Abstract
Despite guidelines recommending against its routine use, perioperative imaging for distant metastases is frequently performed in newly diagnosed breast cancer patients, uncovering incidental findings of uncertain significance. We assessed the clinical significance of incidental findings by determining if their presence is associated with disease recurrence. A retrospective review of staging imaging was performed in patients with stage II or III invasive breast cancer diagnosed during 2008–2009 at a large academic medical center. Data related to perioperative imaging and disease recurrence were abstracted from the medical record. Kaplan–Meier curves and Cox proportional hazards models were used to assess the association between incidental findings and time to disease recurrence. A total of 169 of 340 patients (49.7 %) underwent staging evaluation for distant metastases (CT chest, abdomen, pelvis, bone scan, and/or PET-CT). Of these, 146 (86.4 %) had at least one suspicious or indeterminate finding. Follow-up studies were performed in 73 (43.2 %) patients. Nineteen patients were diagnosed with metastatic disease at diagnosis, 18 of whom had stage III disease. In patients without metastatic disease at diagnosis, 32 later developed recurrence. Non-calcified pulmonary nodules were associated with shorter time to disease recurrence (hazard ratio 2.51, 95 % CI 1.13–5.57, p = 0.02). Imaging for distant metastases frequently reveals indeterminate findings, most of which are not associated with disease recurrence. The association between pulmonary nodules and recurrence warrants validation in an independent cohort. Overall, these findings support current guidelines recommending against routine extent of disease evaluation in patients with newly diagnosed stage II breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients newly diagnosed with invasive breast cancer often undergo imaging scans to assess for the presence of distant metastatic disease, even though only about 6 % of patients have evidence of metastases at diagnosis. Detection of distant metastases can change clinical management, since treatment in this setting is administered with palliative, as opposed to curative, intent. Extent of disease evaluation usually consists of a CT scan of the chest and abdomen, radionuclide bone scan, and/or PET-CT. Multiple studies have demonstrated that the yield of imaging for distant metastases is low, particularly in patients with early stage breast cancer without signs or symptoms of metastatic disease [1–4]. As part of the Choosing Wisely Campaign, the American Society of Clinical Oncology (ASCO) recommended against the use of routine PET, CT, and radionuclide bone scans for evaluation of asymptomatic patients with stage I–II breast cancer, since in that setting there is a low risk of having identifiable metastatic disease [5]. National Comprehensive Cancer Network (NCCN) guidelines similarly recommend advanced imaging scans for evaluation of metastatic disease in patients with stage I and II breast cancer only in the presence of concerning signs and symptoms [6]. For patients with stage III disease, the NCCN guidelines recommend consideration of imaging for distant metastases due to the higher prevalence of occult metastatic disease in these individuals.
Despite recommendations against screening for metastatic disease in early stage breast cancer, perioperative staging scans continue to be performed [7–9]. Staging scans frequently yield false positive or indeterminate findings, which can lead to further non-invasive and invasive testing, delays in care, increased healthcare costs, and patient anxiety [4, 10–12]. Furthermore, as imaging technology has improved, the rate of detection of incidental findings such as small pulmonary nodules has increased [13], raising many questions regarding the clinical significance of such findings [14, 15]. This retrospective study aims to address the clinical significance of incidental findings on extent of disease imaging by evaluating if the presence of incidental findings is associated with a higher risk of distant breast cancer recurrence in patients with newly diagnosed stage II and stage III breast cancer.
Methods
Patient population
All patients with stage II, III, and IV invasive breast cancer diagnosed in 2008 or 2009 at a large U.S. academic medical center were identified using the tumor registry. Patients listed as having stage IV disease at initial diagnosis of breast cancer were individually reviewed and were included if they had initially presented with a primary breast mass and were subsequently discovered to have metastatic disease based on staging imaging. These patients were classified as stage II or stage III based on their clinical or pathologic (if they had received surgery) stage prior to assessment of distant metastases. Patients who did not undergo definitive surgery and were not thought to have metastatic disease were excluded. Patients who received neoadjuvant therapy were staged clinically. Institutional Review Board approval was granted for this retrospective study, and a waiver of informed consent was obtained.
Study design
Details about patient demographics, tumor characteristics (TNM staging, hormone receptor and HER2 status, grade, histology, angiolymphatic invasion, and surgical margins), treatments received, dates and sites of recurrent disease, and perioperative staging scans (CT chest, CT abdomen/pelvis, bone scintigraphy, and PET-CT) to evaluate for distant metastases were abstracted from the medical record in late 2014. When available, radiology reports from staging scans performed at outside institutions were included in our analysis. Abnormal findings on imaging called suspicious by the radiologist in the radiology report were classified as suspicious; all other abnormal findings were classified as indeterminate. Benign findings including calcified pulmonary nodules, renal cysts, and degenerative changes or dental disease on bone scan were considered to be normal. Subsequent imaging studies and procedures that were performed to further evaluate and/or follow-up indeterminate or suspicious findings were recorded.
Statistical methods
T-tests or Wilcoxon rank sum tests were used to compare continuous characteristics, and Chi-square or Fisher’s exact tests were used to compare categorical characteristics between those who did and did not undergo staging imaging. To further evaluate factors associated with undergoing staging imaging, a multivariable model was performed using backward model selection technique. Kaplan–Meier curves and log-rank tests were used to identify associations between the presence of incidental findings (indeterminate or suspicious findings in patients not determined to have metastases at diagnosis) and time to disease recurrence. Cox proportional hazards models were used to control for time to disease recurrence according to stage, hormone receptor status, and HER2 status.
Results
Patient characteristics
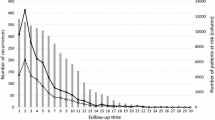
Demographic and clinical characteristics of the patients who did and did not undergo extent of disease evaluation are provided in Table 1. A total of 169 out of 340 patients (49.7 %) underwent evaluation for distant metastases, including CT chest (n = 152), CT abdomen/pelvis (n = 153), bone scan (n = 140), and PET-CT (n = 15) (Fig. 1). Based on review of medical records, reasons for ordering staging scans included stage III disease (n = 86), tumor characteristics or physician preference (n = 42), patient symptoms (n = 12), abnormal laboratory values (n = 6), previous abnormal imaging (n = 6), abnormal physical exam findings (n = 5), planned neoadjuvant therapy (n = 54), patient request (n = 1), and unknown (n = 21). On multivariable analysis, factors associated with undergoing extent of disease evaluation included age [odds ratio (OR) 0.98, 95 % confidence interval (CI) (0.95–1.00), p = 0.041], higher stage [OR 12.33 (3.48–43.75), p < 0.001], higher nodal status [pN1 v. pN0 OR 1.61 (0.89–2.91), pN2-3 v. pN0 OR 24.0 (2.45–235.05), p = 0.014], and receipt of neoadjuvant therapy [OR 4.40 (2.24–8.63), p < 0.001]. Further details regarding patient and clinical characteristics can be found in Online Resource 1.
Findings on initial extent of disease evaluation
Of patients who underwent staging scans, 26 (15.4 %) had findings reported as suspicious for metastases, 120 (71.0 %) had indeterminate findings without suspicious findings, and 23 (13.6 %) did not have indeterminate or suspicious findings. There were 94 (61.8 %) patients with at least one indeterminate or suspicious finding on CT chest, 105 (68.6 %) on CT abdomen pelvis, 43 (30.7 %) on bone scan, and 10 (66.7 %) on PET-CT (Fig. 2). The most common findings included non-calcified pulmonary nodules (n = 73), liver lesions (n = 57), borderline or enlarged lymph nodes (n = 30), renal lesions (n = 26), adnexal lesions (n = 25), and abnormal uptake on bone scan (n = 39). A complete list of findings, which highlights findings determined to represent metastatic disease, can be found in the online supplemental material (Online Resource 2).
To further evaluate abnormalities on staging imaging, 53 of 120 (44.2 %) patients with indeterminate findings and 20 of 26 (76.9 %) patients with suspicious findings underwent follow-up imaging or procedures (Fig. 1). Identification of indeterminate or suspicious findings led to follow-up studies in 25.7 % of all patients who underwent CT chest, in 22.9 % of patients who underwent CT abdomen/pelvis, in 9.3 % of patients who underwent bone scan, and in 26.7 % of patients who underwent PET-CT (Fig. 2). Details regarding the numbers and types of follow-up studies can be found in Online Resource 3.
In total, 19 patients were determined to have metastatic disease at diagnosis, all of whom had findings described as “suspicious for metastases” on initial extent of disease imaging. Of patients with metastases, 8 were diagnosed with metastatic disease based on biopsy, 5 were diagnosed based on the results of follow-up imaging studies for suspicious findings, and 6 were presumed to have metastases based on initial imaging without further imaging studies or biopsies. Metastatic disease was present in 1 (1.3 %) and 18 (19.6 %) of stage II and stage III patients, respectively, who underwent staging imaging. The single clinical stage II patient with metastases had multiple breast masses and a clinically positive axilla, and was determined to have biopsy-proven metastatic disease prior to completion of surgical staging. Of the 18 patients with stage III breast cancer who were found to have distant metastases, 5 had abnormal symptoms, exam features, laboratory values, or prior imaging to prompt imaging. Of the 84 patients with stage III breast cancer who did not have signs or symptoms that led to assessment with scans, 13 (15.5 %) were found to have metastatic disease. On univariate analysis, factors associated with having metastases at diagnosis included higher stage (those with metastases and stage III: 94.7 % v. those without metastases and stage III: 49.3 %, p < 0.001), larger tumor size (those with metastases and T2-T3: 57.8 %, T4: 42.1 %; those without metastases and T2-T3: 68.0 %, T4: 7.3 %, p < 0.001), and the presence of angiolymphatic invasion (those with metastases: 81.8 % v. those without metastases: 38.9 %, p = 0.009). Grade, age, hormone receptor status, triple-negative phenotype, and HER2 positivity were not associated with metastatic disease at diagnosis.
Associations between initial imaging findings and disease recurrence
In the 321 patients without known metastases at the time of breast cancer diagnosis, 46 (14.3 %) were diagnosed with distant disease recurrence with a median duration of follow-up of 5.1 years. Thirty-two of the 46 (69.6 %) patients had undergone imaging at diagnosis. Of those 32 patients, disease recurrence was identified following presentation with abnormal symptoms (n = 25, 78.1 %), abnormal physical exam findings (n = 6, 18.8 %), and abnormal laboratory values (n = 1, 3.1 %). No episodes of disease recurrence were discovered as a result of surveillance imaging for indeterminate findings. Sixteen of the 32 (50.0 %) patients had recurrence within the same organ system as the initial indeterminate finding. Seven of these patients had previously had documented stability of the finding on interval imaging.
Controlling for stage, HER2, and hormone receptor status, the presence of an indeterminate or suspicious finding on extent of disease evaluation at diagnosis was not associated with time to disease recurrence (Hazard Ratio (HR) 1.44, 95 % CI [0.51–6.03], p = 0.51) (Fig. 3a). Indeterminate and suspicious findings were not associated with time to recurrence with any single imaging modality, including CT chest [HR 2.06 (0.89–4.76), p = 0.09], CT abdomen pelvis [HR 1.39 (0.63–3.06), p = 0.42], or bone scan [HR 1.04 (0.44–2.46), p = 0.93] (Fig. 4). The PET-CT cohort (n = 11) was too small to analyze. The presence of non-calcified pulmonary nodules was associated with a significant difference in time to disease recurrence [HR 2.51 (1.13–5.57), p = 0.024] (Fig. 3b). The presence of other specific types of incidental findings, including liver lesions and borderline or enlarged lymph nodes, was not associated with time to disease recurrence (Fig. 4).
Time to disease recurrence. Kaplan–Meier curves comparing time to disease recurrence between patients with and without (a) any indeterminate or suspicious finding (N = 150) and (b) non-calcified pulmonary nodules on CT chest (N = 136). Only patients without metastatic disease at diagnosis are included. Univariable log-rank p values are shown at the bottom right of each graph
Time to disease recurrence by imaging modality or type of incidental finding. Forest plot depicting the association between time to disease recurrence and the presence of indeterminate or suspicious findings on specific imaging modalities (top section) or the presence of specific types of incidental findings (bottom section). Hazard ratios were adjusted using a multivariable model to control for hormone receptor status, HER2 status, and stage
Discussion
Although national guidelines recommend consideration of extent of disease evaluation with advanced imaging in patients with newly diagnosed stage III breast cancer, routine imaging of asymptomatic patients with clinical stage II breast cancer is not recommended because of the low likelihood of identification of metastatic disease. Despite these recommendations, patients often undergo imaging [7–9, 16, 17]. Indeed, about 40 % of patients who underwent imaging had testing performed that was not in accordance with current recommendations. Only a single patient (1.3 %) with stage II disease was found to have metastatic disease. In contrast, over 15 % of patients with stage III disease who did not have signs or symptoms of metastases were upstaged to stage IV. These data are comparable to a recent systematic review, which estimated the median prevalence of asymptomatic distant metastases to be 1.2 and 13.9 % in stages II and III breast cancer patients, respectively [18].
Many of the recommendations against use of imaging at diagnosis are based on older studies performed using tests with low sensitivity, including chest X-ray and liver ultrasound [1, 2, 4, 11]. More sensitive imaging techniques could potentially result in increased detection of metastatic disease at diagnosis, although this has not yet been demonstrated in more modern studies [19]. There is concern about performing extent of disease evaluation with more sensitive advanced imaging because of the high incidence of indeterminate findings [3], which could potentially necessitate additional imaging and/or invasive procedures with associated risks including radiation exposure, bleeding, and infection. Lending merit to this concern, the vast majority of patients (86 %) in our study had at least one indeterminate or suspicious finding on staging imaging for metastatic disease, half of whom underwent additional imaging or procedures.
While previous studies have evaluated the significance of specific types of incidental findings [3, 15, 20], to our knowledge, no study has evaluated the association between such a broad array of indeterminate findings across multiple imaging modalities and the risk of disease recurrence. Overall, we failed to demonstrate a significant association between the presence of indeterminate findings on staging imaging and time to disease recurrence, although the presence of non-calcified pulmonary nodules was associated with a slightly increased risk of recurrence in sub-group analysis. Notably, surveillance imaging for indeterminate findings did not result in a single diagnosis of metastatic disease. With the possible exception of non-calcified pulmonary nodules, our data suggest that the clinical relevance of indeterminate findings is minimal.
At first glance, the association between non-calcified pulmonary nodules and disease recurrence would seem to suggest that more aggressive surveillance of pulmonary nodules is warranted. However, only a single patient with disease recurrence in our cohort had demonstrated growth of previously identified pulmonary nodules, and this was incidentally discovered on a CT-angiogram to evaluate dyspnea. About half of patients with indeterminate pulmonary nodules underwent routine surveillance, which did not lead to a diagnosis of metastatic disease. In addition, based on currently available data, early detection of metastatic disease does not improve survival in breast cancer [21]. However, those studies were conducted decades ago. Additional studies would need to be performed to determine if early detection and treatment of metastatic disease in the modern era of more sensitive cross-sectional imaging technology and more effective therapies would result in improved survival.
A recent retrospective study in Canada suggested that the publication of the Choosing Wisely guidelines had little impact on the frequency with which physicians order imaging studies for distant metastases. In their study, 75 % of patients underwent imaging evaluation that was not in keeping with the spirit of the published guidelines [7]. Another study reported that 55 % of early stage breast cancer patients underwent unnecessary staging imaging at their institution [9]. In our study, based on the reasons documented in the medical record for why scans were performed, about 25 % of all stage II patients could have avoided staging imaging had current guidelines been followed. This could have led to significantly fewer follow-up studies and procedures. Improving physician adherence to guidelines regarding staging imaging has the potential to save costs and reduce radiation exposure, and should be an area of further quality improvement efforts.
All patients in this study who were determined to have metastatic disease at diagnosis had findings on staging imaging that were interpreted as being “suspicious for metastases.” The presence of a suspicious finding had a 73.1 % positive predictive value and a 100 % negative predictive value for metastatic disease. These numbers may be subject to bias since a radiologist’s interpretation can be dependent upon the provided clinical history, and his or her interpretation may influence the treating clinician’s assessment of metastatic disease (58 % of patients with presumed metastases did not undergo confirmatory biopsy). However, several factors affirm the accuracy of the radiologist and clinician assessment of metastatic disease. First, only 2 patients determined to have metastatic disease based on imaging alone were known to be alive at the time of analysis. Second, with the possible exception of non-calcified pulmonary nodules, patients with indeterminate findings did not have a higher risk of disease recurrence. Lastly, none of the 123 imaging studies and 3 procedures/biopsies that were performed to follow-up indeterminate findings led to a diagnosis of metastatic disease, suggesting that many of these studies could have potentially been avoided.
Standardization of language in the radiology report has the potential to better inform clinician decisions regarding follow-up of indeterminate findings. Others have hypothesized that the expression of doubt in a radiology report (for instance, describing a finding with the phrase, “cannot rule out metastatic disease,” rather than “likely benign”) would result in improved follow-up of indeterminate findings [22]. Currently, there is no standard language that is used to characterize a radiologist’s level of suspicion regarding an indeterminate finding. Additionally, placing recommendations for follow-up directly in the radiology report has the potential to better guide clinicians, acknowledging that such guidelines do not exist for every type of incidental finding. Efforts to guide appropriate follow-up of indeterminate findings are ongoing [23, 24].
This study has several limitations. First, although we included a large number of patients with high risk of disease recurrence and approximately 5 years of follow-up, the high prevalence of indeterminate findings limited the power of the study. Second, classification of indeterminate findings was dependent on the radiologist’s interpretation, which could vary from institution to institution, and the majority of imaging tests were performed at a single institution. There were few PET-CT scans performed in this study, which limits the generalizability of results to patients who undergo PET-CT.
As a retrospective study, this study is inherently subject to biases. Information is limited to what was recorded in the medical record, which may not always accurately reflect clinical reasoning. In addition, because not all patients had imaging studies performed at a single institution, we may be unaware of some tests that were performed. However, this potential omission is not likely to affect the overall findings.
In conclusion, evaluation for distant metastasis at the time of diagnosis in patients with clinical stage III breast cancer is reasonable due to the high prevalence of metastatic disease in this population. Extent of disease evaluation at the time of diagnosis for patients with clinical stage II breast cancer is not indicated due to low incidence of detection of metastatic disease. Advanced imaging yields a significant number of indeterminate findings that may require additional imaging and invasive procedures, and these findings appear to be of minimal clinical significance. Identification of effective methods to reduce overuse of testing is required.
References
Puglisi F, Follador A, Minisini AM et al (2005) Baseline staging tests after a new diagnosis of breast cancer: further evidence of their limited indications. Ann Oncol 16(2):263–266
Kasem AR, Desai A, Daniell S, Sinha P (2006) Bone scan and liver ultrasound scan in the preoperative staging for primary breast cancer. Breast J 12(6):544–548
Kim H, Han W, Moon HG et al (2011) The value of preoperative staging chest computed tomography to detect asymptomatic lung and liver metastasis in patients with primary breast carcinoma. Breast Cancer Res Treat 126(3):637–641
Gerber B, Seitz E, Muller H et al (2003) Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat 82(1):29–37
Schnipper LE, Smith TJ, Raghavan D et al (2012) American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol 30(14):1715–1724
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (version 2.2015). National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Mar 9 2015
Simos D, Hutton B, Clemons M (2015) Are physicians choosing wisely when imaging for distant metastases in women with operable breast cancer? J Oncol Pract 11(1):62–68
Crivello ML, Ruth K, Sigurdson ER et al (2013) Advanced imaging modalities in early stage breast cancer: preoperative use in the United States Medicare population. Ann Surg Oncol 20(1):102–110
Han D, Hogeveen S, Sweet Goldstein M et al (2012) Is knowledge translation adequate? A quality assurance study of staging investigations in early stage breast cancer patients. Breast Cancer Res Treat 132(1):1–7
James JJ, McMahon MA, Tennant SL, Cornford EJ (2012) CT staging for breast cancer patients with poor prognostic tumours. Breast 21(6):735–738
Morris PG, O’Connor M, O’Rafferty C et al (2009) The excessive cost of baseline diagnostic imaging in early breast cancer. Ir Med J 102(5):149–152
Yeh KA, Fortunato L, Ridge JA, Hoffman JP, Eisenberg BL, Sigurdson ER (1995) Routine bone scanning in patients with T1 and T2 breast cancer: a waste of money. Ann Surg Oncol 2(4):319–324
Truong MT, Ko JP, Rossi SE et al (2014) Update in the evaluation of the solitary pulmonary nodule. Radiographics 34(6):1658–1679
MacMahon H, Austin JH, Gamsu G et al (2005) Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: a Statement from the Fleischner Society. Radiology 237(2):395–400
Lee B, Lim A, Lalvani A et al (2008) The clinical significance of radiologically detected silent pulmonary nodules in early breast cancer. Ann Oncol 19(12):2001–2006
Hahn EE, Tang T, Lee JS et al (2015) Use of imaging for staging of early-stage breast cancer in two integrated health care systems: adherence with a choosing wisely recommendation. J Oncol Pract. 11(3):e320–e328
Ramsey SD, Henry NL, Gralow JR et al (2015) Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol 33(2):149–155
Brennan ME, Houssami N (2012) Evaluation of the evidence on staging imaging for detection of asymptomatic distant metastases in newly diagnosed breast cancer. Breast 21(2):112–123
Brennan M, Houssami N (2012) Newly diagnosed early breast cancer—an update on pre-operative assessment and staging. Aust Fam Physician 41(11):871–874
Daglar G, Yuksek YN, Gozalan U, Tutuncu T, Kama NA (2010) The significance of pulmonary nodule in breast cancer patients. Bratisl Lek Listy 111(5):280–283
Khatcheressian JL, Wolff AC, Smith TJ et al (2006) American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 24(31):5091–5097
Al-Mutairi A, Meyer AN, Chang P, Singh H (2015) Lack of timely follow-up of abnormal imaging results and radiologists’ recommendations. J Am College Radiol 12(4):385–389
Blagev DP, Lloyd JF, Conner K et al (2014) Follow-up of incidental pulmonary nodules and the radiology report. J Am College Radiol 11(4):378–383
Dutta S, Long WJ, Brown DF, Reisner AT (2013) Automated detection using natural language processing of radiologists recommendations for additional imaging of incidental findings. Ann Emerg Med 62(2):162–169
Funding
Dr. Henry was supported in part by an NCI Clinical Cancer Investigator Team Leadership Award (supplement to 3-P30-CA046592, PI M Wicha).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Henry has previously received research funding from BioMarin, Celldex, and Sanofi. No other authors have conflicts of interest to report.
Informed consent
Institutional Review Board approval was granted for this study and a waiver of informed consent was obtained.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brothers, J.M., Kidwell, K.M., Brown, R.K.J. et al. Incidental radiologic findings at breast cancer diagnosis and likelihood of disease recurrence. Breast Cancer Res Treat 155, 395–403 (2016). https://doi.org/10.1007/s10549-016-3687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3687-1