Abstract
Purpose
The optimal time interval from neoadjuvant chemotherapy (NAC) to surgery in patients with breast cancer has not been established. We investigated whether different time intervals impact the rate of pathologic complete response (pCR), disease free survival (DFS), overall survival (OS), surgical complications, and rates of conversion from mastectomy to breast conserving surgery (BCS) in this population.
Methods
We identified patients who received NAC at the BC Cancer Agency followed by surgery from May 2012 to April 2018. Patients were grouped based on time interval between NAC and surgery: < 4 weeks, 4–8 weeks, and > 8 weeks. Kaplan Meier method was used to estimate DFS and OS. Rates of pCR between the time intervals were also compared.
Results
Of the 343 patients, 78 (22.8%) received surgery < 4 weeks, 233 (67.9%) received surgery between 4–8 weeks, and 32 (9.3%) received surgery > 8 weeks after NAC, with a median time to surgery (TTS) of 5.0 weeks. pCR was observed in 32.1%, 32.2%, and 28.1%, respectively (p = 0.90). Median follow-up time was 3.3 years. The 5-year DFS was 76%, 78%, and 70% (p = 0.89), respectively. The 5-year OS was 83%, 82%, and 78% (p = 0.33), respectively. No statistically significant differences were seen in surgical complications (p = 0.90), or rates of conversion from mastectomy to BCS (p = 0.19).
Conclusions
There were no statistically significant differences in pCR, DFS, OS, surgical complications, and rates of conversion from mastectomy to BCS, among breast cancer patients receiving surgery < 4 weeks, 4–8 weeks, or > 8 weeks after the last dose of NAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neoadjuvant approach has been well established for the curative treatment of breast cancer. Although neoadjuvant treatment does not confer an overall survival benefit compared to adjuvant treatment [1, 2], the neoadjuvant approach has several advantages, including potentially rendering inoperable breast cancer operable, providing the ability to assess a tumor’s response to systemic treatment, and increasing surgical options [3, 4]. The increase in surgical options may allow patients to undergo less invasive surgery, such as converting from an originally planned mastectomy to breast conserving surgery (BCS). This may be beneficial in terms of post-operative complications, cosmetic outcomes, and resource utilization [5, 6]. In addition, pathologic complete response (pCR) has been shown in a pooled analysis and a meta-analysis to be an excellent prognostic marker for long term outcomes, correlating with significantly improved disease free survival (DFS) and overall survival (OS) results [7, 8].
The administration of NAC necessitates the efficient coordination of care across the medical, surgical, and radiation oncology modalities. Patient care can sometimes be delayed when there is a transition between care providers and care systems, with many factors contributing to the time interval before getting surgery after receiving NAC. Whether time to surgery after NAC has an impact on patient outcomes has not been fully characterized.
A single study by Sanford et al. found that a prolonged interval between NAC and surgery might affect survival outcomes [9]. Their data suggests that patients who received surgery > 8 weeks after NAC have worse overall survival, which implies that an optimal time interval between NAC and surgery may exist. In a multivariable analysis, patients who underwent surgery ≤ 4 weeks, 4–6 weeks, and > 6 weeks had equivalent OS, locoregional recurrence-free survival (LRFS), and recurrence-free survival (RFS), although a sensitivity analysis suggested worse OS in patients who underwent surgery at > 8 weeks.
Reported clinical trials in the neoadjuvant setting have varying time intervals between the completion of chemotherapy and surgery ranging between 1 and 5 weeks from last dose of NAC. Wildiers et al. describes time to surgery as early as 1–3 weeks after NAC in patients with locally advanced breast cancer [10]. Sparano et al. reported a time interval of about 4 weeks between the completion of neoadjuvant chemotherapy and surgery in patients with stage IIB-IIIC breast cancer [11]. Three other studies describe a time interval of 28 days between NAC and surgery [12,13,14]. Finally, a retrospective study of a single surgeon’s immediate breast reconstructions by Azzawi et al. noted a median time to surgery of 37 days from last dose of NAC to surgery [15]. Conventional practice is to undergo surgery following the neutropenic window post-NAC to avoid morbidity related to healing [16]. Theoretically, there must be a balance between allowing a patient to suitably recover from any potential neutropenia and pursuing surgery to prevent the systemic progression of disease. We sought to determine whether different time intervals to surgery impact pCR, DFS, OS, surgical complications, and rates of conversion from mastectomy to breast conserving surgery (BCS) in the breast cancer population receiving NAC in British Columbia.
Methods
A cohort study was conducted using the BC Cancer Agency’s neoadjuvant treatment (NAT) database. The NAT database prospectively collects data on individual patients undergoing NAC in the BC Cancer Agency Vancouver center. Variables collected include patient and treatment characteristics. The database is consistently maintained with weekly, monthly, and quarterly reviews to ensure that the data is accurate. Ethics approval was obtained through the University of British Columbia’s Research Ethics Boards (REB). Patients were included in the study if they had undergone NAC followed by surgical resection between May 2012 and April 2018 at the BC Cancer Agency. Patients were excluded if they had received neoadjuvant radiation or neoadjuvant hormone therapy, if they were not treated with curative intent, or if they had not undergone surgery by April 2018.
Patients were then divided into three groups: those who had surgery < 4 weeks from last dose, 4–8 weeks from last dose, and > 8 weeks from last dose of NAC. These intervals were chosen based on the outcomes from Sanford et al., which noted decreased OS in patients undergoing surgery > 8 weeks after NAC. Time to surgery (TTS) was calculated from the date of the last dose of NAC to the date of surgery. Charts were audited and reviewed for demographic data, comorbidities, tumor characteristics, complications from surgery, and rates of conversion from mastectomy to breast conserving surgery after NAC. Patient comorbidities were stratified using the Charlson Comorbidity Index (CCI) scoring system. The scores ranged from 2 to 7, as all our patients have localized solid tumors.
With respect to outcome measures, pCR was determined based on the pathology report upon completion of surgery and was defined as no invasive disease in the breast and lymph nodes. A pre-specified difference of ≥ 5% in pCR rate was considered to be clinically meaningful between the subgroups. DFS was measured from the date of core biopsy to the date of recurrence. OS was calculated from the date of core biopsy to the date of death, with living subjects censored at last follow-up. Subgroup analyses of tumor subtypes [hormone receptor positive (HR-positive)/HER2-negative, HER2-positive, or triple negative breast cancer (TNBC)] and stage of the initial tumor were also performed. The p-values for pCR, DFS, OS, surgical complications, and rates of conversion from mastectomy to BCS between the three time intervals were calculated using Chi-Squared tests. DFS and OS were calculated using the Kaplan Meier method, and the log-rank test was used to compare groups. Multivariate Cox proportional hazards models were also used to account for any potential confounders that may affect the relationship between time to surgery and survival outcomes. Variables included were selected based on clinical significance: age, pCR, clinical stage, receptor type, comorbidities (measured by CCI), surgery type, and surgical complications. All statistics were carried out using SPSS software version 25 (SPSS Inc, Chicago, Ill).
Surgical complications were defined as infection, hematoma, seroma, or delayed would healing, and were identified through chart review. This data was then coded as being absent or present, and occurring within 4 weeks of surgery or beyond 4 weeks of surgery. The 4 week time point was chosen in consultation with our surgical oncology colleagues, who agree that any complications occurring more than 4 weeks after surgery are unlikely to be directly related to the time interval between completion of chemotherapy and surgery. Data on the rates of conversion from mastectomy to BCS, and the proportion of patients who were rendered eligible for BCS were also collected. These data were analyzed using a Chi-Squared test. Down-staging data were also compiled. We defined a tumor as being down-staged if the pathologic stage of the tumor after surgery was lower than the clinical stage of the tumor before NAC.
Results
We identified 458 cases in the NAT database where patients received NAC with curative intent followed by surgical resection between May 2012 and April 2018. After excluding patients that received adjuvant chemotherapy, neoadjuvant radiation treatment, and neoadjuvant hormonal treatment, 343 patients were analyzed (Fig. 1). The median age of patients in our study was 56 years (range 28–87 years) (Table 1). The Charlson Comorbidity score was used to stratify patients by their comorbidities, and since all patients in our population have a solid tumor, scores ranged from 2 to 7 (Table 1). There were 123 (35.9%) HR-positive/HER2-negative cases, 143 (41.7%) HER2-positive cases, and 77 (22.4%) TNBC cases. Pre-treatment AJCC stages were: 13 (3.8%) stage I, 196 (57.2%) stage II, and 134 (39.0%) stage III (Table 1). In terms of nuclear grade, 5 (1.6%) cases had a grade I tumor, 121 (38.7%) cases had a grade II tumor, 187 (59.7%) cases had a grade III tumor, and 30 (8.7%) cases were unknown on pathology.
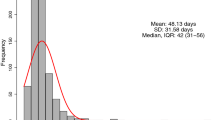
TTS after last dose of chemotherapy ranged from 0.9 to 19.5 weeks, with a median TTS of 5 weeks. The delay in surgery for the patient with the longest wait time was a result of intolerance of the patients’ NAC. The interquartile range for TTS for the overall cohort was 4.0 weeks (28 days) to 6.1 weeks (43 days). In the overall cohort, 78 patients (22.8%) had a TTS < 4 weeks, 233 patients (67.9%) had a TTS of 4–8 weeks, and 32 patients (9.3%) had a TTS > 8 weeks (Table 1). Of the 32 patients with a TTS of > 8 weeks, 8 cases were due to early termination of NAC because of complications and/or adverse side effects, 6 cases were due to patients’ requests and/or non-compliance, and the remaining 18 cases did not have a specific reason. The median TTS for patients who achieved a pCR was 4.9 weeks, and the median TTS for patients who did not achieve a pCR was 5.0 weeks.
The median follow-up time was 3.3 years. The percentage of patients that achieved a pCR was 32.1%, 32.2%, and 28.1% in the < 4 weeks, 4–8 weeks, and > 8 weeks groups (p = 0.90), respectively (Table 2). The 5-year DFS was 76%, 78%, and 70% (p = 0.89) with respect to the three time intervals (Fig. 2). The 5-year OS was 83%, 82%, and 78%, respectively (p = 0.33) (Fig. 3). At the time of analysis, 54 patients had relapsed (15.7%) and 39 patients had died (11.3%). Surgical complications < 4 weeks occurred in 15.4%, 15.9%, and 18.8%, respectively (p = 0.90). Surgical complications occurring > 4 weeks were rare and did not differ between groups.
Subgroup analysis revealed no statistical differences in pCR between the three groups based on receptor status. The HR-positive/HER2-negative subgroup (n = 123) had pCR rates of 4.8%, 9.6%, and 0% (p = 0.53); the HER2-positive subgroup (n = 143) had pCR rates of 47.4%, 51.7%, and 50% (p = 0.90), and the TNBC subgroup (n = 77) had pCR rates of 31.6%, 40.4%, and 0% (p = 0.14), respectively, in each of the < 4 week, 4–8 week, and > 8 week group (Table 2). Conversion from mastectomy to BCS (n = 228) occurred in 9.2% of the < 4 week group, 5.6% in the 4–8 weeks group, and 0% in the > 8 week group (p = 0.19) (Table 2). Down-staging was observed in 75.6% of the < 4 week group, 75.1% in the 4–8 weeks group, and 65.8% in the > 8 week group (p = 0.49) (Table 2). In multivariable analysis, using patients with TTS < 4 weeks as reference, patients who underwent surgery at 4–8 weeks and > 8 weeks had equivalent OS and DFS (Table 3). Multivariable analysis including TTS as a continuous variable did not demonstrate a statistically significant difference in OS and DFS (data not shown).
Discussion
This study found no statistically significant difference in pCR, DFS, OS, surgical complication rates, or rates of conversion from mastectomy to BCS in patients receiving surgery < 4 weeks, 4–8 weeks, or > 8 weeks after last dose of NAC in patients with breast cancer.
The majority of our patients (90.7%) underwent surgery < 8 weeks after completion of NAC. These findings provide additional evidence that delays to surgery up to 8 weeks do not have a meaningful impact on outcomes. This builds on the findings of Sanford et al. that found equivalent OS, LRFS, and RFS outcomes in ≤ 4 weeks, 4–6 weeks, and > 6 week cohorts, with a sensitivity analysis suggesting worse OS in patients who underwent surgery after 8 weeks. The reasons for an extended time to surgery of > 8 weeks are as follows: 6 patients reported medical complications including diverticulitis, congestive heart failure, cellulitis, and hospital admission due to Methicillin-resistance Staphylococcus aureus (MRSA) bacteremia, and 2 patients reported intolerable side effects such as fatigue, and peripheral neuropathy. Patient non-compliance and scheduling conflicts resulted in 6 patients having a TTS of > 8 weeks due to reluctance to undergo surgery, missing NAC doses, and accommodating for patients needing to travel out of the country. While the remaining 18 patients with a TTS of > 8 weeks did not have any reasons documented, it may be explained by limits on operating-room time and constraints on other resources in a publically funded health system. In our cohort, the 32 (9.3%) patients that received surgery > 8 weeks after NAC had a higher median age of 59 years, with a median CCI of 3. Although the patient issues mentioned above were not limited to the TTS > 8 weeks cohort, further investigation would be warranted to provide greater insight into the survival outcomes in this group.
No guidelines currently exist recommending an optimal interval from NAC to surgery. Clinical trials in the neoadjuvant setting have reported variable durations between the completion of NAC and surgery often ranging from 3 to 5 weeks. Several factors may influence a clinician’s decision on when to proceed to surgery after NAC. There is a theoretical risk of performing surgery too early if a patient is still within the neutropenic window after NAC, which may lead to increased morbidity. In addition, significant delays in surgery after NAC may lead to the systemic progression of disease.
In the absence of evidence and guidelines, clinicians have often extrapolated from the adjuvant setting in which several observational studies have demonstrated that delays from surgery to initiation of chemotherapy can impact survival outcomes if treatment is delayed beyond 8 weeks [17, 18]. In certain breast cancer subtypes, such as in triple negative breast cancer, poorer outcomes have been observed in patients with delays greater than 30 days from surgery [19].
Surgical outcomes, although not statistically significant, show a pattern of increasing complications in patients undergoing delayed surgery, which is counter to what one would have expected, given that an increased TTS usually correlates to an immune system that has had more time to recover from NAC. The lack of statistical significance in surgical complications between the three groups may suggest that this time interval (e.g. between 4–8 weeks) is a good balance between the immunosuppressive window after NAC and patient recovery before surgery. Down-staging of the tumor showed a general trend of occurring most frequently in patients that had surgery < 8 weeks after NAC, which may warrant further investigation into whether a specific subtype or stage is more appropriate to undergo later versus earlier surgery. There was also a tendency for patients who received surgery earlier to undergo BCS as opposed to mastectomy, which may be a surrogate marker for down-staging.
This study was subject to limitations inherent in a cohort study performed at a single institution. It is also limited by a median follow-up time of 3.3 years. A randomized controlled trial looking at varying time intervals between NAC and surgery would be challenging to conduct, if not unethical, primarily due to the lack of clinical equipoise. While retrospective, the data collected in this study is robust and detailed. In an era of increasing healthcare demands and surgical wait times, this study does provide additional evidence that patient outcomes were not meaningfully affected within an 8-week window from the completion of NAC.
As breast cancer patients are treated with multiple modalities, it is important to have an evidence-based approach to ensure appropriate timelines are followed between the different treatments, specifically between the medical team and surgical team. In light of the growing number of patients with breast cancer, it may become more difficult to ensure that everyone is treated within a rigid timeframe. But with this evidence, we can help reassure patients and health care professionals alike that a slightly more flexible schedule need not negatively impact patient outcomes. Knowing that a time to surgery of up to 8 weeks will most likely not affect DFS or OS, physicians can properly allocate their time and resources to balance the ever-increasing load of patients that we must schedule for medical and surgical treatments.
References
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr 30:96–102
van der Hage JA, van de Velde CJ, Julien JP et al (2001) Peroperative chemotherapy in primary operatble breast cancer: results from the European organization for research and treatment of cancer trial 10902. J Clin Oncol 19(22):4224
Simmons CE, Hogeveen S, Leonard R et al (2015) A Canadian national expert consensus on neoadjuvant therapy for breast cancer: linking practice to evidence and beyond. Curr Oncol 22(Suppl 1):S43–53
Mauri D, Pavlidis N, Ioannidis JPA (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97(3):188–194
Gralow JR, Burstein HJ et al (2008) Peroperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26(5):814
Shannon C, Smith I (2003) Is there still a role for neoadjuvant therapy in breast cancer? Crit Rev Oncol Hematol 45(1):77
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled anaylsis. Lancet 384(9938):164–172
Spring LM, Fell G, Arfe A, et al. Pathological complete response (pCR) after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival, stratified by breast cancer subtypes and adjuvant chemotherapy usage: patient-level meta-analyses of over 27, 000 patients. In: Proceedings from the 2018 San Antonio Breast Cancer Symposium (SABCS); Dec. 4–8; San Antonio, Texas
Sanford RA, Lei X, Vvarcenas CH, Mittendorf EA, Caudle AS, Valero V, Tripathy D, Giodrano SH, Chavez-MacGregor M (2016) Impact of time from completion of neoadjuvant chemotherapy to surgery on survival outcomes in breast cancer patients. Ann Surg Oncol 23:1515–1521
Wildiers H, Neven P, Christiaens MR, Squifflet P, Amant F, Weltens C, Smeets A, van Limbergen E, Debrock G, Renard V, Van Eeno L, Wynendaele W, Paridaens R (2010) Neoadjuvant capecitabine and docetaxel (plus trastuzumab): an effective non-anthracycline-based chemotherapy regimen for patients with locally advanced breast cancer. Ann Oncol 22(3):588–594
Sparano JA, Moulder S, Kazi A, Coppola D, Negassa A, Vahdat L, Li T, Pellegrino C, Fineberg S, Munster P, Malafa M, Lee D, Hoschander S, Hopkins U, Hershman D, Wright JJ, Kleer C, Merajver S, Sebti SM (2009) Phase II trial of the farnesyl transferase inhibitor tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin Cancer Res 15(8):2942–2948
Honkoop AH, Luykx-de Bakker SA, Hoekman K, Meyer S, Meyer OW, van Groeningen CJ, van Diest PJ, Boven E, van der Wall E, Giaccone G, Wagstaff J, Pinedo HM (1999) Prolonged neoadjuvant chemotherapy with GM-CSF in locally advanced breast cancer. Oncologist 4(2):106–111
Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, Smith I, Walker LG, Eremin O, Aberdeen Breast Group (2002) Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer 3(Suppl 2):S69–74
Balduzzi A, Montagna E, Bagnardi V, Torrisi R, Bertolini F, Mancuso P, Scarano E, Viale G, Veronesi P, Cardillo A, Orlando L, Goldhirsch A, Colleoni M (2009) Infusional fluorouracil, epirubicin, and cisplatin followed by weekly paclitaxel plus bevacizumab in locally advanced breast cancer with unfavorable prognostic features. Anticancer Drugs 20(3):197–203
Azzawi K, Ismail A, Earl H, Forouhi P, Malata CM (2010) Influence of neoadjuvant chemotherapy on outcomes of immediate breast reconstruction. Plast Reconstr Surg 126(1):1–11
Yoo TK, Moon HG, Han W, Noh DY (2017) Time interval of neoadjuvant chemotherapy to surgery in breast cancer: how long is acceptable? Gland Surg 6(1):1–3
Lohrisch C, Paltiel C, Gelmon K et al (2006) Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 24(30):4888–4894
Shin DW, Cho J, Kim SY, Guallar E, Hwang SS, Cho B et al (2013) Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not long or thyroid cancer. Ann Surg Oncol 20:2468–2476
Morante Z, Ruis R, De la Cruz-Ku G, et al. Impact of the delayed initiation of adjuvant chemotherapy in the outcomes of triple negative breast cancer: proceedings from the 2018 San Antonio Breast Cancer Symposium (SABCS); Dec. 4–8, 2018; San Antonio, Texas
Acknowledgements
We would like to acknowledge the neoadjuvant breast cancer database team at the BC Cancer Agency Vancouver Centre, and all patients undergoing neoadjuvant breast cancer treatment at the BC Cancer Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any disclosures or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lai, V., Hajjaj, O., Le, D. et al. Impact of wait time from neoadjuvant chemotherapy to surgery in breast cancer: Does time to surgery affect patient outcomes?. Breast Cancer Res Treat 184, 755–762 (2020). https://doi.org/10.1007/s10549-020-05894-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05894-x