Abstract
It is not well known to what extent carrying a BRCA2 mutation affects the survival of women with breast cancer and prognostic factors among BRCA2-positive women warrant investigation. Using a record linkage approach we compared the long-term survival in carriers and noncarriers of an inherited BRCA2 founder mutation (999del5), and sought to identify prognostic factors among the BRCA2 mutation-positive subset, including markers of genetic instability (aneuploidy) and mitotic activity (S-phase fraction). We established the genetic status of 2,967 Icelandic breast cancer patients (215 mutation carriers and 2,752 noncarriers) diagnosed from 1955 to 2004, representing 72 % of all cases diagnosed in the country during this period. Tumour ploidy and S-phase fraction were assessed on tumour cells by DNA flow cytometry. Prognostic factors were assessed blindly with respect to mutation status. Univariate and multivariate hazard ratios (HR) were estimated for breast cancer-specific survival by BRCA2 status, using Cox regression. After a median follow-up of 9.5 years, BRCA2 mutation carriers had a higher risk of death from breast cancer than noncarriers (HR 1.64, 95 % CI 1.24–2.16, p < 0.001). The risk increase was restricted to women with diploid tumours (HR 3.03, 95 % CI 1.91–4.79, p < 0.001). Among breast cancer patients with aneuploid tumours, survival of carriers was similar to that of noncarriers (HR 0.76, 95 % CI 0.41–1.41, p = 0.38). Increased tumour size and a positive nodal status predicted worse prognosis in all patients, whereas the highly correlated prognostic factors diploidy, low proliferative activity and a positive estrogen receptor status had reverse effects in mutation carriers and noncarriers. Breast cancer patients who carry the Icelandic founder BRCA2 mutation have inferior long-term survival than noncarriers, but the adverse prognosis is restricted to mutation carriers with diploid, slowly proliferating tumours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited mutations in the BRCA2 gene increase the risk of breast cancer by approximately tenfold [1–3]. A founder mutation in BRCA2 (995del5) is present in 0.6 % of the general population of Iceland and in 6–7 % of unselected breast cancer patients. This is the only BRCA2 mutation detected in the Icelandic population, a five base pair deletion leading to a premature stop codon [4]. Recently, it has been proposed that women with breast cancer and a BRCA2 mutation have a higher case fatality than women with sporadic disease (Hazard ratios (HR) 1.81, 95 % CI 1.15–2.86, p = 0 .01) [5], but the mortality disadvantage was attenuated after adjustment for age and other prognostic factors (HR 1.12; 95 % CI, 0.70–1.79, p = 0.64). There are few other studies of the prognosis of women with BRCA2-mutation-associated breast cancers and little is known about the features that are predictive of survival in this hereditary subgroup. Population-based studies with long-term follow-up will help resolve this question [5–7]. Genetic instability is a hallmark of solid tumours and has been described as ‘the engine of both tumour progression and tumour heterogeneity’ [8]. In cancer cells, it is sometimes manifest at the nucleotide level, but it is also observed at the chromosomal level [8]. Aneuploidy is an aberrant chromosome number that deviates from a multiple of the haploid. Aneuploidy is one of the most common characteristics of cancer cells and is present in approximately 85 % of solid tumours [9, 10]. The BRCA2 protein has an important function in DNA repair via homologous recombination [11] and genetic instability is increased in the cells of mutation carriers [12, 13]. Breast cancer cells in BRCA2 mutation carriers often exhibit complex chromosomal changes, including DNA copy number variations [13–15]; however, it is currently not known to what extent BRCA2-mutation-associated tumours are aneuploid or whether aneuploidy impacts upon patient survival.
In Iceland, information is available for all breast cancers diagnosed since 1955 and for the majority, genetic BRCA2 mutation status has been established. Information on tumour ploidy and S-phase fraction (a proliferative index indicating the percentage of cells in a tumour that are in the S-phase of the cell cycle) is available for the majority of cases diagnosed since 1981. We used data from the national population-based Cancer Registry of Iceland to compare the long-term survival experiences of breast cancer patients with and without a BRCA2 mutation and we studied the effects of tumour size, nodal status, tumour grade, estrogen receptor (ER) status, tumour ploidy and S-phase fraction on survival in the hereditary and non-hereditary subgroups.
Methods
Study population
Iceland is a country of 320,000 individuals and currently approximately 190 cases of breast cancer are diagnosed annually. The Icelandic Cancer Registry [16], founded in 1954, was the main source of information for the breast cancer patients in this study. The present study group consists of 2,967 patients diagnosed from 1955 to 2004 with primary invasive breast cancer. They were screened for the Icelandic BRCA2 founder mutation 999del5 in connection with a breast cancer research project conducted at the Icelandic Cancer Society in 1987–2004, by using a blood sample donated by participants (n = 968 cases), a stored paraffin-embedded tumour sample (n = 963), or both types of samples (n = 1036). As the Icelandic mutation is a five base pair deletion, there is complete correlation between the BRCA2 status of the paraffin embedded tumour samples and the blood samples. The 2,967 cases represent 71.7 % of all cases of breast cancer diagnosed in Iceland during this time period. Of these women, 1,856 have now died (62 %).
The blood samples were drawn at or after diagnosis; for 1,046 women the sample was obtained more than 2 years after diagnosis. Including these patients in the study would potentially introduce survivorship bias [17]. For 881 of these 1,046 cases, a paraffin sample was also available. In sensitivity analyses, neither the inclusion of these 881 cases, nor the remaining 165 cases affected the results, so they were included in all analyses.
Information on histology has been recorded on all cases, information on tumour size and nodal involvement has been recorded since 1974, information on ER and progesterone receptor (PR) status has been recorded since 1981 and information on tumour grade was recorded from 1981 to 1984 in the context of a research study [18], and since 1991. Routine assessment of HER2 receptor status began in Iceland in 2004 and information on HER2 was not available for the patients in the current study.
Markers of genetic instability
In 1991, routine assessment of S-phase and DNA ploidy by flow cytometry was initiated at the Department of Pathology at the University Hospital, using paraffin-embedded breast tumours rendered for histology [19]. Ploidy and S-phase had also been recorded for breast cancer patients diagnosed from 1981 to 1984 [18]. Tumours were classified as diploid if the DNA index was 1.00 + 0.15 and aneuploid if the DNA index was <0.85 or >1.15 [20]. Eight tetraploid tumours (DNA index = 2.0) were included in the aneuploid group. S-phase fraction was divided into two groups based on the proportion of dividing cells (low: <7.0 %; intermediate to high ≥7.0 %). Approval was obtained for this study from the Icelandic Data Protection Authority (2006050307) and the National Bioethics Committee of Iceland (VSNb2006050001/03-16).
Statistical analysis
The mean values for continuous variables were compared for mutation carriers and noncarriers, using the t test statistic. The Wilcoxon rank-sum test was used to compare median values and the Chi square test was used for comparing proportions. All statistical tests were two-sided and p values less than 0.05 were considered to be statistically significant. The Kaplan–Meier method was used for generating univariable survival curves and the Log-Rank test was used for comparing them. Relative hazards were estimated using the Cox proportional hazards model. Patients were followed from the date of diagnosis until death or last date of follow-up (December 31st, 2009). The outcome was breast cancer-specific survival, defined as the time from diagnosis to death from breast cancer, as registered on death certificates. Patients who died of causes other than breast cancer were censored at the date of death.
Initially, we compared the crude survival in the carriers and the noncarrier comparison group. We then conducted a series of adjusted survival analyses. In the initial analysis, adjustment was made for year of birth and year of diagnosis. In a subsequent multivariable analysis, we also adjusted for tumour size (≤20 mm, 20–50 mm, 51+ mm), lymph node status (positive/negative), grade (I, II, III), and ER status (negative/positive).
We then conducted additional analyses to evaluate the markers of genetic instability (tumour ploidy (diploid/aneuploid) and S-phase fraction (low/intermediate to high). We evaluated the impact of tumour ploidy and S-phase fraction on survival in the hereditary and non-hereditary cancers separately. All analyses were performed using STATA Statistical Software Stata 10 for Windows.
Results
Characteristics of patients and their tumours according to mutation status
Genetic testing was performed on 2,967 breast cancer cases diagnosed between 1955 and 2004. The study group represents 71.7 % of all cases diagnosed in that period. Of the tested cases, 215 (7.2 %) were found to be BRCA2 mutation carriers and 2,752 (92.8 %) were found to be noncarriers. Mutation carriers were on average younger at diagnosis than noncarriers (49.5 vs. 57.6 years) (Table 1). Tumours from mutation carriers were on average larger (28.1 vs. 23.1 mm, p < 0.001) and were more often node-positive (52.4 vs. 41.9 %, p = 0.008) than tumours from noncarriers.
Information was available for grade, ER status and markers of genetic instability for most patients diagnosed since 1981 (Table 1). Tumours in mutation carriers were more often grade 3 than in noncarriers (50.0 vs. 31.4 %, p < 0.001). Carriers and noncarriers were similar for the proportion of ER-positive tumours (70.6 % in carriers and 75.7 % in noncarriers, p = 0.23) and for the proportions with diploid tumours (52.0 % in mutation carriers and 49.1 % in noncarriers, p = 0.57). The median S-phase fraction was higher for carriers (6.5 % dividing cells) than for noncarriers (4.4 %; p < 0.001).
In the data set, low S-phase fraction (<7 %) was highly correlated with tumour diploidy (OR 12.5, 95 % CI 9.1–17.0, p < 0.001). ER-positivity and diploid status were also positively correlated, but to a lesser extent (OR 1.6, 95 % CI 1.2–2.1, p = 0.001) as were ER-positivity and low S-phase fraction (OR 3.9, 95 % CI 2.9–5.2, p < 0.001). These associations were present in both carriers and noncarriers.
Mutation status and survival
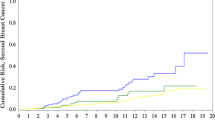
The median follow-up time was 9.5 years. The actuarial 10-year survival was 53 % (95 % CI 0.46–0.60 %) for carriers and was 72 % (95 % CI 0.70–0.74 %) for noncarriers (Fig. 1). The HR of death from breast cancer associated with a BRCA2 mutation was 1.61 (95 % CI 1.32–1.96, p < 0 .001) in the overall group, adjusting for year of birth and year of diagnosis. After adjustment for tumour size and nodal status as well, the HR was 1.32 (95 % CI 1.02–1.73, p = 0.038). Among the subgroup of patients (diagnosed 1981–2004) for whom information on other tumour characteristics was available (grade, ER status, diploidy), the unadjusted and adjusted (for all factors) HR associated with a BRCA2 mutation were 1.64 (95 % CI 1.24–2.16, p < 0.001) and 0.98 (95 % CI 0.64–1.48, p = 0.91), respectively (Table 2).
The HR associated with the various tumour characteristics in the mutation-negative and mutation-positive subgroups are provided in Table 3 (adjusted for year of birth and age) and in Table 4 (adjusted for all prognostic factors). Among noncarriers, an increased risk of death was associated with larger tumour size, positive lymph nodes, high tumour grade, negative ER status, aneuploidy and a high S-phase fraction. Among mutation carriers, only the associations with tumour size and lymph node status were as seen in noncarriers. In carriers, there was no significant association with grade, and the associations with ER status, with tumour ploidy and with S-phase fraction were opposite to what was expected. In the multivariable analysis (Table 4), among BRCA2 mutation carriers, tumour diploidy was an independent and statistically significant predictor of poor prognosis (HR 4.86, 95 % CI 1.65–14.30, p = 0.004). Similarly, in the multivariable analysis, among BRCA2 mutation carriers, low tumour S-phase fraction (<7.0 %) was highly predictive of poor survival (HR 12.7, 95 % CI 2.40–67.4, p = 0.003) (because of co-linearity we did not include both aneuploidy and S-phase fraction in the same model). In noncarriers, low S-phase fraction was associated with good prognosis (HR 0.71, 95 % CI 0.50–1.01, p = 0.06).
Table 5 shows tumour characteristics by mutation status and ploidy. The proportion of patients with positive lymph nodes was highest (63.5 %) among mutation carriers with diploid tumours. Both among mutation carriers and noncarriers, diploidy was associated with lower grades, and a considerably higher proportion of ER-positive tumours and tumours with a low S-phase fraction, than aneuploidy.
BRCA2 mutation carriers with ER-positive, diploid tumours with a low S-phase fraction represent a clinical high-risk subgroup, which comprises 34 % of the carriers. The 10-year crude survival rate for BRCA2 mutation carrier women with this phenotype was only 51 %, compared to 91 % for women with tumours with none of these features. Among carriers, diploidy was associated with increased mortality both in the ER-positive (HR 3.90, 95 % CI 1.27–11.98, p = 0.017) and ER-negative subgroups (HR 4.48, 95 % CI 0.89–22.51, p = 0.069), whereas a positive ER status was neither associated with a significantly increased risk in the subgroup of women with diploid tumours (HR 0.81, 95 % CI 0.29–2.27, p = 0.69) nor aneuploid tumours (HR 2.25, 95 % CI 0.30–16.87, p = 0.43). This suggests that diploidy rather than ER-positivity is the more relevant of the two factors in terms of patient survival among BRCA2 mutation carriers.
Fifty percent of mutation carriers with diploid tumours died within 10 years of diagnosis, versus 22 % of carriers with aneuploid tumours (unadjusted HR 3.24, 95 % CI 1.57–6.69, p = 0.001) (Fig. 2a; Table 2). In contrast, among noncarriers diploidy was a favourable prognostic factor (unadjusted HR 0.77, 95 % CI 0.60–0.99, p = 0.041) (Fig. 2b; Table 2). Among mutation carriers a positive (versus negative) ER status was associated with increased mortality (HR 2.05, 95 % CI 0.96–4.39, p = 0.064), contrasting with what was observed for noncarriers (HR 0.63, 95 % CI 0.51–0.78, p < 0.001) (Fig. 2c, d).
Breast cancer-specific survival probability, subset diagnosed 1981–2004. A Survival in patients with diploid versus aneuploid tumours among BRCA2 mutation carriers and B among noncarriers. C Survival in patients with estrogen receptor (ER) positive tumours versus ER negative tumours among BRCA2 mutation carriers and D among noncarriers
Among women with diploid tumours, BRCA2 mutation status was a strong predictor of mortality (unadjusted HR 3.03, 95 % CI 1.91–4.79, p < 0.001) (Table 2). Among women with aneuploid tumours, there was no survival disadvantage associated with carrying a BRCA2 mutation (unadjusted HR 0.76, 95 % CI 0.41–1.41, p = 0.38).
Discussion
We found that breast cancer patients who carry a founder BRCA2 mutation presented with more aggressive breast cancers than noncarriers and had an inferior 10-year survival rate (53 vs. 72 %). The survival disadvantage for BRCA2 mutation carriers was contingent on tumour ploidy and was present only for women with diploid cancers—approximately one-half of the patients. Tumour diploidy was protective in the noncarrier subgroup.
Tumour diploidy, low S-phase fraction and a positive ER status were highly correlated, both in BRCA2 mutation carriers and noncarriers. In a multivariable analysis of BRCA2 mutation carriers, the strong association between diploidy and mortality was not attenuated by adjustment for these and other prognostic variables. The same was true for the association between low S-phase fraction and the risk of death. In contrast, ER status was not predictive of survival when the analysis was adjusted for tumour ploidy. The majority of patients with diploid tumours in mutation carriers were node-positive (63.5 %), but otherwise diploid tumours had characteristics that are typically favourable, i.e., relatively low grade, ER-positivity and low proliferative activity (a low S-phase fraction).
In noncarriers, aneuploidy was associated with a significantly worse prognosis than diploidy, in keeping with what has been reported for the general population [20]. However, recent CGH-based studies have made it possible to subdivide diploid tumours by the presence of other, often complex genetic changes and these sub-chromosomal abnormalities may also influence survival [21]. In a previous study from Iceland, diploid tumours arising in BRCA2 mutation carriers were commonly found to carry complex chromosomal rearrangements, according to CGH analysis [15]. In these patients, small structural rearrangements, rather than gross changes in chromosome number, appear to be the dominant type of genomic instability associated with aggressive behaviour. It is also possible that specific genetic changes (e.g., driver mutations) have a role. If there is a common mutation in a single gene that is correlated with adverse prognosis, and if this gene is typically the driver of metastatic potential in diploid cancers with low proliferation, but not in aneuploid cancers, the pattern might be explained. It is possible that through whole genomic sequencing of tumour DNA from diploid and aneuploid BRCA2-mutation-associated breast cancers, such a driver mutation might be identified.
Approximately 50 % of tumours were aneuploid both among mutation carriers, and noncarriers, in contrast to expectations of an excess of aneuploidy in cancers of BRCA2 mutation carriers [22]. The superior survival associated with aneuploidy versus diploidy in the mutation-positive patients supports the notion that if cancer cells of mutation carriers acquire changes at the chromosomal level (aneuploidy) on top of their complex nucleotide level changes, they may become less fit, resulting in better survival in association with aneuploidy. Genetic instability is generally associated with poor prognosis in cancer patients, but it has been hypothesized that, at a certain point, excessive genetic instability may surpass a threshold compatible with cell viability [23]. In support of this hypothesis are recent findings which indicate that aneuploidy can inhibit tumourigenesis in animals with a genetic tendency for tumour formation [24], and that extreme chromosomal instability in ER-negative tumours can be associated with improved prognosis of breast cancer patients [25].
Our study has several strengths, including a large number of patients and a long follow-up period. Carriers and noncarriers were derived from the same source population (Icelandic Cancer Registry) and the majority of cancer cases in the country were included. Previous findings of studies on survival in BRCA2 mutation carriers with breast cancer have generally not been indicative of an association between mutation status and prognosis, although results have been inconsistent [6, 7], but large studies with long-term follow up have been lacking. Other studies have not included markers of genomic instability. In a recent, relatively large, long-term study, BRCA2 mutation-positivity was associated with adverse survival [5]. In that study, as in our study, there was also an indication of a worse outcome associated with a positive ER status among BRCA2 mutation carriers.
In the BRCA2-mutation-positive subgroup, we found that size and nodal status were both predictive of survival, but ER status and tumour grade were not (the associations were opposite to what is expected and to what was seen in the noncarriers). It is important that these observations are confirmed, but if true, these results imply that prognosis in BRCA2 mutation carriers should be estimated, based on size and nodal status and that other canonical prognostic factors (e.g., grade and ER status) are not informative and should not be used to guide treatment decisions regarding chemotherapy.
In this study, we did not have information regarding chemotherapy and hormonal therapies and these will be topic of future studies. However, the practice in Iceland since 1981 has been that over 90 % of breast cancer patients with node positive tumours receive chemotherapy, and as 63.5 % of the mutation carriers with diploid tumours had positive lymph nodes, their poor survival can not be explained by a low application rate of chemotherapy. We did not have information on HER2 status or other markers of mitotic activity such as Ki-67. It is of interest to evaluate the BRCA2 mutation-related cancers in terms of canonical phenotype (luminal A, luminal B, etc.) and to see if these categories are predictive of mortality in BRCA2 mutation carriers.
We studied a single mutation (999del5) and the results should be confirmed for patients with other mutations. The unadjusted HR comparing BRCA2 mutation carriers to noncarriers was similar in our study (1.64, 95 % CI 1.24–2.16) to the unadjusted HR observed in a recent study by Goodwin et al. [5] with median follow-up time 7.9 years (HR 1.81, 95 % CI 1.15–2.86). This could indicate that the effects of the Icelandic mutation are not much different from effects of other BRCA2 mutations.
In the light of the unorthodox association between diploid tumours and survival among mutation carriers, and the fact that those ‘diploid’ tumours have complex genomic changes, one might reason that ploidy assessment by flow cytometry is not useful in this context. We argue that on the contrary, the results clearly demonstrate that diploidy defines a biologically important phenotype being strongly associated with low mitotic activity and a positive ER status, and even more importantly, in mutation carriers this phenotype was higly predictive of a poor survival outcome, and remained so after adjustment for other prognostic factors.
In conclusion, our data suggest that ER-status, S-phase and diploidy define two distinct subclasses of breast cancers among BRCA2 mutation carriers and that women with ER-positive, diploid cancers of low proliferation are at a high risk of recurrence and death. It will be important to confirm these observations in other populations and to study the impacts of various treatments in carriers, stratified by ploidy status. Given the relatively small proportion of BRCA2 mutation carriers among breast cancer patients, it is hoped that these studies can be planned in the context of multi-centre collaborations.
References
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G (1995) Identification of the breast cancer susceptibility gene BRCA2. Nature 378(6559):789–792
Tavtigian SV, Simard J, Rommens J, Couch F, Shattuck-Eidens D, Neuhausen S, Merajver S, Thorlacius S, Offit K, Stoppa-Lyonnet D, Belanger C, Bell R, Berry S, Bogden R, Chen Q, Davis T, Dumont M, Frye C, Hattier T, Jammulapati S, Janecki T, Jiang P, Kehrer R, Leblanc JF, Goldgar DE (1996) The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet 12(3):333–337
Tryggvadottir L, Sigvaldason H, Olafsdottir GH, Jonasson JG, Jonsson T, Tulinius H, Eyfjord JE (2006) Population-based study of changing breast cancer risk in Icelandic BRCA2 mutation carriers, 1920–2000. J Natl Cancer Inst 98(2):116–122
Thorlacius S, Sigurdsson S, Bjarnadottir H, Olafsdottir G, Jonasson JG, Tryggvadottir L, Tulinius H, Eyfjord JE (1997) Study of a single BRCA2 mutation with high carrier frequency in a small population. Am J Hum Genet 60(5):1079–1084
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, O’Malley FP, Milne RL, Andrulis IL, Friedlander ML, Southey MC, Apicella C, Giles GG, Longacre TA (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30(1):19–26. doi:10.1200/JCO.2010.33.0068
Bordeleau L, Panchal S, Goodwin P (2010) Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat 119(1):13–24
Lee EH, Park SK, Park B, Kim SW, Lee MH, Ahn SH, Son BH, Yoo KY, Kang D, Group KR, Korean Breast Cancer S (2010) Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat 122(1):11–25
Lengauer C, Kinzler KW, Vogelstein B (1998) Genetic instabilities in human cancers. Nature 396(6712):643–649
Weaver BA, Cleveland DW (2006) Does aneuploidy cause cancer? Curr Opin Cell Biol 18(6):658–667. doi:10.1016/j.ceb.2006.10.002
Rajagopalan H, Lengauer C (2004) Aneuploidy and cancer. Nature 432(7015):338–341. doi:10.1038/nature03099
Venkitaraman AR (2002) Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108(2):171–182
Gudmundsdottir K, Ashworth A (2006) The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25(43):5864–5874
Gretarsdottir S, Thorlacius S, Valgardsdottir R, Gudlaugsdottir S, Sigurdsson S, Steinarsdottir M, Jonasson JG, Anamthawat-Jonsson K, Eyfjord JE (1998) BRCA2 and p53 mutations in primary breast cancer in relation to genetic instability. Cancer Res 58(5):859–862
Stefansson OA, Jonasson JG, Johannsson OT, Olafsdottir K, Steinarsdottir M, Valgeirsdottir S, Eyfjord JE (2009) Genomic profiling of breast tumours in relation to BRCA abnormalities and phenotypes. Breast Cancer Res 11(4):R47
Stefansson OA, Jonasson JG, Olafsdottir K, Bjarnason H, Johannsson OTh, Bodvarsdottir SK, Valgeirsdottir S, Eyfjord JE (2011) Genomic and phenotypic analysis of BRCA2 mutated breast cancers reveals co-occurring changes linked to progression. Breast Cancer Res 13(5):R95. doi:10.1186/bcr3020
Sigurdardottir LG, Jonasson JG, Stefansdottir S, Jonsdottir A, Olafsdottir GH, Olafsdottir EJ, Tryggvadottir L (2012) Data quality at the Icelandic Cancer Registry: comparability, validity, timeliness and completeness. Acta Oncol 51(7):880–889. doi:10.3109/0284186X.2012.698751
Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, vd Ouweland A, Menke-Pluymers MB, Bartels CC, Kriege M, van Geel AN, Burger CW, Eggermont AM, Meijers-Heijboer H, Klijn JG (2007) Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 43(5):867–876. doi:10.1016/j.ejca.2006.12.009
Agnarsson BA, Jonasson JG, Bjornsdottir IB, Barkardottir RB, Egilsson V, Sigurdsson H (1998) Inherited BRCA2 mutation associated with high grade breast cancer. Breast Cancer Res Treat 47(2):121–127
Jonasson JG, Hrafnkelsson J (1994) Nuclear DNA analysis and prognosis in carcinoma of the thyroid gland. A nationwide study in Iceland on carcinomas diagnosed 1955–1990. Virchows Archiv 425(4):349–355
Ross JS, Linette GP, Stec J, Ross MS, Anwar S, Boguniewicz A (2003) DNA ploidy and cell cycle analysis in breast cancer. Am J Clin Pathol 120:S72–S84
Hicks J, Krasnitz A, Lakshmi B, Navin NE, Riggs M, Leibu E, Esposito D, Alexander J, Troge J, Grubor V, Yoon S, Wigler M, Ye K, Borresen-Dale AL, Naume B, Schlicting E, Norton L, Hagerstrom T, Skoog L, Auer G, Maner S, Lundin P, Zetterberg A (2006) Novel patterns of genome rearrangement and their association with survival in breast cancer. Genome Res 16(12):1465–1479
Venkitaraman AR (2009) Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol 4:461–487. doi:10.1146/annurev.pathol.3.121806.151422
Cahill DP, Kinzler KW, Vogelstein B, Lengauer C (1999) Genetic instability and Darwinian selection in tumours. Trends Cell Biol 9(12):M57–M60 S0962-8924(99)01661-X
Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW (2007) Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11(1):25–36
Birkbak NJ, Eklund AC, Li Q, McClelland SE, Endesfelder D, Tan P, Tan IB, Richardson AL, Szallasi Z, Swanton C (2011) Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res 71(10):3447–3452. doi:10.1158/0008-5472.CAN-10-3667
Acknowledgements
We are indebted to the women who participated in the family studies at the Icelandic Cancer Society, Prof. Helga M. Ogmundsdottir and Dr. Tryggvi Thorgeirsson for inspiring and constructive discussions and Holmfridur Hilmarsdottir for mutation analyses. This study was supported by the Icelandic Cancer Society and the US Army Medical Research Acquisition Activity (DAMD17-97-1-7002 and DAMD17-99-1-9216).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tryggvadottir, L., Olafsdottir, E.J., Olafsdottir, G.H. et al. Tumour diploidy and survival in breast cancer patients with BRCA2 mutations. Breast Cancer Res Treat 140, 375–384 (2013). https://doi.org/10.1007/s10549-013-2637-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2637-4