Abstract
Patients who achieve a pathological complete response (pCR) after neoadjuvant therapy, including chemotherapy with or without trastuzumab (NAT) have better outcomes than patients with residual disease. Despite the excellent prognosis associated with achieving a pCR, tumors still recur. The objective of this study was to evaluate factors associated with tumor recurrence and survival among patients achieving pCR after NAT. We identified 749 patients with primary breast cancer who achieved pCR after NAT between 1988 and 2009. pCR was defined as no evidence of invasive cancer in the breast and ipsilateral axillary lymph nodes on pathological evaluation. The Kaplan–Meier product limit method and multivariate Cox proportional hazards models were used to determine the association between clinical and demographic factors and outcomes. Median follow-up was 35 months (range, 1–258 months). Overall 5-year distant metastasis-free survival was 93 % (95 % confidence interval [CI], 90–95 %) and 5-year overall survival (OS) was 96 % (95 % CI, 93–97 %). In the multivariable model, we observed that patients >50 years had significantly decreased risk of distant metastasis (hazard ratio [HR] 0.47; 95 % CI, 0.22–0.98) and that patients with clinical stage at diagnosis IIIB–C cancer had both an increased risk of distant metastasis (HR 3.92; 95 % CI, 1.54–10.00) and lower OS (HR 4.75; 95 % CI, 1.60–14.08). Patients with pCR after NAT have excellent outcomes. However, our data show that younger patient and those with clinical stage at diagnosis IIIB and IIIC cancers are at increased risk of developing distant metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant therapy (NAT) is used to treat patients with locally advanced breast cancer, and large operable breast cancer to reduce tumor size and improve the rate of breast-conserving surgery [1–4]. Clinicians can also use NAT to determine the efficacy of systemic treatment by monitoring the in vivo response of the primary tumor, [5, 6]. The NAT model is also used to accelerate the development and approval of treatments for early breast cancer by allowing rapid assessment of drug efficacy [7].
After NAT, the pathological evaluation of the residual tumor provides important early prognostic information; it has been shown that patients who achieve a pathological complete response (pCR) at surgery have a survival advantage [8–11]. pCR has been defined in different ways; however, a recent pooled individual patient data analysis established that eradication of invasive tumor from both the breast and lymph nodes was associated with better disease-free and overall survival (OS) compared with eradication from the breast alone [9]. When the stricter definition of pCR was applied in the pooled analysis trials, the average pCR rate decreased from 22 % ypT0/is (absence of invasive cancer from breast alone) to 18 % ypT0/is ypN0 (absence of invasive cancer in the breast and axillary lymph nodes) [9]. The study also re-enforced several earlier studies showing that the relationship between tumor pCR and long-term outcome was strongest among patients with rapidly proliferating tumor subtypes such as human epidermal growth factor receptor type 2 (HER2+) and triple receptor-negative breast cancer.
Research to date has mainly identified factors related to initial achievement of pCR. Little work has examined risk factors associated with recurrence after pCR. Despite the excellent prognosis associated with pCR, some patients still develop recurrence. Previous studies have shown 5-year recurrence rates ranging from 13 to 25 % [9, 12–14] in patients who achieve pCR. In this retrospective study of 749 patients treated with anthracycline with or without taxane-based NAT, we sought to identify risk factors associated with recurrence after pCR.
Methods
Patients with primary breast cancer who received NAT and achieved pCR were retrospectively identified in the Breast Cancer Management System database at The University of Texas MD Anderson Cancer Center. We identified 824 patients who were treated with anthracycline with or without taxane-based neoadjuvant chemotherapy from February 1988 to December 2009. After we excluded inflammatory breast cancer and patients treated with lapatinib-containing regimens, our final cohort included 749 patients.
Patient demographic characteristics, disease stage and subtype, treatment, and outcomes (distant metastasis-free survival [DMFS] and OS) were extracted from the database and summarized using frequencies. Breast cancer subtypes were defined as hormone receptor (HR)-positive (>10 %) breast cancer (including estrogen receptor-positive and/or progesterone receptor-positive); overexpression of HER2-positive breast cancer (regardless of HR status); and triple receptor-negative breast cancer (TNBC; estrogen receptor-negative, progesterone receptor-negative, and HER2-negative). We defined pCR according to the criteria (ypT0/is ypN0) that Cortazar et al. found to be an adequate surrogate for outcome [9]. DMFS was measured from the date of surgery to the first documented distant recurrence or last follow-up. Patients who died before experiencing a distant recurrence were considered censored at the date of death. OS was measured from the date of surgery to the date of death or last follow-up.
The Kaplan–Meier product limit method was used to estimate DMFS and OS; groups were compared with the log-rank test. Multivariate Cox proportional hazards models were used to identify the predictive factors associated with outcome. Results were expressed in hazard ratios (HR) and 95 % confidence intervals (CI). Factors that were significantly associated with outcomes (univariate P ≤ 0.05) were considered in the multivariate models. In addition, adjustment was made for factors that may have lacked statistical significance but have clinical relevance. The final variables in the model included age (>50 vs. ≤50), clinical stage (I–IIA, IIB–IIIA, and IIIB–C), tumor subtype (HR-positive, HER2-positive, and TNBC), number of lymph nodes resected (≥10 vs. <10), and adjuvant radiation therapy. P values less than 0.05 were considered statistically significant, and all tests were 2-sided. Statistical analyses were carried out using SAS 9.1 (SAS Institute Inc., Cary, NC), S-Plus 7.0 (Insightful Corporation, Seattle, WA), and R 2.9.0.
Results
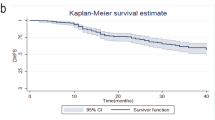
The demographic and clinical characteristics of the 749 patients included in our analysis are summarized in Table 1. The median age was 49 years (range, 21–81 years). 20.7 % of patients had HR-positive tumors, 44.4 % had HER2-positive, and 34.9 % had TNBC. At a median follow-up of 35 months (range, 1–258 months), 5.9 % (n = 44) patients developed distant metastasis and 4.9 % (n = 37) died. Univariate associations between survival outcomes and patient and clinical characteristics are shown in Table 2. The 5-year DMFS rate was 93 % (95 % CI, 90–95 %) and the 5-year OS rate 96 % (95 % CI, 93–97 %) (Table 2). Younger age (≤50) was associated with significantly worse DMFS (P = 0.05), and patients with clinical stage IIIB–C disease had the worst DMFS (P = 0.01) (Fig. 1a, b). Neither tumor subtype nor type of NAT regimen used showed any significant effect on outcome.
Multivariate Cox proportional hazards models showed that, compared to patients who were 50 years old or younger, patients who were older than 50 years had a less risk of developing distant metastasis (HR 0.47; 95 % CI, 0.22–0.98) (Table 3). However, age had no statistically significant impact on OS (HR 0.68; 95 % CI, 0.30–1.54). Compared to patients with stage I–IIA tumors, patients with stage IIIB–C breast cancer had both an increased risk of developing distant metastasis (HR 3.92; 95 % CI, 1.54–10.00) and increased risk of death (HR 4.75; 95 % CI, 1.60–14.08). Tumor subtype, number of lymph nodes resected, and adjuvant radiation showed no statistical significance as markers for recurrence and OS.
Discussion
Several studies have established that achievement of a pCR after NAT is a surrogate marker associated with favorable outcomes in patients with breast cancer [9, 15–18]. Such studies have evaluated the factors associated with achievement of pCR, including HR status, tumor size, histology, intrinsic subtype, and grade [19–22]. However, only a handful of studies have evaluated factors associated with risk of recurrence after pCR. In our large, single-institution, retrospective analysis, we observed a much lower rate of recurrence (7 %) than what has been previously reported (10–25 %) [13, 14, 23, 24]. This is likely due to improvement associated with the chemotherapy regimens used, particularly those targeted against HER2-positive tumors [25]. It is possible that our high pCR rates are influenced by the highly selective nature of our patient population since a large proportion of the population included TNBC and HER2-positive tumors. This phenomenon likely speaks of our ability to select patients most likely to respond to NAT but we cannot rule out some potential distortion of the outcomes. Despite the excellent outcomes associated with achieving a pCR, younger patients and those with locally advanced stage IIIB and IIIC cancer are at increased risk of developing distant metastasis after pCR.
Recent studies have differed on the factors contributing to the risk of distant metastasis after pCR. A previous study of 226 patients by our group found that stage IIIB–C and inflammatory breast cancer, premenopausal status (a variable closely related to age), and resection of fewer than 10 lymph nodes were associated with an increased risk of developing distant metastasis [24]. Our study also found that more advanced disease stage (IIIB–C) and younger age (≤50 years) were predictors of recurrence; however, neither the number of lymph nodes resected nor clinically relevant variables like HR or HER2 status showed any prognostic significance in patients who had achieved a pCR. In a similar study of 8 patients who achieved a pCR after NAT, Tanioka et al. identified HER2-positive status and axillary metastasis as predictors of recurrence [23]. These findings contrast with ours since neither of the latter found HER2 status to be a predictor of recurrence after pCR.
One possible explanation for these differences is that in the study by Tanioka et al. 37 % of patients with HER2-positive disease did not receive trastuzumab and thus did not benefit from anti-HER2 therapy [25]. In our study, 294 patients had HER2-positive tumors, of whom 232 received trastuzumab. Of these, only 11 had distant metastases and 4 died. Our contemporary series of patients therefore reflects the use of anti-HER2 therapies as part of the standard of care. Research on the links between tumor subtype and recurrence after pCR has, likewise, produced mixed conclusions. In a retrospective study of 1731 patients, Guarneri et al. observed that HR status had a significant effect on achievement of pCR; however, among their 225 patients with pCR, HR status was not associated with differential outcomes [26, 27]. Our observations are consistent with this study, since we found no association between HR status and DMFS or OS. Other studies have suggested that the relationship between pCR and outcome does differ according to breast cancer subtypes. These studies have found that pCR appears to be a valid and strong surrogate for long-term survival, mainly in patients with HER2-positive cancer and TNBC. In the German pooled analysis [15, 16] and in the more recent and largest to date CTNeoBC pooled analysis [9], the strongest association between pCR and long-term outcome was observed among patients with aggressive breast cancer subtypes (TNBC, high-grade and HER2-positive tumors). We found no difference in DMFS or OS based on HR status or HER2 status in patients who achieved pCR, suggesting that patients who have no evidence of invasive cancer in the breast and lymph nodes after NAT have an excellent prognosis, regardless of tumor subtype. It is possible, however, that difference in findings could be due to sample size or with longer follow-up, differences will be observed.
Given the retrospective nature of our study design, residual confounding cannot be completely excluded, but we minimized this risk by adjusting for clinically relevant covariates. Despite this limitation, we report on a large series of patients treated with contemporary NAT regimens. Our results highlight the importance of volume of disease in anticipating long-term outcomes. This finding is partly consistent with previous studies in which advanced stage/clinical tumor size was associated with higher risk of distant metastasis [14, 24, 28, 29]. Similarly, premenopausal status has also previously been associated with poorer prognosis, but our study is unique in its specific finding that age 50 years or younger is a predictor of distant metastasis among patients achieving a pCR. These findings may have implications in the design of contemporary trials testing novel agents in the post-neoadjuvant setting in higher risk patients with residual disease. Inclusion of younger patients and those with clinical Stage IIIB–C regardless of pCR status might be warranted.
In conclusion, patients who achieve pCR after NAT have an excellent long-term clinical outcome, DMFS, and OS, in all subgroups. However, we observed that among patients who achieved a pCR after NAT, those younger than age 50 and those with stage IIIB–C disease have a higher risk of developing distant metastasis.
References
Chia S, Swain SM, Byrd DR, Mankoff DA (2008) Locally advanced and inflammatory breast cancer. J Clin Oncol 26:786–790
Liu SV, Melstrom L, Yao K et al (2010) Neoadjuvant therapy for breast cancer. J Surg Oncol 101:283–291
Berruti A, Brizzi MP, Generali D et al (2008) Presurgical systemic treatment of nonmetastatic breast cancer: facts and open questions. Oncologist 13:1137–1148
Kaufmann M, von Minckwitz G, Bear HD et al (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18:1927–1934
Mieog JS, van der Hage JA, van de Velde CJ (2007) Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev Cd005002
Teshome M, Hunt KK (2014) Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin N Am 23:505–523
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366:2438–2441
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778–785
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Bonnefoi H, Litiere S, Piccart M et al (2014) Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol 25:1128–1136
Mieog JS, van der Hage JA, van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94:1189–1200
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Kuerer HM, Newman LA, Smith TL et al (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17:460–469
Fei F, Messina C, Slaets L et al (2015) Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase III trial. Eur J Cancer 51:301–309
von Minckwitz G, Untch M, Nuesch E et al (2011) Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 125:145–156
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21:4165–4174
Feldman LD, Hortobagyi GN, Buzdar AU et al (1986) Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res 46:2578–2581
Tubiana-Hulin M, Stevens D, Lasry S et al (2006) Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol 17:1228–1233
Luangdilok S, Samarnthai N, Korphaisarn K (2014) Association between pathological complete response and outcome following neoadjuvant chemotherapy in locally advanced breast cancer patients. J Breast Cancer 17:376–385
Wu K, Yang Q, Liu Y et al (2014) Meta-analysis on the association between pathologic complete response and triple-negative breast cancer after neoadjuvant chemotherapy. World J Surg Oncol 12:95
Tan MC, Al Mushawah F, Gao F et al (2009) Predictors of complete pathological response after neoadjuvant systemic therapy for breast cancer. Am J Surg 198:520–525
Tanioka M, Shimizu C, Yonemori K et al (2010) Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer 103:297–302
Gonzalez-Angulo AM, McGuire SE, Buchholz TA et al (2005) Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol 23:7098–7104
Gianni L, Eiermann W, Semiglazov V et al (2014) Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 15:640–647
Guarneri V, Broglio K, Kau SW et al (2006) Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 24:1037–1044
Ring AE, Smith IE, Ashley S et al (2004) Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer 91:2012–2017
Jeruss JS, Mittendorf EA, Tucker SL et al (2008) Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol 26:246–252
Mittendorf EA, Jeruss JS, Tucker SL et al (2011) Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 29:1956–1962
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Chaudry, M., Lei, X., Gonzalez-Angulo, A.M. et al. Recurrence and survival among breast cancer patients achieving a pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 153, 417–423 (2015). https://doi.org/10.1007/s10549-015-3533-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3533-x