Abstract
Background
At the present time, there is no consensus on the association between benign thyroid diseases and breast cancer (BC). Therefore, the aim of this study is to help shed some light on the association between hyperthyroidism, hypothyroidism, and thyroiditis and breast cancer risk.
Methods
Use of the Disease Analyzer database (IQVIA) enabled us to perform a retrospective case–control study of 7408 women aged between 18 and 80, who were treated for an initial breast cancer diagnosis in a general practice in the United Kingdom between 2006 and 2015 (index date). Patients with a previous cancer diagnosis and an observation time of less than 12 months prior to the index date were excluded. The control group consisted of 7408 healthy women, who were matched to cases 1:1 by age, body mass index, hormone replacement therapy, and physician. The main outcome parameters of this study were the presence of thyroid disease (hypothyroidism, hyperthyroidism, struma, and thyroiditis) and the TSH values in the two groups. A univariate logistic regression model was used to investigate the association between benign thyroid diseases, TSH values, and BC.
Results
The mean age was 58.4 years in both groups. We found a significant association between thyroiditis and BC (OR: 1.91, p = 0.01) and were able to refute the association between hyperthyroidism/hypothyroidism and BC. We also found that thyroid-stimulating hormone (TSH) had no significant effect on breast cancer risk.
Conclusion
Many experimental studies suggest a link between hyperthyroidism/hypothyroidism and BC. We were able to demonstrate an epidemiological association between thyroiditis and an increased BC risk. This shows the need for close monitoring for BC in women with thyroiditis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most common types of cancer in women worldwide [1]. In many cases, the etiology of the disease is unclear. In addition to some known risk factors such as age, menopausal status, and hormone therapies, it is suspected that the thyroid gland has an influence on the development of breast cancer [1, 2].
The thyroid gland produces hormones that are responsible for proper metabolic function, cell differentiation, and calcium balance. The thyroid hormones triiodothyronine (T3) and tetraiodothyronine (T4) influence various organs and tissues by binding to a thyroid hormone receptor (TR) [3]. Tang et al. showed that thyroid hormones have a proliferating effect by activating the estrogen receptors in mammary gland tissue [4]. This suggests that thyroid diseases may promote the development of breast cancer.
Various studies on thyroid dysfunction and its association with breast cancer risk have been published in recent years (2.5–13). Søgaard et al. described an increased risk of developing breast cancer in women with hyperthyroidism and a lower risk of developing breast cancer in women with hypothyroidism [5]. In 2012, however, Angelousi et al. were unable to statistically demonstrate the influence of hypothyroidism on breast cancer risk [6]. In a case–control study performed in Taiwan, Weng et al. also described an increased breast cancer risk in patients < 55 years diagnosed with hyperthyroidism. However, this study also showed an increased breast cancer risk in patients with hypothyroidism [7]. Khan et al. described an increased risk of developing solid tumors and breast cancer if increased levels of the free form of thyroid hormone (T4) were found in the circulatory system [2]. Contrary to the epidemiological results, the findings of many experimental studies suggest associations between thyroid diseases and breast cancer on a molecular level.
Although some studies have addressed this issue, there is still no consensus. The present case–control study therefore aims to examine the association between the two diseases and also includes thyroiditis in addition to hyper- and hypothyroidism in order to identify additional risk factors for the development of breast cancer.
Materials and methods
Database
This study was based on data from the Disease Analyzer database (IQVIA), which compiles drug prescriptions, diagnoses, and basic medical and demographic data obtained directly and in anonymized format from computer systems used in the practices of general practitioners and specialists [8]. Diagnoses (according to International Classification of Diseases, 10th revision [ICD-10]), prescriptions (according to Anatomical Therapeutic Chemical [ATC] Classification system), and the quality of reported data are monitored regularly by IQVIA. In Germany, the sampling methods used to select physicians' practices are appropriate for obtaining a representative database of general and specialized practices [8].
Study population
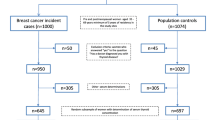
This retrospective case–control study included patients aged 18–80 years with an initial diagnosis of breast cancer (ICD-10: C73) in 200 general practices in the United Kingdom (UK) between January 2006 and December 2015 (index date; Fig. 1). One further inclusion criterion was an observation time of at least 12 months prior to the index date. Patients with cancer diagnoses (ICD-10: C00-C99) prior to the index date were excluded.
Breast cancer patients were matched to non-cancer patients by age (± 1 year), physician, index year, body mass index (BMI) (± 1 kg/m2), and hormone replacement therapy (HRT) status prior to the index date. Matching by age was necessary because the risk of cancer differs with age; matching by physician was necessary as diagnosis behavior differs between physicians; matching by index year was necessary to allow a similar pre-observation time for cases and controls; finally, matching by BMI and HRT status was necessary as BMI and HRT are known to be important risk factors for breast cancer.
For the controls, the index date was that of a randomly selected visit between January 2006 and December 2015 (Fig. 1).
Study outcomes and covariates
The main outcome of the study was the association between different thyroid gland diseases and breast cancer. All thyroid gland disorders found in at least 0.1% of study patients were included in the analyses. These diagnoses were as follows: hypothyroidism [ICD-10: E03], other nontoxic goiter [ICD-10: E04], thyrotoxicosis [hyperthyroidism] [ICD-10: E05], thyroiditis [ICD-10: E06], and other disorders of the thyroid [ICD-10: E07]. Of the patients with thyroiditis, 80% had autoimmune thyroiditis [ICD-10: E06.3]. The mean TSH value per patient based on all documented TSH values prior to the index date was also calculated.
Statistical analyses
Differences in the sample characteristics between subjects with and those without breast cancer were tested using Chi-squared tests by age groups, BMI category, and HRT status, and Wilcoxon tests for mean age and mean BMI. Univariate logistic regression models were used to study the association between the thyroid gland disorders and TSH values and breast cancer incidence. Three different models were calculated. The first of these contained the seven main thyroid gland diseases. The second model included mean TSH values as a continuous variable. Finally, the third model included TSH values grouped as < 0.3, 0.3–4.2 (reference value), 4.3–6.0, 6.1–10.0, and > 10.0 units per liter. p-values < 0.01 were considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Basic characteristics of the study sample
The present study included 7408 patients with breast cancer and 7408 non-cancer controls. The baseline characteristics of study patients after 1:1 matching are displayed in Table 1. The mean age [SD] was 58.4 [12.2] years, the mean BMI was 27.0 (5.7) kg/m2, and 31.1% had received HRT in the past.
Association between thyroid gland disorders and breast cancer
Table 2 shows the proportions of patients diagnosed with various thyroid gland disorders and the results of the regression analyses. A total of 11.0% of cases versus 10.5% of controls had at least one of the thyroid gland disorders, and having at least one thyroid gland disorder was not associated with a higher risk of breast cancer (OR: 1.05, p = 0.218). The only diagnosis significantly associated with breast cancer was thyroiditis (OR: 1.91, p = 0.01), whereby the absolute frequency of this disorder in cases (0.3%) and controls (0.2%) was relatively low.
Association between TSH values and breast cancer
The mean TSH value was 2.2 (SD: 3.0) in breast cancer cases and 12.3 (SD: 2.7) in non-cancer controls. The odds ratio for breast cancer was not significantly higher or lower than that in the reference group of 0.3–4.2 units per liter; it was 2.94 in the groups with a mean TSH value of < 0.3 units per liter, 4.3–6.0 units per liter, 6.1–10.0 units per liter, and 15.87 units per liter (Table 3).
Discussion
Various studies have been conducted to try to better understand the relationship between thyroid dysfunction and the occurrence of breast cancer in women. A causal relationship between the two diseases was suggested as early as the middle of the nineteenth century [9]. However, there is still no consensus in the literature on this issue. This retrospective case–control study showed a significant association between thyroiditis and BC. By contrast, no association between hyperthyroidism and BC or hypothyroidism and BC could be demonstrated.
In a cross-sectional study, Prinzi et al. described a significantly increased risk of developing breast cancer in women under the age of 45 who had been diagnosed with a thyroid disease [10]. Muller et al. (2011) demonstrated a significantly higher prevalence of BC in patients over the age of 49 with benign thyroid disease than in the general population [11]. Chiappa et al. arrived at a similar conclusion in their study examining 867 patients, showing that there was a significant association between BC and benign thyroid diseases in postmenopausal women [12]. Neither study differentiated between hyperthyroidism and hypothyroidism, however. In addition, the number of patients was low in both studies. By contrast, Søgaard et al. were unable to demonstrate any significant effect of hyperthyroidism or hypothyroidism on breast cancer risk in a large cohort study in Denmark in 2016 [5].
Our results also show no significant association between BC and hypothyroidism or hyperthyroidism. The three following meta-analyses came to the same conclusions [6, 13, 14]. Fang et al. [13], who conducted a meta-analyses of 12 studies, were unable to demonstrate an association between hyper- or hypothyroidism and BC. In another meta-analysis, Angelousi et al. concluded that hypothyroidism was not associated with an increased risk of breast cancer [6]. However, they did not draw any conclusions regarding hyperthyroidism in this meta-analysis. Three years later, Angelousi et al. stated in a case–control study that having a history of thyroid disease was not associated with a higher risk of BC. Nevertheless, it should be noted that this study only included 97 BC patients and 48 healthy controls, which meant that statistical tests could not be performed and no significant effect could be established [15].
Furthermore, we concluded that goiter, more specifically referred to as nontoxic goiter, is also not significantly associated with BC. This result contradicts the meta-analysis by Hardefeldt et al. from 2012 [14], in which an increased risk of BC with a pooled OR of 2.26 (95% CI 1.39–3.69) for diffuse and nodular goiters was calculated on the basis of various studies. However, this analysis included five studies with significant heterogeneity. In parallel to the incidence analyses in patients with goiter, Hardefeldt et al. also examined the risk of developing BC when diagnosed with autoimmune thyroiditis. The authors determined that the risk of BC was significantly increased in the presence of an autoimmune thyroid disease (pooled OR of 2.92; 95% CI 2.13–4.01). Chiappa et al. found a significant association between premenopausal patients with autoimmune thyroiditis and the risk of breast cancer [12]. Hashimoto's thyroiditis is an immune-mediated disorder of the thyroid. In 2015, Anil et al. showed an association between Hashimoto's thyroiditis and benign breast diseases [16]. Although this study was not designed to examine the association between thyroid diseases and the simultaneous development of breast cancer, it nevertheless demonstrated a clear connection between the two.
The present study also shows a comparably significant association between thyroiditis and BC. It is important to note that the diagnosis process differed between the two studies. While the present study is based on international ICD-10 coding, the inclusion criteria in the study by Hardefeldt et al. included the diagnostic criteria of increased serum thyroid antibodies, thyroid dysfunction, and histological confirmation of the diagnosis. Therefore, antibody-triggered inflammation of the thyroid gland was detected more specifically, while the present study included acute, subacute, drug-induced, and other thyroid infections in addition to autoimmune thyroiditis, which accounted for 80% of cases, and was thus much more extensive.
Thus, a purely epidemiological view suggests only a vague association between thyroid diseases and the occurrence of breast cancer. When the physiology of both organs is taken into account, however, further associations and mutual influences are revealed.
Although multiple studies have focused on thyroid hormone levels in BC patients, their findings are also controversial. Tosovic et al. examined the T3 levels in 2185 patients as part of a prospective cohort study in 2014 [17]. In addition to increased T3 levels in patients with BC, the authors demonstrated a correlation between increased serum T3 and the size of breast tumors as well as the number of metastases. This contrasts with the findings of a 2018 case–control study by Ortega-Olvera et al. [18], which identified an association between low T3 levels and BC in pre- and postmenopausal patients. They also described the presence of increased serum T4 in pre- and postmenopausal patients and an effect modification whereby the influence of T4 decreased with increasing BMI in premenopausal women. Conde et al. [19] and Khan et al. [2] also identified an association between increased free T4 and an increased occurrence of BC. Both studies also examined the TSH values of patients with BC compared with healthy patients but did not find any significant association between TSH values and BC. Furthermore, this study did not show an association between increased or decreased TSH values and the incidence of BC. The findings of Angelousi et al. are in agreement with this result, as the authors of that study also found that an increased TSH value had no significant effect in patients with BC [15].
The mammary gland and thyroid show similarities at the cellular level, which may be the reason behind the causality of the association. The thyroid peroxidase (TPO) is immunologically similar to lactoperoxidase, both of which can be detected in breast and thyroid tissues [7, 15]. In this respect, Godlewska et al. examined thyroid peroxidase (TPO) expression levels in breast cancer tissue and healthy surrounding tissue in a number of patients [20]. In particular, they showed that considerably less TPO is transcribed in poorly differentiated breast cancer (grade 3). Muller et al. hypothesized that patients with breast cancer have a better prognosis if they have TPO-antibodies (TPO-AB) [21]. A year later, Kemal Y et al. published a study in which they demonstrated an association between TPO-AB and a lower incidence of BC metastases [22]. The value of this study is limited by the small number of patients included, however. The question of whether TPO-AB can be declared a protective factor in breast cancer must therefore be examined in further studies, taking into account the molecular overlaps.
In addition, thyroid hormone receptors (TR) were detected on the surfaces of cancer cells, which suggests that thyroid hormones have an influence on the metabolism of tumor cells [23, 24]. Heublein et al. found contrary prognoses for the presence of thyroid receptors alpha or beta [25]. The effect of thyroid hormones on tumor cells and especially on breast cancer cells has been investigated in several studies. In 2019, Liu et al. described the influence of thyroid hormones on cancer cells due to the stimulation of cell proliferation and angiogenesis [26]. Sar et al. however, attributed a pro-apoptopic effect to T3. This invalidates the hypothesis regarding the pro-carcinogenicity of thyroid hormones. By contrast, Tang et al. demonstrated that thyroid hormones (TH) had a proliferative effect via the estrogen receptors [4]. This in turn shows that thyroid hormones can partially imitate the effects of estrogen. As early as 2002, Dinda et al. demonstrated that TH had estrogen-like effects in breast cancer cells [27]. In a more recent 2014 cohort study, Conde et al. examined the effects of T3 and estradiol in the tissues of breast cancer patients and concluded that T3 can also activate the genes that are otherwise stimulated by estradiol [19].
In summary, this study revealed a significant association between thyroiditis and the simultaneous occurrence of breast cancer in patients. The rare presence of thyroiditis in the case and control groups should be noted as a limitation. Therefore, there is a need to explore this hypothesis in a larger study population. Our study refutes the theory that there is an association between hyperthyroidism or hypothyroidism and an increased or decreased risk of BC.
The lack of information on some potential confounding factors (e.g., marital status, number of children, smoking, alcohol use, body mass index, physical activity, menopausal status), tumor staging using TNM classification, family history, menopause status, mortality data, and T3 and T4 values is another limitation of this study. Moreover, cancer and thyroid disorder diagnoses cancer and depression diagnoses relied solely on ICD codes provided by GPs. The major strengths of this work are the number of patients available for analysis and the length of the follow-up period.
Although many experimental studies indicate a relationship between breast cancer and benign thyroid diseases, the association between the two diseases remains controversial. There is a need for additional studies that combine the pathophysiological aspects and the epidemiological facts in order to be able to demonstrate a causal connection between the diseases.
References
Momenimovahed Z, Salehiniya H (2019) Epidemiological characteristics of and risk factors for breast cancer in the world. Breast cancer 11:151–164
Khan SR, Chaker L, Ruiter R, Aerts JGJV, Hofman A, Dehghan A et al (2016) Thyroid function and cancer risk: the rotterdam study. J Clin Endocrinol Metab 101(12):5030–5036
Cheng S-Y, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31(2):139–170
Tang HY, Lin HY, Zhang S, Davis FB, Davis PJ (2004) Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 145(7):3265–3272
Søgaard M, Farkas DK, Ehrenstein V, Jørgensen JOL, Dekkers OM, Sørensen HT (2016) Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. Eur J Endocrinol 174(4):409–414
Angelousi AG, Anagnostou VK, Stamatakos MK, Georgiopoulos GA, Kontzoglou KC (2012) Mechanisms in endocrinology: primary HT and risk for breast cancer: a systematic review and meta-analysis. Eur J Endocrinol 166(3):373–381
Weng CH, Chen YH, Lin CH, Luo X, Lin TH (2018) Thyroid disorders and breast cancer risk in Asian population: a nationwide population-based case-control study in Taiwan. BMJ Open 8(3):020194
Rathmann W, Bongaerts B, Carius H-J, Kruppert S, Kostev K (2018) Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther 56(10/2018):459–466
Loeser A (1954) A new therapy for prevention of post-operative recurrences in genital and breast cancer. Br Med J 2:1380
Prinzi N, Baldini E, Sorrenti S, de Vito C, Tuccilli C, Catania A et al (2014) Prevalence of breast cancer in thyroid diseases: results of a cross-sectional study of 3,921 patients. Breast Cancer Res Treat 144(3):683–688
Muller I, Pinchera A, Fiore E, Belardi V, Rosellini V, Giustarini E et al (2011) High prevalence of breast cancer in patients with benign thyroid diseases. J Endocrinol Invest 34(5):349–352
Chiappa C, Rovera F, Rausei S, del Ferraro S, Fachinetti A, Lavazza M et al (2017) Breast cancer and thyroid diseases: analysis of 867 consecutive cases. J Endocrinol Invest 40(2):179–184
Fang Y, Yao L, Sun J, Yang R, Chen Y, Tian J et al (2017) Does thyroid dysfunction increase the risk of breast cancer? A systematic review and meta-analysis. J Endocrinol Invest 40(10):1035–1047
Hardefeldt PJ, Eslick GD, Edirimanne S (2012) Benign thyroid disease is associated with breast cancer: a meta-analysis. Breast Cancer Res Treat 133(3):1169–1177
Angelousi A, Diamanti-Kandarakis E, Zapanti E, Nonni A, Ktenas E, Mantzou A et al (2017) Is there an association between thyroid function abnormalities and breast cancer? Arch Endocrinol Metab 61(1):54–61
Anil C, Guney T, Gursoy A (2015) The prevalence of benign breast diseases in patients with nodular goiter and Hashimoto’s thyroiditis. J Endocrinol Invest 38(9):971–975
Tosovic A, Bondeson A-G, Bondeson L, Ericsson U-B, Manjer J (2014) T3 levels in relation to prognostic factors in breast cancer: a population-based prospective cohort study. BMC cancer 14(1):536
Ortega-Olvera C, Ulloa-Aguirre A, Ángeles-Llerenas A, Mainero-Ratchelous FE, González-Acevedo CE, de Hernández-Blanco ML et al (2018) Thyroid hormones and breast cancer association according to menopausal status and body mass index. Breast Cancer Res 20(1):94
Conde SJ, de Luvizotto AMR, de Síbio MT, Nogueira CR (2014) Thyroid hormone status interferes with estrogen target gene expression in breast cancer samples in menopausal women. ISRN Endocrinol 2014:1–8
Godlewska M, Arczewska KD, Rudzińska M, Łyczkowska A, Krasuska W, Hanusek K et al (2017) Thyroid peroxidase (TPO) expressed in thyroid and breast tissues shows similar antigenic properties. PLoS ONE 12(6):e0179066
Muller I, Giani C, Zhang L, Grennan-Jones FA, Fiore E, Belardi V et al (2014) Does thyroid peroxidase provide an antigenic link between thyroid autoimmunity and breast cancer? Int J Cancer 134(7):1706–1714
Kemal Y, Demirag G, Ekiz K, Yucel I (2015) Antithyroid peroxidase antibody positivity is associated with lower incidence of metastasis in breast cancer. Mol Clin Oncol 3(3):629–632
Davis PJ, Tang H-Y, Hercbergs A, Lin H-Y, Keating KA, Mousa SA (2018) Bioactivity of thyroid hormone analogs at cancer cells. Front Endocrinol (Lausanne) 9:739
Glinskii AB, Glinsky GV, Lin HY, Tang HY, Sun M, Davis FB et al (2009) Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 8(21):3562–3570
Heublein S, Mayr D, Meindl A, Angele M, Gallwas J, Jeschke U et al (2015) Thyroid hormone receptors predict prognosis in BRCA1 associated breast cancer in opposing ways. PLoS ONE 10(6):e0127072
Liu YC, Yeh CT, Lin KH (2019) Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int J Mol Sci 20(20):1–27
Dinda S, Sanchez A, Moudgil V (2002) Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene 21:761–768
Funding
The authors have received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LB, MK, LS, KK declares that they have no conflict of interest.
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Informed consent
German law allows the use of anonymous electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bach, L., Kostev, K., Schiffmann, L. et al. Association between thyroid gland diseases and breast cancer: a case–control study. Breast Cancer Res Treat 182, 207–213 (2020). https://doi.org/10.1007/s10549-020-05675-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05675-6