Abstract
Purpose
A recent study reported that time to adjuvant chemotherapy (TTC) > 30 days was significantly associated with worse OS and DFS in triple-negative breast cancer (TNBC). Earlier studies, however, found that worse outcomes were associated with TTC > 60 days or > 90 days. As the trend for mastectomy with reconstruction continues to rise, TTC of < 30 days is often not feasible due to wound-healing issues in some of these patients. To elucidate the impact of TTC, we sought to evaluate the clinical outcomes associated with TTC in a contemporary cohort treated for TNBC at a single institution.
Methods
A single-institution database was queried to identify nonmetastatic TNBC patients who received adjuvant chemotherapy from 2009 to 2018. TTC was defined as interval between date of surgery and adjuvant chemotherapy start date. Median TTC was used to divide our cohort into four quartiles; ≤ 31, 32–42, 43–56, and > 56 days. Logrank, Kaplan–Meier, and inverse probability weighting (IPW) tests were used to analyze disease-free (DFS) and overall survival (OS).
Results
The mean TTC of our study cohort (n = 724) was 48 days (median TTC = 42 days). Black race, mastectomy without adjuvant radiation, and mastectomy with immediate reconstruction were associated with delayed TTC (all p-values < 0.01). In multivariate IPW analysis, TTC > 56 (n = 173) days did not impact DFS or OS compared to TTC ≤ 31 (n = 198) days (p = 0.27 and p = 0.21, respectively). Similar results were seen during subgroup analysis for groups identified as higher risk for delayed TTC.
Conclusion
Our results demonstrated that TTC was not significant or significantly associated with DFS or OS in patient receiving chemotherapy for operable TNBC. Our results were reassuring for patients electing mastectomy with immediate reconstruction, who may experience a longer TTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancers (TNBCs), characterized by lack of expression of hormone and HER2 receptors, account for ~ 15% of all breast cancer subtype diagnoses [1]. Although adjuvant chemotherapy has improved recurrence and mortality rates, TNBC patients continue to have a worse prognosis following chemotherapy than other breast cancer subtypes [2]. While adjuvant chemotherapy usually starts within 12 weeks after surgery, no definitive treatment timeline exists [3, 4].
The relationship between time to chemotherapy (TTC) and outcomes has been evaluated retrospectively with conflicting results regarding how long is considered too long for chemotherapy to commence [3,4,5,6,7,8,9,10,11,12]. While the majority of studies on this topic have not shown detrimental effects of postponing chemotherapy, two recent studies reported that a delay in initiating adjuvant chemotherapy was associated with adverse outcomes [3, 4]. In the first study, beginning chemotherapy at 61 or more days after surgery was associated with diminished survival [3]. This association was the strongest in the HER2 + and TNBC breast cancer subtypes [3]. The second report found that initiating chemotherapy more than 90 days post surgery impacted both overall survival (OS) and breast cancer-specific survival for patients with TNBC, but not the patients with HER2 + or hormone receptor-positive breast cancer [4]. A recent abstract indicated that TNBC patients experienced significantly worse disease-free survival (DFS) outcomes when TTC was longer than 30 days [13]. The 30-day interval may not be a feasible time interval to start adjuvant chemotherapy in patients who experience postoperative complications after their definitive surgery.
To elucidate whether the effects of delayed TTC is associated with worse outcomes in TNBC, we performed a retrospective study on a patient cohort diagnosed with nonmetastatic TNBC treated at our institution between 2009 and 2018. We evaluated factors associated with delayed TTC and evaluated the impact of delayed treatment on clinical outcomes.
Methods
Data sources and study cohort
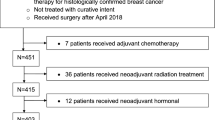
After obtaining Institutional Review Board approval, our institution cancer registry database was queried to identify patients diagnosed with nonmetastatic triple-negative breast cancer (TNBC) who were treated with surgery followed by adjuvant chemotherapy between January 2009 and June 2018. Although our institutional registry has been prospectively collecting information since 2004, we elected to begin our study at 2009, given the improved consistency of electronic medical record data at that time. We identified 796 patients who met inclusion criteria. We excluded those with missing pathologic staging (n = 38) or treatment information (n = 8) as well as lumpectomy patients who did not complete adjuvant radiation (n = 26). Our final cohort comprised 724 patients.
Time to chemotherapy (TTC)
TTC was defined as the time, in days, from first surgery to initiation of adjuvant chemotherapy. Since treatment delay was defined in previous study using cutoffs that were arbitrarily set, we evaluated the TTC within our institution to identify when the majority of patients began treatment. Rather than arbitrarily setting TTC as 30-day intervals, we used the median TTC (42 days) as the cutoff to define four intervals or quartiles.). Our four TTC quartiles were ≤ 31, 32–42 days, 43–56 days, or > 56 days. Those who underwent chemotherapy in the fourth quartile (> 75th percentile TTC) were considered to have delayed treatment.
Outcome measures
Our primary outcome measures were disease-free survival (DFS) and overall survival (OS) of patients stratified by TTC. Overall survival was calculated from the date of diagnosis to the date of death or last contact as of January 21, 2019. Disease-free survival was calculated from the date of diagnosis to the date of first recurrence or last contact. DFS and OS stratified by TTC categories of ≤ 31, 32–42 days, 43–56 days, or greater than 56 days were compared using the Kaplan–Meier method. We then evaluated the impact of delayed TTC on survival using the inverse probability weighting analysis.
Statistical analysis
Demographic and clinical characteristics of our cohort were compared according to TTC group using Chi-squared tests for categorical variables and ANOVA tests for continuous variables. For univariate outcome analysis, we used the Kaplan–Meier survivor function to estimate the mean OS and DFS according to TTC group, with TTC of ≤ 31 days as the reference group.
We used inverse probability-weighted (IPW) analysis based on the propensity score to adjust for imbalances in baseline characteristics between groups [14,15,16]. A logistic regression model was fitted to estimate each individual’s propensity score. When performing the IPW analysis, weights are applied to individuals in each group to create a pseudo-population in which potential confounders are balanced between groups [16]. Patients with TTC > 56 days were considered to have delayed chemotherapy treatment, and patients with TTC ≤ 31 day s were used as the reference group. In secondary analysis, TTC delays of > 60 or > 90 days were used as the delayed chemotherapy group and compared to a baseline of 30 days or less using an IPW model. Weights were used to improve accuracy of our model and estimate the average effect of delayed TTC on DFS and OS [15]. We ran IPW models after adjusting for confounders included in Table 1: age at diagnosis, race, year diagnosed, pathologic stage, and definitive treatment (lumpectomy with radiation or mastectomy with or without radiation). Receipt of reconstruction could not be used in the overall IPW model as many of the patients who had mastectomy and no post-mastectomy radiation (PMRT) and those who had mastectomy with immediate reconstruction were the same, causing colinearity. When doing the subanalysis of just no PMRT patients, however, we were able to adjust for this potential confounder.
Kaplan–Meier and IPW tests were conducted for variables including black race, no PMRT, and mastectomy with reconstruction which were shown to be associated with delayed TTC in our analyses. All hypothesis tests were two-sided, and p ≤ 0.05 was considered statistically significant. STATA 15/SE was used to carry out all statistical analyses (StataCorp LLC, College Station, TX).
Results
Demographics
Among the 724 patients included in our analyses, the median TTC was 42 days (Fig. 1). Two-thirds of our cohort began chemotherapy within 48 days, and only 5.66% of patients underwent chemotherapy treatment beyond 90 days (Fig. 1). The patient, tumor, and treatment characteristics of our cohort stratified by TTC group are listed in Table 1. We observed that compared to whites, blacks were significantly more likely to begin chemotherapy > 56 days after surgery (p < 0.01). Patients who underwent mastectomy and no PMRT had significantly delayed TTC compared to those who underwent breast-conserving surgery or those who had PMRT (p < 0.01). Patients electing mastectomy with immediate reconstruction also experienced significantly longer TTC (p < 0.01). TTC did not vary by pathological stage, age at diagnosis, or year diagnosed.
Primary analysis
Median follow-up was 45 months. Survival analyses using the Kaplan–Meier method for DFS and OS according to TTC are presented in Fig. 2 and 3, respectively. Compared to TTC ≤ 31 days, TTC of 32–42 days did not have significant impact on DFS or OS (p = 0.73 and p = 0.48, respectively). Similar results were seen for TTC of 43–56 days (p = 0.36 and p = 0.20, respectively) and TTC > 56 days (p = 0.84 and p = 0.95, respectively) compared to TTC ≤ 31 days in univariate analysis. We then carried out weighted survival analysis using variables associated with delayed TTC shown in Table 1. Results were similar to the overall analysis (Fig. 2 and 3). Again, black race, no PMRT, and mastectomy with immediate reconstruction were more likely to result in delayed TTC. However, the delayed TTC did not impact survival (Fig. 2 and 3).
Using a reference of TTC ≤ 31 days, we preformed multivariate analyses for OS and DFS to estimate the impact of TTC > 56 days on survival (Table 2). After adjusting for potential confounders using an IPW model, we observed that a TTC > 56 days did not significantly impact DFS or OS (p = 0.27 and p = 0.21, respectively). We performed subset IPW analyses for black race, no PMRT, and mastectomy with immediate reconstruction. In all the groups, delayed TTC did not impact OS or DFS compared to TTC of ≤ 31 days (Table 2).
As the benchmark for treatment delays has been defined as TTC > 60 days or 90 days in prior studies, we repeated our outcome analyses using quartiles 30 days or less, 31–60 days, 61–90 days, or more than 90 days used in other studies (Supplementary Fig. 1 and Table 1) [3, 4]. DFS and OS stratified by these four TTC quartiles were compared using the Kaplan–Meier method. We also evaluated the impact of delayed TTC > 60 days or > 90 days compared to reference quartile of < 30 days using IPW. As shown in Supplementary Fig. 1, we did not observe an impact of TTC on DFS or OS in TTC > 60 days, TTC > 90 days or any other TTC intervals.
Discussion
Two recent studies reported that delayed TTC in breast cancer patients was associated with worse outcomes [3, 4]. The impact of TTC appeared to be most prominent for those with TNBC. Subgroup analysis of TNBC patients who had TTC > 60 days (n = 156, 17.5%) had worse OS (HR: 1.54, p = 0.02) and distant relapse-free survival (HR: 1.36, p = 0.06) compared to those who had TTC < 31 days [3]. In the second report, a TTC of ≥ 91 days (p = 371, 7.9%) was associated with diminished OS (HR: 1.53, CI (1.17–2.00)) for TNBC [4]. Results of a recent study further narrowed the optimal TTC window to 30 days by showing that TTC > 30 days for TNBC was associated with significantly worse outcomes. In contradiction to these previous reports, we did not see an association between TTC and outcome during secondary analysis when delayed TTC was defined as > 60 days or as > 90 days (Supplementary Table 1).
Our results also showed that outcomes are similar when TTC is defined using quartiles: ≤ 31, 32–42 days, 43–56 days, or > 56 days. These results, however, aligned with results from several other studies [5, 8, 10, 12, 17]. In one study, Cold and coauthors reported that 98% of the analyzed patients began chemotherapy within 3 months following surgery. Of these patients, those who initiated chemotherapy 13 weeks after surgery had similar OS to those who began treatment within 3 weeks [5]. A group from Spain reported that OS was similar for patients with a TTC of > 9 weeks compared to < 3 weeks. The majority of patients in this study began chemotherapy within 6 weeks, and only 8% delayed treatment beyond 9 weeks [8]. Shannon et al. found no differences in DFS or OS when TTC was assessed as a continuous variable or when using a 21, 28, or 35 days cutoff [10]. Finally, data from the British Columbia Cancer Agency suggested that chemotherapy is equally effective if initiated within 12 weeks from surgery [7].
Our study was retrospective by design similar to other prior studies. Our cohort size was also similar to several prior studies [3, 4, 13]. Ours is, however, one of the earliest studies to focus on the clinical impact of TTC on patients diagnosed with one breast cancer subtype, TNBC. To adjust for the inherent bias of our retrospective study design, we used an inverse probability weighting (IPW) model to control for baseline covariate differences between groups. A notable difference between our study and others was the way in which we defined TTC. We used the median time to chemotherapy and interquartile range of days to define TTC quartiles as ≤ 31, 32–42 days, 43–56 days, or > 56 days. Other studies had used arbitrary quartiles of 30 days or less, 31–60 days, 61–90 days, or more than 90 days. We compared clinical outcomes using TTC quartiles defined in this study or other studies and did not observe a deleterious clinical impact of any of the TTC quartiles examined. Our inability to demonstrate an association between TTC and outcomes may be because our sample size was not powered to detect differences or TTC is not a prognostic factor in TNBC. The lack of clinical impact of TTC may also reflect the relatively homogeneous clinical practice of a single institution with an established patient navigation and clinical care pathway.
Our study identified several factors associated with TTC > 56 days which included black race, no PMRT, and mastectomy with immediate reconstructions including both implant-based and autologous tissue-based reconstructions. As noted in other studies, patients who underwent mastectomy with immediate reconstruction have been shown to have delayed TTC [18, 19]. Our results, however, did not show that these patients with TTC > 56 days, who had mastectomy with immediate reconstruction, had worse outcomes. Besides TTC, several other time intervals, prior to receipt of chemotherapy, may also play a role in treatment delay. These time intervals include the time between detection of abnormal exam or imaging findings and access to diagnostic breast imaging, time interval from tissue diagnosis to oncologic and/or plastic surgery consultation, and time interval from surgical consultation to definitive surgery. Whether any of these time intervals individually or in total affect clinical outcomes is uncertain and warrants further investigation.
In conclusion, we demonstrated that TTC > 56, TTC > 60, and TTC > 90 days did not impact DFS or OS in a contemporary TNBC cohort treated at a single institution.
References
Yamamoto Y, Iwase H (2010) Clinicopathological features and treatment strategy for triple-negative breast cancer. Int J Clin Oncol 15:341–351. https://doi.org/10.1007/s10147-010-0106-1
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948. https://doi.org/10.1056/NEJMra1001389
De Melo Gagliato D, Gonzalez-Angulo AM, Lei X et al (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32:735–744. https://doi.org/10.1200/JCO.2013.49.7693
Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH (2016) Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2:322–329. https://doi.org/10.1001/jamaoncol.2015.3856
Cold S, Düring M, Ewertz M et al (2005) Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer 93:627–632. https://doi.org/10.1038/sj.bjc.6602734
Colleoni M, Coates AS, Gelber RD et al (2000) Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. J Clin Oncol 18:584–584. https://doi.org/10.1200/jco.2000.18.3.584
Lohrisch C, Paltiel C, Gelmon K et al (2006) Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 24:4888–4894. https://doi.org/10.1200/jco.2005.01.6089
Jara Sánchez C, Ruiz A, Martín M et al (2006) Influence of timing of initiation of adjuvant chemotherapy over survival in breast cancer: a negative outcome study by the spanish breast cancer research group (GEICAM). Breast Cancer Res Treat 101:215–223. https://doi.org/10.1007/s10549-006-9282-0
McLaughlin JM, Anderson RT, Ferketich AK et al (2012) Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 30:4493–4500. https://doi.org/10.1200/jco.2012.39.7695
Shannon C, Ashley S, Smith IE (2003) Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol 21:3792–3797. https://doi.org/10.1200/JCO.2003.01.073
Hershman DL, Jacobson JS, Wang X et al (2006) Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat 99:313–321. https://doi.org/10.1007/s10549-006-9206-z
Bellon JR, Come SE, Gelman RS et al (2005) Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol 23:1934–1940. https://doi.org/10.1200/JCO.2005.04.032
Morante Z, R R, De la Cruz G Impact of the delayed initiation of adjuvant chemotherapy in the outcomes of triple negative breast cancer
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34:3661–3679. https://doi.org/10.1002/sim.6607
Cole SR, Hernán MA (2008) Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168:656–664. https://doi.org/10.1093/aje/kwn164
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Buzdar AU, Smith TL, Powell KC et al (1982) Effect of timing of initiation of adjuvant chemotherapy on disease-free survival in breast cancer. Breast Cancer Res Treat 2:163–169
Vandergrift JL, Niland JC, Theriault RL et al (2013) Time to adjuvant chemotherapy for breast cancer in national comprehensive cancer network institutions. J Natl Cancer Inst 105:104–112. https://doi.org/10.1093/jnci/djs506
Khan F, Cranmer D, Kachajian J et al (2016) Access to care in vermont: factors linked with time to chemotherapy for women with breast cancer—a retrospective cohort study. J Oncol Pract 12:e848–e857. https://doi.org/10.1200/jop.2016.013409
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2019_5282_MOESM1_ESM.tif
Supplementary material 1—Kaplan–Meier curves for overall survival (OS) and disease free survival (DFS)according to time to chemotherapy (TTC), in months, for TNBC patients, with a TTC of ≤ 30 days as a reference group (TIFF 929 kb)
Rights and permissions
About this article
Cite this article
Pomponio, M.K., Keele, L.J., Fox, K.R. et al. Does time to adjuvant chemotherapy (TTC) affect outcomes in patients with triple-negative breast cancer?. Breast Cancer Res Treat 177, 137–143 (2019). https://doi.org/10.1007/s10549-019-05282-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05282-0