Abstract
Background
Studies evaluating role of BRCA mutations on the survival outcomes in breast cancer (BC) patients have given confounding results and hence, in this meta-analysis, we assessed the impact of BRCA mutations on survival in BC patients.
Methods
Studies comparing survival outcomes of BC patients having BRCA mutations against wildtype BRCA phenotype were retrieved from PubMed, EMBASE, and Cochrane Library. Overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS), and breast cancer-specific survival (BCCS) were the outcomes. Hazard ratio (HR) with 95% confidence interval (CI) was used for analysis. Subgroup analysis was performed for survival based on triple negative breast cancer (TNBC) and follow-up durations. The meta-analysis was performed as per PRISMA guidelines.
Results
Altogether, 30 articles with 35,972 patients (mean age 45.6 years) were included. Patients with BRCA 1 mutation had significantly lower OS (HR [95% CI] 1.2 [1.08, 1.33]; P < 0.001), BRCA 2 mutation had significantly lower DFS (HR [95% CI] 1.35 [1.1, 1.67]; P = 0.0049) and BCSS (HR [95%CI] 1.46 [1.26, 1.7]; P < 0.0001), and TNBC patients with BRCA 1 mutation had significantly poor DFS (HR [95% CI] 1.65 [1.08, 2.54]; P = 0.0216). Based on follow-up duration, the OS in BRCA 1-mutated patients revealed significantly poorer outcomes in studies with ≤ 5 years (HR 1.48) and > 5 years (HR 1.14) of follow-up. In BRCA 2 -mutated patients, the OS was significantly poorer in studies with > 5 years of follow-up (HR 1.39, P < 0.05).
Conclusion
BC patients with BRCA 1 or BRCA 2 mutations had poor survival outcomes and hence screening patients with BC for BRCA mutations might help in strategizing their treatment and improving their survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, breast cancer (BC) is the second most frequent cancer and first most frequent gynecological cancer affecting women with a relatively low case-mortality rate [1]. The incidence of breast cancer has been previously reported to be influenced by diet, reproductive pattern, and social economy [2,3,4]. Genetic mutation in specific genes increases both the probability of acquiring breast cancer and may influences the severity [5]. BReast CAncer genes (BRCA) 1 and 2 are genes encoding DNA repair enzymes and were named due to their association with breast cancer [6,7,8]. Germline mutation in BRCA 1 and 2 increases the probability of breast and ovarian cancer in comparison to women with wildtype BRCA [9, 10]. However, there is no consensus on the severity and prognosis of breast cancer in patients with somatic or germline BRCA mutations with contradictory reports [9, 11]. The incidence of germline BRCA mutations is reported to be affected by ethnicity and Ashkenazi Jews were more prone to acquiring BRCA mutations [12]. Previous studies have also observed varying prognosis in different populations with BRCA mutations which also complicates the clinical course of BC in BRCA-mutated patients with BC. The management of BC in BRCA-mutated patients was addressed by a previous systematic qualitative review by Liebens et al. who concluded that BRCA mutation may not provide additional prognostic information. They included a total of 20 relevant studies providing information about BRCA mutation status and therapeutic management [13]. But no quantitative appraisal of evidences was done in their study. Another systematic review by Lee et al. with 11 studies concluded that BRCA 1 mutation decreases short-term and long-term overall survival (OS) whereas BRCA2 mutation neither affects short-term nor long-term survival rate [14]. But the study did not account for triple negative breast cancer (TNBC) which is a subtype of BC with an incidence of 10–20% of all BC cases. Similarly, a recent meta-analysis by Baretta et al. addressed the prognostic ability of BRCA mutation testing in patients with germline BRCA mutation. Although the analysis was extensive with 60 studies included for analysis in multiple subgroup of patients, they used statistical methods to extrapolate the effect estimates from the Kaplan Meier curves for survival which may not provide a precise effect estimate. Nevertheless, the study had higher statistical power owing to the large number of included studies [15].
The studies included in the previous meta-analyses provided diverse endpoints which may impact the overall conclusion. Among the different factors that influence survival outcomes, TNBC status and BRCA mutation status are reported to be independent risk factors. TNBC patients are particularly difficult to treat because of the aggressive nature of the cancer which has high probability for local and distant recurrence. Further, inefficacy of hormone and endocrine inhibitors in TNBC patients reduces the available therapeutic repertoire [16], whereas BRCA mutation presents with a different set of challenges with respect to severity that impedes the clinical outcomes. In previous studies, TNBC patients with BRCA mutation were found to be younger with non-significant findings on the survival outcomes [17]. The difference in the association of BRCA mutations in TNBC and HR+/ER+/HER+ patients suggests a role for BRCA mutations in driving TNBC status.
The main reasons for the lack of consensus with respect to the precise role of BRCA mutations in predicting survival are the TNBC status, variable end points assessed in each study, and the follow-up period. These factors influence the prognostic ability of BRCA mutations. TNBC patients have been reported to experience better outcomes in the presence if BRCA mutations in a few retrospective studies. Except for the study by Baretta et al., all the other meta-analyses utilized OS as the study endpoint which may not give a comprehensive overview of BRCA mutation on different survival endpoints. Moreover, previous studies have also not accounted for the difference in survival endpoints based on the length of follow-up. Hence, in this study, the relationship between BRCA mutations and prognosis in patients with breast cancer was assessed using comprehensive endpoints in different patient subgroups including TNBC and follow-up period.
Materials and methods
Search strategy and inclusion criteria
Systematic search was performed in PubMed, EmBase, and Cochrane with the following search terms: “breast” AND (“neoplasms” or “cancer” or “tumor” or “carcinoma” or “neoplasms”) AND (“BRCA1” or “BRCA2” or “BRCA”) AND (“survival” or “outcome” or “mortality” or “relapse”). The databases were searched for studies published in English providing BRCA mutational status published till June 2019. References of the included studies were manually screened for additional studies. The systematic search and screening were performed by two independent reviewers and the inconsistencies were settled by mutual consensus or by a third reviewer. Studies were included based on the following criteria: (1) study population: breast cancer patients; (2) study design: observational studies (retrospective cohort, prospective cohort, and case–control studies); (3) studies reporting BRCA1 or BRCA2 mutation on exposure; and (4) studies reporting survival outcomes along with the hazards ratio (HR) for the comparison of patients with BRCA 1 or 2 mutation and wild type. Reviews, case reports, meta-analysis, studies with insufficient data, and those reporting outcomes other than the ones specified above were excluded. If same cohort of patients were used in multiple studies, then only the study with the updated or comprehensive data was included for analysis. Secondary search was also done in the reference list of all the included articles for relevant studies missed in the initial search. The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement and was registered in PROSPERO (CRD42019134563) [18, 19]. We intended to include only observational studies, since interventional studies (randomized controlled studies on a particular intervention) will provide outcomes based on the presence or absence of a particular intervention and not on the basis of BRCA mutation. Even if studies report outcomes stratified by BRCA mutation status, the intervention used in multiple studies will lead to significant confounding which may not be possible to avoid or minimize.
Data collection and quality assessment
Data collection and quality assessment were performed by two independent authors and disparities were settled by a third independent reviewer. The data extracted include demographic data including sample size, number and nature of BRCA mutants, disease status, mean age, investigated outcomes, and adjusted factors. The survival endpoints analyzed in this study included overall survival (OS), disease-free survival (DFS), breast cancer-specific survival (BCSS), and distant metastatic-free survival (DMFS). The hazards ratios (HRs) and the corresponding 95% confidence intervals (CIs) for different endpoints were extracted. The methodological quality was assessed with the Newcastle–Ottawa Scale (NOS) based on selection (four items with a total of four stars), comparability (one item with a total of two stars), and outcome (three items with a total of three stars) with a total of nine stars that were developed for assessment [20]. Studies with a score of > 7 were considered to be of high quality. Publication bias was assessed by visual inspection of the funnel plots.
Statistical analysis
The effect of BRCA1/2 mutation on the survival outcomes was evaluated in patients with breast cancer based on the effect estimates (HR) and corresponding 95% CIs in individual studies. The HRs derived from univariate/multivariable Cox proportional hazards model were used to arrive at the cumulative HRs by either fixed-effects or random-effect model based on the inter study heterogeneity assessed by the I2 statistic. The overall HRs for the comparison groups were calculated using random-effects model if the heterogeneity was higher (I2 > 50%), and using fixed-effects model in case of lower heterogeneity (I2 ≤ 50%). Based on the nature of the included studies, the analysis was divided into three parts: (1) BRCA1-mutated vs BRCA 1 wild type, (2) BRCA 2-mutated vs BRCA 2 wild type, and (3) BRCA 1/2-mutated vs BRCA 1/2 wild type. Each analysis part included the following survival endpoints: (1) OS, (2) DFS, (3) BCSS, and (4) DMFS. Studies with TNBC patients were analyzed as a separate subgroup. Two tailed P values were computed and P value < 0.05 was considered statistically significant. Statistical analyses were conducted using R software (version 3.4.2; https://www.r-project.org/).
Results
Nature of included studies and patient population
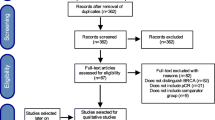
Based on the search strategy, a total of 8136 studies were retrieved from the databases and after removal of duplicates, a total of 4646 studies were included for screening (Fig. 1). Based on the abstracts, 85 articles were further selected for full text evaluation and 30 articles with data comparing survival outcomes of patients with BRCA-mutated BC and patients with wildtype BRCA BC were included for the analysis. A total of 35,972 patients (32,308 wildtype BRCA patients and 3402-mutated BRCA patients) were included in the analysis (Table 1). Of these studies, 8 studies were prospective cohort studies, whereas 22 were retrospective cohort studies. The mean age of the population was 45.6 years (Table 1). All the included studies considered only known BRCA mutations of clinical significance as per (as per BRCA exchange database).
Assessment of heterogeneity
Since the studies included for the systematic appraisal of evidences were all observational studies, significant heterogeneity, with respect to assessment of the endpoints in the individual studies, was expected. There was no clinical heterogeneity among the included studies with respect to the PICO framework. TNBC, which could be a source of heterogeneity, was assessed in subgroup analysis. The other probable sources of heterogeneity include therapeutic standard of care and the mode of assessing BRCA mutations. We could not ascertain the heterogeneity introduced by the mode of BRCA testing and the therapeutic standard of care because of lack of necessary data in the included studies.
The risk of bias/methodological assessment by NOS revealed all except the study by Bayraktar et al. [21], and Lang et al. [22], to be of high quality. Both the studies had a follow-up duration of < 3 years. Publication bias, as estimated by visual inspection of funnel plots for OS, revealed relatively lesser publication bias as depicted in the resultant funnel plots as standard error for all were within acceptable range (funnel), except for one study included in the analysis in BRCA1 group (Fig. 2).
Association of BRCA 1 mutation on survival outcomes
A total of 13 studies had compared OS of BC patients with BRCA 1 mutation with that of patients with wildtype BRCA 1. The analysis using FE model (I2 = 14.68%; P = 0.1628) revealed that BC patients with BRCA 1 mutation had significantly poor OS in comparison to that of patients with wildtype BRCA 1 (HR 1.2; 95% CI 1.08, 1.33; P = 0.0008) (Fig. 3). A total of 7, 9, and 9 studies evaluated DFS, BCSS, and DMFS, respectively. Although the effect estimates were better in wildtype BRCA 1 patients, there was no statistical significance (Table 2).
Association of BRCA 2 mutation on survival outcomes
A total of nine studies have compared survival outcomes of BC patients with BRCA 2 mutation with those of patients with wildtype BRCA 2. The meta-analysis (FE model; I2 = 0%; P = 0.3479) revealed better OS in patients with wildtype BRCA 2 than patients with BRCA 2 mutation (HR 1.17; 95% CI 0.99, 1.39; P = 0.0591) (Fig. 4a), although statistically non-significant. Four studies evaluated BRCA 2 mutation-associated DFS in patients with BC. The analysis using FE model (I2 = 0%; P = 0.4746) showed significantly better DFS of patients with wildtype BRCA 2 compared with that of patients with BRCA 2 mutation (HR 1.35; 95% CI 1.1, 1.67; P = 0.0049) (Fig. 4b). Comparison of BCSS was assessed by five studies and the analysis by FE model revealed significantly better BCSS in patients with wildtype BRCA 2 than patients with BRCA 2 mutation (HR 1.46; 95% CI 1.26, 1.7; P < 0.0001) (Fig. 4c). DMFS, which was assessed in four studies, did not reveal statistically significant difference in both the groups (Table 2).
Association of BRCA 1/2 mutation on survival outcomes
Studies included in the meta-analysis also compared survival outcomes in patients with either BRCA 1 mutation or BRCA 2 mutation in comparison to patients with wildtype BRCA without stratifying the patients into BRCA 1 mutation and BRCA 2 mutation. OS, DFS, and DMFS were not statistically significant among the groups (Table 2).
Survival outcomes in triple negative breast cancer
The DFS was significantly poor in TNBC patients with BRCA 1 mutation as compared to TNBC patients with BRCA 1 wild type (n = 2; FE model; HR 1.65; 95% CI 1.08, 2.54; P = 0.0216). Statistically significant difference was not observed for the other endpoints (Table 3.)
Follow-up duration associated with survival outcomes
Follow-up duration was evaluated as possible factor associated with survival in BRCA-mutated patients. Studies were divided based on the reported median follow-up into ≤ 5 years and > 5 years. A total of 4 studies with ≤ 5 years of follow-up and 8 studies with > 5 years of follow-up reported the OS in BRCA 1-mutated vs BRCA 1 wildtype patients. While the summary HR was 1.48 (P < 0.05) in studies with ≤ 5 years of follow-up, it was reduced to 1.14 (P < 0.05) in studies with > 5 years of follow-up. Similarly, a total of 3 studies with ≤ 5 years of follow-up and 6 studies > 5 years of follow-up reported OS in BRCA 2 -mutated vs BRCA 2 wildtype patients. The summary HR in studies with ≤ 5 years of follow-up did not reveal significant difference (0.68, P > 0.05) whereas in studies with > 5 years of follow-up duration, the summary HR was 1.39 (P < 0.05) signifying worst outcomes and recurrence in BRCA 2-mutated patients after longer follow-up (Table 4).
Discussion
BRCA 1 and BRCA 2 genes are tumor suppressor genes whose protein products plays an important role in the homologous recombination that predominantly carries out repair of replication-associated DNA double-strand breaks (DSBs) [50]. Since the other DNA repair mechanisms rectifying DSBs are error prone, homologous recombination and BRCA proteins help in maintaining genomic integrity [51]. Germline mutation in BRCA 1 or BRCA 2 genes leads to increased incidence of a variety of malignancies with higher preponderance to breast and ovarian cancers [52]. Studies have also reported the incidence of BC in BRCA 1-mutated patients who have been previously diagnosed with ovarian cancer. In a study by Gangi et al., 8.9% of patients with BRCA 1/2-mutated ovarian cancers subsequently developed BC with a median time to diagnosis of BC of 50.5 months. This suggests the mechanism of action may primarily involve accumulation of DSBs due to inefficient homologous recombination. The harmful effects of BRCA mutation not only lead to increased incidence of BC, but also lead to pathological progression with higher tumor grade in comparison to patients with wildtype BRCA [53]. A variety of other tumor markers has also been reported to have differential expression pattern in BRCA-mutated BC patients. In a previous study by Aleskandarany et al., DNA damage/proliferation markers like Ki67 was expressed more in BRCA 1-mutated patients in comparison to wildtype BRCA 1 patients. They also reported no statistical significance in Ki67 expression in patients with BRCA 1 and 2 mutation [54].
The role of BRCA mutation testing in predicting prognosis in BC patients is unclear. Previous studies have provided contradictory findings which makes it difficult to assess the importance of BRCA gene testing [15]. Although previous meta-analyses have compared the survival outcomes in BRCA-mutated patients and wildtype BRCA patients, in the present meta-analysis, we have included recent long-term follow-up studies which were not included in previous analyses. Further, we have performed the analysis on multiple survival outcomes in different subgroups of patients to provide the precise role of BRCA mutation testing in patients with BC. We found that OS, DFS, BCSS, and DMFS were better in BRCA wildtype patients than in BRCA-mutated patients further substantiating the role BRCA mutation testing for predicting prognosis in BC patients.
With respect to BC patients with BRCA 1 mutation, the results revealed significantly poor OS when compared to BC patients with BRCA 1 wild type. However, there was no significant difference in DFS, BCSS, and DMFS between BRCA 1-mutated and BRCA 1 wildtype patients with BC. However, the summary effect estimates suggested poorer survival outcomes in patients with mutated BRCA 1 BC. These results are in line with the previously published reports [15]. A larger study on germline BRCA mutations by Baretta et al. reported a pooled OS estimate of 1.30 (95% CI 1.11–1.52) favoring BRCA 1 wildtype patients which was statistically significant [15]. This is in accordance with the results of our current analysis.
In case of BRCA 2-mutated patients, the survival outcomes were poor in comparison to BRCA 2 wildtype patients, but statistical significance was observed only for BCSS and DFS. In case of BRCA 2-mutated patients, Baretta et al. reported an OS effect estimate of 0.98 (95% CI 0.76–1.25) whereas the current meta-analysis favored patients with BRCA 2 mutation (HR 1.17; 95% CI 0.99, 1.39; P = 0.0591) [15]. Although statistical significance could not be established in both the studies, the disparity in results could suggest a differential effect of germline BRCA 2 mutations on survival outcomes which requires further studies for confirmation.
The analysis of studies that included patients with BRCA 1 or 2 mutations revealed that OS was similar in BRCA 1/2-mutated and BRCA 1/2 wildtype patients. This is in line with the findings of Bonadona et al. who reported similar survival in BRCA 1/2-mutated and wildtype patients with BC [35]. On the contrary, a study by Wang et al. reported statistically significant HRs for DFS and DMFS favoring BRCA 1/2 wildtype patients and non-significantly better HRs for OS [45]. A probable reason for this variation could be due to differences in the ethnicity of the included patients. The study by Wang et al. included Chinese patients, whereas the study by Bonadona et al. included French patients [35, 45]. Further, in the current study, DFS and DMFS suggested poorer outcomes in BRCA-mutated patients which substantiates the role of BRCA mutation in disease progression.
Since follow-up time may also influence the outcomes, we performed analysis stratified based on ≤ 5 years and > 5 years of follow-up. While studies with ≤ 5 years of follow -up reported significantly poorer OS in BRCA 1-mutated patients, the difference seems to balance out after longer follow-up, despite significantly favoring BRCA 1 wildtype patients (1.48 vs 1.14). In case of BRCA 2-mutated patients, there was no difference in OS in studies with ≤ 5 years of follow-up whereas in studies with > 5 years of follow-up, OS was significantly poorer in BRCA 2 -mutated patients (0.68, P > 0.05 vs 1.39, P < 0.05). A probable reason for the difference in survival in BRCA 1- and BRCA 2-mutated patients based on follow-up duration could be the fact that BRCA 1 mutation occurs predominantly in TNBC patients whereas BRCA 2 mutation occurs predominantly in HR-positive patients [16]. Previous studies have reported early recurrence in TNBC patients and late recurrence in HR-positive patients which is corroborated by our study findings [55, 56]. In a previous study by Aleskandarany et al. in BRCA-mutated patients, 68% patients are reported with BRCA 1 mutation to be triple negative. A high proportion of TNBC, BRCA 1-mutated patients were also showing a basal (basal cytokeratin expression) phenotype suggesting the association between TNBC, BRCA mutation, and a distinct molecular marker profile [54]. Further, TNBC patients with BRCA 1 mutation were also reported to have a large tumor burden (> 2 cm) and high expression of nuclear grade compared to TNBC patients with BRCA 2 mutation and TNBC patients with wildtype BRCA [16]. Among TNBC patients, a previous meta-analysis, reported marginally better survival outcomes in BRCA 1-mutated patients in comparison to BRCA 1 wildtype patients and a cumulative OS HR significantly favoring BRCA-mutated patients [15]. In the current study, in TNBC patients, it was revealed that BRCA mutation may lead to better OS, BCSS and DMFS. But statistical significance was not observed with any of the endpoints. This is substantiated by a study by Huszno et al. who reported non-significantly better OS in TNBC patients with BRCA 1 mutation with (HR 1.08; P = 0.883) in comparison to those without TNBC [45].
Irrespective of the follow-up duration, BRCA 1- and BRCA 2-mutated patients experienced poorer prognosis which emphasizes the need for early novel therapeutic options like poly-ADP ribose polymerase (PARP) inhibitors. PARP inhibitors have reported promising outcomes in BRCA--mutated patients. Considering the mode of action of PARPi, which also targets DNA repair pathway, they may offer differential efficacy in BRCA-mutated patients. Hence, early use of PARP inhibitors may enhance prognosis in patients with BRCA-mutated BC.
The previous studies did not consider the diverse treatment regimens used, leading to hidden heterogeneity that cannot be quantified. Although a few of the included studies provided adjusted effect estimates for taxanes and other drugs used predominantly in their study, the results were not stratified based on BRCA mutation status. Differential efficacy of drugs in patients with BRCA mutation has been reported in real-world studies. Data from the German PRAEGNANT registry study revealed 1st-line chemotherapy treatment in metastatic BC could be more effective in BRCA-mutated HER 2-negative patients in terms of progression-free survival (HR 0.7) and OS (HR 0.41) [57]. On the contrary, treatment with CDK4/6 inhibitors has reported worse prognosis in patients with BRCA mutation in comparison to patients with BRCA wild type [58]. The differential effects of drug regimens in BRCA-mutated BC further substantiate the usefulness of BRCA mutation testing.
The current study did not take into account the timing of BRCA testing and diagnosis of BC since most of the studies were retrospective in nature. Further, majority of the studies assessed germline mutation which may compensate the effect of timing of BRCA testing on the overall results. The study by Copson et al. included patients who had undergone BRCA testing within 1 year of BC diagnosis and reported a HR of 0.96 (P = 0.76) for OS [11]. Similarly, the study by Deng et al. reported a median time from BC diagnosis to BRCA testing of 9.2 months and reported a HR of 2.20 (P = 0.017) for DFS significantly favoring patients with wildtype BRCA [43]. The timing of BRCA mutation testing with reference to BC diagnosis is not provided in the other studies.
Among the included studies, some studies have reported the number of patients who had undergone salpingo-oophorectomy, but stratified effect estimates were not provided for analysis. Only one study by Tung et al. reported that BRCA 1-mutated, TNBC patients who had undergone salpingo-oophorectomy had better OS in comparison to patients who had not undergone salpingo-oophorectomy (HR 0.30, P < 0.05) [24]. Since the reason for better OS in patients undergoing salpingo-oophorectomy was not clear in the previous study, we did not analyse the confounding influence of salpingo-oophorectomy.
The study is not without limitations. Firstly, we included observational studies hence the influence of confounders cannot be ruled out completely. Secondly, the study did not account for the treatment modality which is not reported in most of the studies included in the meta-analysis. Although we performed subgroup analysis for TNBC status and follow-up period, there are other known confounding factors which cannot be accounted for in this study. One such confounding factor could be the incidence of ovarian cancer in the patients included for the meta-analysis. However, this is a limitation due to the non-availability of uniformly stratified effect estimates and not due to the study methodology. Nevertheless, wherever available, effect estimates adjusted for the confounding variables were taken for the analysis which could, to some extent, negate the overall effect of the confounders. This could also be the reason for the observed heterogeneity and hence the results need to be interpreted with caution. The limitations are due to the nature of evidences available and not due to the study methodology. Nevertheless, combining the available evidences provides higher statistical power which is the main strength of the study. Further, the studies included for the meta-analysis range from those published 2 decades back to the recent ones which also adds the changes in treatment paradigm and landscape of BRCA mutational testing as additional confounders. Nevertheless, excluding such studies would have led to inclusion bias and reduced the statistical power of the study.
In conclusion, this meta-analysis showed patients with BRCA 1-mutated and/or BRCA 2-mutated patients to have poor prognosis compared with that in wildtype BRCA 1/2 patients. Multiple end points and subgroups were analyzed in this study and all the statistically significant results suggested poor survival outcomes in BRCA 1-mutated and/or BRCA 2-mutated patients. These results suggest that BRCA gene testing maybe a prognostic marker for predicting survival. The confounding effect of other factors needs to be addressed in future prospective studies. Additionally, BRCA-mutated BC patients should be actively treated for better survival outcomes.
Data availability
All data generated or analyzed during this study are included in this article.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Murtaugh MA, Sweeney C, Giuliano AR et al (2008) Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr 87:978–984. https://doi.org/10.1093/ajcn/87.4.978
Aktipis CA, Ellis BJ, Nishimura KK, Hiatt RA (2015) Modern reproductive patterns associated with estrogen receptor positive but not negative breast cancer susceptibility. Evol Med Public Health 2015:52–74. https://doi.org/10.1093/emph/eou028
Rojas K, Stuckey A (2016) Breast cancer epidemiology and risk factors. Clin Obstet Gynecol 59(4):651–672. https://doi.org/10.1097/GRF.0000000000000239
Ruiz de Sabando A, Urrutia Lafuente E, García-Amigot F et al (2019) Genetic and clinical characterization of BRCA-associated hereditary breast and ovarian cancer in Navarra (Spain). BMC Cancer 19:1145. https://doi.org/10.1186/s12885-019-6277-x
BRCA: The Breast Cancer Gene. In: National Breast Cancer Foundation. https://www.nationalbreastcancer.org/what-is-brca. Accessed 26 Febr 2020
Miki Y, Swensen J, Shattuck-Eidens D et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71. https://doi.org/10.1126/science.7545954
Wooster R, Bignell G, Lancaster J et al (1995) Identification of the breast cancer susceptibility gene BRCA2. Nature 378:789–792. https://doi.org/10.1038/378789a0
Easton DF, Ford D, Bishop DT (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56:265–271
Ford D, Easton DF, Stratton M et al (1998) Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 62:676–689. https://doi.org/10.1086/301749
Copson ER, Maishman TC, Tapper WJ et al (2018) Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 19:169–180. https://doi.org/10.1016/S1470-2045(17)30891-4
Walsh T, Mandell JB, Norquist BM et al (2017) Genetic predisposition to breast cancer due to mutations other than BRCA1 and BRCA2 founder alleles among Ashkenazi Jewish women. JAMA Oncol 3:1647–1653. https://doi.org/10.1001/jamaoncol.2017.1996
Liebens FP, Carly B, Pastijn A, Rozenberg S (2007) Management of BRCA1/2 associated breast cancer: a systematic qualitative review of the state of knowledge in 2006. Eur J Cancer 43:238–257. https://doi.org/10.1016/j.ejca.2006.07.019
Lee E-H, Park SK, Park B et al (2010) Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat 122:11–25. https://doi.org/10.1007/s10549-010-0859-2
Baretta Z, Mocellin S, Goldin E et al (2016) Effect of BRCA germline mutations on breast cancer prognosis. Medicine (Baltimore). https://doi.org/10.1097/MD.0000000000004975
Chen H, Wu J, Zhang Z et al (2018) Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00909
Pogoda K, Niwińska A, Sarnowska E et al (2020) Effects of BRCA germline mutations on triple-negative breast cancer prognosis. J Oncol 2020:10. https://doi.org/10.1155/2020/8545643
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 6:e1000100. https://doi.org/10.1371/journal.pmed.1000100
Wells GA, Shea B, O’connell D et al (2015) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Bayraktar S, Gutierrez-Barrera AM, Lin H et al (2013) Outcome of metastatic breast cancer in selected women with or without deleterious BRCA mutations. Clin Exp Metastasis 30:631–642. https://doi.org/10.1007/s10585-013-9567-8
Lang G-T, Shi J-X, Hu X et al (2017) The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer 141:129–142. https://doi.org/10.1002/ijc.30692
Wang C, Zhang J, Wang Y et al (2015) Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann Oncol 26:523–528. https://doi.org/10.1093/annonc/mdu559
Tung N, Gaughan E, Hacker MR et al (2014) Outcome of triple negative breast cancer: comparison of sporadic and BRCA1-associated cancers. Breast Cancer Res Treat 146:175–182. https://doi.org/10.1007/s10549-014-2995-6
Sambiasi D, Lambo R, Pilato B et al (2014) BRCA1/2 and clinical outcome in a monoinstitutional cohort of women with hereditary breast cancer. Oncol Rep 31:365–369. https://doi.org/10.3892/or.2013.2802
Huzarski T, Byrski T, Gronwald J et al (2013) Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 31:3191–3196. https://doi.org/10.1200/JCO.2012.45.3571
Tryggvadottir L, Olafsdottir EJ, Olafsdottir GH et al (2013) Tumour diploidy and survival in breast cancer patients with BRCA2 mutations. Breast Cancer Res Treat 140:375–384. https://doi.org/10.1007/s10549-013-2637-4
Goodwin PJ, Phillips K-A, West DW et al (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30:19–26. https://doi.org/10.1200/JCO.2010.33.0068
Arun B, Bayraktar S, Liu DD et al (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29:3739–3746. https://doi.org/10.1200/JCO.2011.35.2682
Bayraktar S, Gutierrez-Barrera AM, Liu D et al (2011) Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat 130:145–153. https://doi.org/10.1007/s10549-011-1711-z
Lee LJ, Alexander B, Schnitt SJ et al (2011) Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 117:3093–3100. https://doi.org/10.1002/cncr.25911
Budroni M, Cesaraccio R, Coviello V et al (2009) Role of BRCA2 mutation status on overall survival among breast cancer patients from Sardinia. BMC Cancer 9:62. https://doi.org/10.1186/1471-2407-9-62
Rennert G, Bisland-Naggan S, Barnett-Griness O et al (2007) Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med 357:115–123. https://doi.org/10.1056/NEJMoa070608
Brekelmans CTM, Tilanus-Linthorst MMA, Seynaeve C et al (2007) Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 43:867–876. https://doi.org/10.1016/j.ejca.2006.12.009
Bonadona V, Dussart-Moser S, Voirin N et al (2007) Prognosis of early-onset breast cancer based on BRCA1/2 mutation status in a French population-based cohort and review. Breast Cancer Res Treat 101:233–245. https://doi.org/10.1007/s10549-006-9288-7
Brekelmans CTM, Seynaeve C, Menke-Pluymers M et al (2006) Survival and prognostic factors in BRCA1-associated breast cancer. Ann Oncol 17:391–400. https://doi.org/10.1093/annonc/mdj095
Robson ME, Chappuis PO, Satagopan J et al (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 6:R8–R17. https://doi.org/10.1186/bcr658
Verhoog LC, Brekelmans CT, Seynaeve C et al (1999) Survival in hereditary breast cancer associated with germline mutations of BRCA2. J Clin Oncol 17:3396–3402. https://doi.org/10.1200/JCO.1999.17.11.3396
Verhoog LC, Brekelmans CT, Seynaeve C et al (1998) Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet 351:316–321. https://doi.org/10.1016/s0140-6736(97)07065-7
Jóhannsson OT, Ranstam J, Borg A, Olsson H (1998) Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol 16:397–404. https://doi.org/10.1200/JCO.1998.16.2.397
Marcus JN, Watson P, Page DL et al (1996) Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer 77:697–709. https://doi.org/10.1002/(sici)1097-0142(19960215)77:4%3c697::aid-cncr16%3e3.0.co;2-w
Loman N, Johannsson O, Bendahl P et al (2000) Prognosis and clinical presentation of BRCA2-associated breast cancer. Eur J Cancer 36:1365–1373. https://doi.org/10.1016/s0959-8049(00)00098-8
Deng M, Chen H-H, Zhu X et al (2019) Prevalence and clinical outcomes of germline mutations in BRCA1/2 and PALB2 genes in 2769 unselected breast cancer patients in China. Int J Cancer 145:1517–1528. https://doi.org/10.1002/ijc.32184
Huszno J, Kołosza Z, Grzybowska E (2019) BRCA1 mutation in breast cancer patients: analysis of prognostic factors and survival. Oncol Lett 17:1986–1995. https://doi.org/10.3892/ol.2018.9770
Wang YA, Jian J-W, Hung C-F et al (2018) Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer 18:315. https://doi.org/10.1186/s12885-018-4229-5
Sun J, Meng H, Yao L et al (2017) Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res 23:6113–6119. https://doi.org/10.1158/1078-0432.CCR-16-3227
Schmidt MK, van den Broek AJ, Tollenaar RAEM et al (2017) Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw329
Kim MC, Choi JE, Lee SJ, Bae YK (2016) Coexistent loss of the expressions of BRCA1 and p53 predicts poor prognosis in triple-negative breast cancer. Ann Surg Oncol 23:3524–3530. https://doi.org/10.1245/s10434-016-5307-z
Zhong X, Dong Z, Dong H et al (2016) Prevalence and prognostic role of BRCA1/2 variants in unselected Chinese breast cancer patients. PLoS One 11:e0156789. https://doi.org/10.1371/journal.pone.0156789
Schlacher K, Christ N, Siaud N et al (2011) Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145:529–542. https://doi.org/10.1016/j.cell.2011.03.041
Roy R, Chun J, Powell SN (2011) BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 12:68–78. https://doi.org/10.1038/nrc3181
Foulkes WD (2008) Inherited susceptibility to common cancers. N Engl J Med 359:2143–2153. https://doi.org/10.1056/NEJMra0802968
Mavaddat N, Barrowdale D, Andrulis IL et al (2012) Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomark Prev 21:134–147. https://doi.org/10.1158/1055-9965.EPI-11-0775
Aleskandarany M, Caracappa D, Nolan CC et al (2015) DNA damage response markers are differentially expressed in BRCA-mutated breast cancers. Breast Cancer Res Treat 150:81–90. https://doi.org/10.1007/s10549-015-3306-6
Steward L, Conant L, Gao F, Margenthaler JA (2014) Predictive factors and patterns of recurrence in patients with triple negative breast cancer. Ann Surg Oncol 21:2165–2171. https://doi.org/10.1245/s10434-014-3546-4
Richman J, Dowsett M (2019) Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat Rev Clin Oncol 16:296–311. https://doi.org/10.1038/s41571-018-0145-5
Fasching PA, Hu C, Hart S et al (2019) Germline BRCA1and BRCA2 mutations in patients with HER2-negative metastatic breast cancer (mBC) treated with first-line chemotherapy: data from the German PRAEGNANT registry. JCO 37:1048–1048. https://doi.org/10.1200/JCO.2019.37.15_suppl.1048
McLaurin K, Dalvi T, Collins JM et al (2019) A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by gBRCA mutation status. JCO 37:1563–1563. https://doi.org/10.1200/JCO.2019.37.15_suppl.1563
Acknowledgements
The authors acknowledge Dr. Kaushik Subramanian, Indegene pvt Ltd, Bangalore, India, for the medical writing assistance.
Funding
The study was funded by AstraZeneca.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, M., Xie, F., Liu, M. et al. Association between BRCA mutational status and survival in patients with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 186, 591–605 (2021). https://doi.org/10.1007/s10549-021-06104-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06104-y