. Breast Cancer Res Treat 138(1):157–165. https://doi.org/10.1007/s10549-013-2426-0
Hosoda M, Yamamoto M, Nakano K, Hatanaka KC, Takakuwa E, Hatanaka Y, Matsuno Y, Yamashita H (2014) Differential expression of progesterone receptor, FOXA1, GATA3, and p53 between pre- and postmenopausal women with estrogen receptor-positive breast cancer. Breast Cancer Res Treat 144(2):249–261. https://doi.org/10.1007/s10549-014-2867-0
Wang SY, Dang W, Richman I, Mougalian SS, Evans SB, Gross CP (2018) Cost-effectiveness analyses of the 21-gene assay in breast cancer: systematic review and critical appraisal. J Clin Oncol 36(16):1619–1627. https://doi.org/10.1200/jco.2017.76.5941
Han S, Choi JY (2021) Impact of 18F-FDG PET, PET/CT, and PET/MRI on staging and management as an initial staging modality in breast cancer: a systematic review and meta-analysis. Clin Nucl Med 46(4):271–282. https://doi.org/10.1097/rlu.0000000000003502
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11(2):85–95. https://doi.org/10.1038/nrc2981
Ahn SG, Lee JH, Lee HW et al (2017) Comparison of standardized uptake value of 18F-FDG-PET-CT with 21-gene recurrence score in estrogen receptor-positive, HER2-negative breast cancer. PLoS One 12(4):e0175048. https://doi.org/10.1371/journal.pone.0175048
Jin S, Kim SB, Ahn JH et al (2013) 18 F-fluorodeoxyglucose uptake predicts pathological complete response after neoadjuvant chemotherapy for breast cancer: a retrospective cohort study. J Surg Oncol 107(2):180–187. https://doi.org/10.1002/jso.23255
Yoo J, Kim BS, Yoon HJ (2018) Predictive value of primary tumor parameters using (18)F-FDG PET/CT for occult lymph node metastasis in breast cancer with clinically negative axillary lymph node. Ann Nucl Med 32(9):642–648. https://doi.org/10.1007/s12149-018-1288-2
Ahn SG, Lee M, Jeon TJ, Han K, Lee HM, Lee SA, Ryu YH, Son EJ, Jeong J (2014) [18F]-fluorodeoxyglucose positron emission tomography can contribute to discriminate patients with poor prognosis in hormone receptor-positive breast cancer. PLoS One 9(8):e105905. https://doi.org/10.1371/journal.pone.0105905
Higuchi T, Nishimukai A, Ozawa H et al (2016) Prognostic significance of preoperative (18)F-FDG PET/CT for breast cancer subtypes. Breast 30:5–12. https://doi.org/10.1016/j.breast.2016.08.003
Groheux D, Martineau A, Teixeira L, Espie M, de Cremoux P, Bertheau P, Merlet P, Lemarignier C (2017) (18)FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res 19(1):3. https://doi.org/10.1186/s13058-016-0793-2
Chae SY, Park SH, Lee HS et al (2022) Association between tumor (18)F-fluorodeoxyglucose metabolism and survival in women with estrogen receptor-positive, HER2-negative breast cancer. Sci Rep 12(1):7858. https://doi.org/10.1038/s41598-022-11603-z
Lee HS, Oh JS, Park YS, Jang SJ, Choi IS, Ryu JS (2016) Differentiating the grades of thymic epithelial tumor malignancy using textural features of intratumoral heterogeneity via (18)F-FDG PET/CT. Ann Nucl Med 30(4):309–319. https://doi.org/10.1007/s12149-016-1062-2
Lasnon C, Desmonts C, Quak E, Gervais R, Do P, Dubos-Arvis C, Aide N (2013) Harmonizing SUVs in multicentre trials when using different generation PET systems: prospective validation in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 40(7):985–996. https://doi.org/10.1007/s00259-013-2391-1
Boellaard R, Delgado-Bolton R, Oyen WJ et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42(2):328–354. https://doi.org/10.1007/s00259-014-2961-x
Sparano JA, Gray RJ, Makower DF et al (2015) Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 373(21):2005–2014. https://doi.org/10.1056/NEJMoa1510764
Sparano JA, Gray RJ, Makower DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Hudis CA, Barlow WE, Costantino JP et al (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25(15):2127–2132. https://doi.org/10.1200/JCO.2006.10.3523
Andre F, Ismaila N, Henry NL et al (2019) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol 37(22):1956–1964. https://doi.org/10.1200/JCO.19.00945
Henry NL, Somerfield MR, Abramson VG et al (2019) Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: update of the ASCO endorsement of the cancer care Ontario guideline. J Clin Oncol 37(22):1965–1977. https://doi.org/10.1200/JCO.19.00948
Jung SU, Sohn G, Kim J et al (2019) Survival outcome of adjuvant endocrine therapy alone for patients with lymph node-positive, hormone-responsive, HER2-negative breast cancer. Asian J Surg 42(10):914–921. https://doi.org/10.1016/j.asjsur.2019.01.003
Bae YK, Gong G, Kang J, Lee A, Cho EY, Lee JS, Suh KS, Lee DW (2012) Hormone receptor expression in invasive breast cancer among Korean women and comparison of 3 antiestrogen receptor antibodies: a multi-institutional retrospective study using tissue microarrays. Am J Surg Pathol 36(12):1817–1825. https://doi.org/10.1097/PAS.0b013e318267b012
Trudeau ME, Pritchard KI, Chapman JA et al (2005) Prognostic factors affecting the natural history of node-negative breast cancer. Breast Cancer Res Treat 89(1):35–45. https://doi.org/10.1007/s10549-004-1368-y
Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, Blamey RW, Ellis IO (2008) Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol 26(19):3153–3158. https://doi.org/10.1200/JCO.2007.15.5986
Wolff AC, Hammond MEH, Allison KH et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/jco.2018.77.8738
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26(5):778–785. https://doi.org/10.1200/JCO.2007.15.0235
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Albain KS, Barlow WE, Shak S et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65. https://doi.org/10.1016/S1470-2045(09)70314-6
Cardoso F, van’t Veer LJ, Bogaerts J et al (2016) 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–729. https://doi.org/10.1056/NEJMoa1602253
Hatzis C, Pusztai L, Valero V et al (2011) A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA 305(18):1873–1881. https://doi.org/10.1001/jama.2011.593
Parker JS, Mullins M, Cheang MC et al (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160–1167. https://doi.org/10.1200/JCO.2008.18.1370
Liedtke C, Hatzis C, Symmans WF et al (2009) Genomic grade index is associated with response to chemotherapy in patients with breast cancer. J Clin Oncol 27(19):3185–3191. https://doi.org/10.1200/JCO.2008.18.5934
Iwamoto T, Lee JS, Bianchini G et al (2011) First generation prognostic gene signatures for breast cancer predict both survival and chemotherapy sensitivity and identify overlapping patient populations. Breast Cancer Res Treat 130(1):155–164. https://doi.org/10.1007/s10549-011-1706-9
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumor imaging. J Nucl Med 48(6):932–945. https://doi.org/10.2967/jnumed.106.035774
Funding
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HR18C0016). The funders had no role in the conceptualization or design of the study; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Material preparation and data collection were performed by SBL, GG, and SYC. Data analyses were performed by SH, JL, and JSO. Conception and interpretation of data were performed by DHM. The first draft of the manuscript was written by SH, and all authors commented on early versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki and was approved by the institutional review board of Asan Medical Center (2020-1648).
Consent to participate
The institutional review board of Asan Medical Center (2020-1648) waived the need for informed consent.
Consent for publication
The institutional review board of Asan Medical Center (2020-1648) approved publication of the study results to the research community.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, S., Lee, S.B., Gong, G. et al. Prognostic significance of pretreatment 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with T2N1 hormone receptor-positive, ERBB2-negative breast cancer who underwent adjuvant chemotherapy. Breast Cancer Res Treat 198, 207–215 (2023). https://doi.org/10.1007/s10549-022-06852-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06852-5