Abstract
The incidence of ductal carcinoma in situ (DCIS) of the breast has increased in recent decades, particularly, in counties offering mammography screening. The aims of the present study are to examine factors that may predict subsequent breast malignancy amongst patients with DCIS, and to compare the incidence of the subsequent malignancy and mortality with that of the general population. This population-based study includes all primary cases of pure DCIS diagnosed in Norway in the period 1993 to 2007 (N = 3167). The patients were followed to subsequent malignancy (DCIS or invasive cancer) or death. Risk estimates within 10 years of follow-up were calculated using Kaplan–Meier methods adjusting for competing risks, Cox regression models and Standard Incidence and Mortality Ratios. Patients with DCIS had a 11.2% risk of being diagnosed with a subsequent breast malignancy within 10 years (9.4% for invasive cancer), implying that they were five times as likely to be diagnosed with breast malignancy as the general female population in Norway. The risk was dependent on the treatment of the DCIS; patients treated with mastectomy and breast-conserving treatment had a 3.8 and 9.8% risk of ipsilateral invasive cancer within 10 years, respectively. Breast cancer mortality was 2.5% within 10 years of follow-up, a fourfold risk compared with the general population. Patients with DCIS have an increased risk of both subsequent breast malignancy and breast cancer death compared with women in the general population. Our results support previous knowledge of DCIS as a heterogeneous disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of ductal carcinoma in situ (DCIS) of the breast has increased in recent decades, particular, in countries that are offering mammography screening [1–3]. DCIS has shown to be a highly heterogeneous disease [4–8], and the question of whether DCIS is an inevitable step in the development of invasive breast cancer or merely a marker of risk remains unanswered [9]. However, the purpose of treating DCIS is to achieve local control of the disease and to prevent subsequent occurrence of an invasive breast cancer. The risk of ipsilateral invasive breast cancer has been shown to be highly dependent on the given treatment [10]. Amongst patients treated with mastectomy 1–2% were diagnosed with local invasive recurrence within 10 years [10, 11] and amongst those treated with breast-conserving therapy (BCT) the rates were 13 and 28% with and without radiation therapy (XRT), respectively [12]. The risk of contralateral invasive breast cancer has also been increased in patients with DCIS [13–15], where the rate was 6–7% within 10 years of follow-up [14, 15]. The optimal treatment for DCIS patients is still a matter of debate, and the decision-making process has become complex and controversial [5, 16]. Other factors of possible influence for the rates of subsequent malignancy are histopathological tumour characteristics (grade, size, presence of necrosis, multifocality, and surgical margins) as well as patients’ age and method of detection [5, 17, 18].
To differentiate the woman’s susceptibility to breast malignancies from what could be caused by her primary diagnosis (and treatment) of DCIS is an interesting issue, but there is lack of knowledge in this area. The State-of-the-Science conference for DCIS in 2009 expressed the need for further research on this topic of diagnosis and management of DCIS [16].
As a DCIS has not spread to the surrounding tissue, it is by definition not able to cause death. However, in larger observational studies of patients with DCIS the 10-year overall survival and breast cancer-specific survival were reported to be 92 and 98–99%, respectively [10, 19]. The results may be biased due to misclassification of the initial disease as non-invasive cancer or reflect the progression of DCIS to invasive disease. To minimize this type of misclassification error, it is of importance to study the mortality amongst patients diagnosed with pure DCIS. Such studies require large populations, long follow-up periods and high quality data. In an effort to elucidate risk factors for later disease amongst DCIS patients, various authors have reported that certain characteristics of the patient or the tumour may be associated with local recurrence, survival and/or mortality. Several of these studies have analysed specific subgroups of patients (e.g. with a defined treatment) [11, 20, 21] or reported just one of the outcomes (e.g. contralateral breast cancer) [14, 15, 21, 22]. Studies reporting joint estimates are less frequent [10]. Using a population-based approach with all outcomes in one study helps to clarify the factors associated with outcomes and their internal relationship.
The aims of this study are to analyse the risk of second events amongst patients with pure DCIS, and to examine factors that may predict subsequent DCIS and invasive breast cancer. Further, to compare the incidence of subsequent malignancy and mortality to that of women in the general population.
Materials and methods
Patients
All women diagnosed with a primary pure DCIS in Norway in the period from 1993 to 2007 were considered as patients in this study. Data were obtained from the incidence database of the Cancer Registry of Norway, which includes all clinical and pathological reports of invasive cancer (and certain pre-invasive conditions) since 1951 [23]. Patients with mixed tumours (DCIS and invasive components), micro-invasion, Paget’s disease, or previous or concurrent (within 4 months) history of breast cancer were excluded. Of the 3167 patients with primary pure DCIS, four cases had no follow-up information. In the remaining 3163 patients, there were 17 bilateral synchronous cases, which resulted in 3180 cases. Furthermore, in the follow-up analysis of subsequent malignancy, 117 patients were excluded because they had fewer than 4 months of follow-up, yielding 3046 patients at risk. All 3163 patients were at risk when death was used as the endpoint.
Characteristics
Baseline data on age and date of diagnosis, laterality, surgical and radiation treatment were obtained from the incidence database at the Cancer Registry of Norway. Screening information was extracted from the screening database of the Norwegian Breast Cancer Screening Programme (NBCSP). Information from the two databases was linked together using the unique 11-digit personal identification number assigned to all inhabitants in Norway. In addition, detailed information about histological tumour size and grading was abstracted through review of the pathological reports, and thus made available for this study. The pathological size of the lesion was reported as the largest diameter, and grade was reported according to a non-uniform classification system before 2000 and to van Nuys classification from 2000 onwards. The joint reporting of tumour size and grade was only 5% in 1993–1995, but increased to 80% in 2005–2007. Treatment was given as the most advanced treatment reported to the registry. Additional information about XRT was abstracted from the radiation units at the hospitals which reports to the Cancer Registry. Missing data were probably due to less knowledge and attention of DCIS, especially in the early years. A case was considered as screen detected if the diagnosis had been made on the basis of the screening examination in the NBCSP. Further description of the data has been provided previously [3]. Table 1 shows the baseline characteristics of the patients and the primary cases included in the study.
Second events
Both subsequent malignancy and death in patients diagnosed with DCIS were regarded as second events. A subsequent malignancy was defined as a DCIS or an invasive breast cancer diagnosed in the contralateral breast or an invasive cancer (either local recurrence or a new primary) in the ipsilateral breast. The registration system does not permit identification of more than one DCIS lesion in each breast, thus only the first DCIS is included. A flowchart of subsequent malignancies within 10 years of follow-up is shown in Fig. 1.
Flowchart of subsequent malignancy by final surgical treatment for the primary DCIS within 10 years of follow-up. The 17 patients with bilateral synchronous DCIS were classified according to the most advanced treatment; no one was diagnosed with subsequent malignancy. DCIS ductal carcinoma in situ; BCT breast-conserving treatment; XRT radiation therapy; N/A not available
Information about status (date of emigration or death) and cause of death was extracted from the incidence database of the Cancer Registry of Norway, which is regularly linked to the Cause of Death Registry at Statistics Norway. We considered a death as related to breast cancer when breast cancer was the underlying cause of death.
Statistics
Two sets of follow-up analysis were performed, one where subsequent malignancy was of interest, and another that focused on death. In the former analysis, the patients were followed from 4 months after the date of primary diagnosis of DCIS until the date of subsequent malignancy. Follow-up times started 4 months after the date of diagnosis due to the inclusion criteria and classification of the cases of DCIS, thus 117 patients were excluded from the analysis because they had follow-up time of less than 4 months. The patients were censored at the date of emigration or at the end of follow-up (31 December, 2007). Death was considered as a competing event. In addition, in the analysis of contralateral DCIS, the occurrence of a contralateral invasive cancer was regarded as a competing event. In the latter analysis, which focused on death, patients were followed from the date of primary diagnosis of DCIS until the first of the following dates: death, emigration or end of follow-up. When studying breast cancer-specific survival, death from other causes than breast cancer was considered as a competing event. Due to competing events, we used the cumulative incidence to estimate the probabilities of the main events to avoid bias in the analyses [24].
The risk estimates were given according to final surgical treatment for the primary DCIS (mastectomy, BCT with XRT, BCT without XRT, and BCT unknown XRT) and for different outcome subgroups (all malignancies, ipsilateral invasive cancer, contralateral invasive cancer, and contralateral DCIS). All results were presented at 10 years of follow-up. Age-standardized results are shown for mortality using the indirect method with the following age-distribution: 0–34, 35–49, 50–69 and 70+ years.
Graphically, the cumulative incidence of subsequent malignancy was derived using the competing risk approach and presented as an adjusted Kaplan–Meier plot; the conditional failure rate was presented as a plot of the smoothed hazard function. Separate figures were shown for subsequent ipsilateral and contralateral invasive cancer during 10 years of follow-up including 95% confidence intervals (CIs).
Cox proportional hazards models were fitted to evaluate the prognostic significance of each of the following factors in relation to the risk of subsequent malignancy: age at diagnosis (≤49, 50–69 and ≥70), period of diagnosis (1993–2000 and 2001–2007), detection method (screen and non-screen detected), tumour size in millimetre (<20, ≥20 and unknown), grade (non-high, high and unknown), and treatment (mastectomy, BCT with XRT, BCT without XRT, BCT unknown XRT and no or unknown surgery). The assumption of proportional hazards was verified graphically, and checked using tests of proportional hazards assumption both for all covariates individually and globally. The Cox proportional hazard models were appropriate for analysing prognostic factors in the presence of competing risks [25]. Frailty models were used to address the issue of unobserved heterogeneity. Unduly influences on the effect estimates were not found and thus the frailty analyses were not presented.
Standardized incidence rates (SIRs) were computed to measure the relative risk of being diagnosed with subsequent malignancy compared with the incidence of primary DCIS in the general female population. With regard to subsequent unilateral disease, the observed number of cases was compared with half the incidence in the general population, whilst when the outcome in focus was all subsequent malignancies, the total incidence was used. Correspondingly, standardized mortality rates (SMRs) were calculated to assess the relative risk of death: overall and breast cancer-specific. Death statistics for the Norwegian population were extracted from Statistics Norway. Reference rates were computed for 3-year calendar periods (1993–1995, 1996–1998, 1999–2001, 2002–2004 and 2005–2007) and 5-year age groups (0–4, 5–9,…, ≥90). Expected numbers were computed by applying the period- and age-specific rates to the observed women–years in the cohort. SIRs and SMRs were computed by taking the ratio of the observed to expected incidence and mortality, respectively. For these estimates, the 95% CI was computed assuming Poisson distribution of the observed number of cases.
All statistical analyses were performed using Stata (version 11, Stata Corporation, College Station, TX, USA).
Results
Of the 3046 patients with 4 months or more of follow-up, 192 were diagnosed with subsequent breast malignancy within 10 years of follow-up: 96 ipsilateral invasive cancer, 30 contralateral DCIS, 65 contralateral invasive cancers and 1 bilateral invasive cancer as the first of the subsequent events (Fig. 1). Two patients had a third event. The median time from diagnosis of DCIS to cessation (minimum of date of death, emigration or end of study) was 5.2 years (max 15.0 years). Cumulative incidence of subsequent malignancy at five and 10 years of follow-up was 5.6% (95% CI 4.7–6.6%) and 11.2% (95% CI 9.6–13.0%), respectively. Classified by outcome, the 10-year cumulative incidence of ipsilateral invasive cancer was 5.5%, contralateral invasive cancer 3.9% and contralateral DCIS 1.8% (Table 2). As expected, the cumulative incidence estimates were a little lower (about 5%) than the regular Kaplan–Meier estimates not adjusted for competing risks.
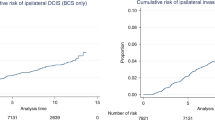
During the 10-year follow-up period, the cumulative incidence was somewhat higher for ipsilateral than for contralateral invasive cancer (Fig. 2a and b), and furthermore, the shape of the hazards by time since diagnosis was different for the two lateralities: the hazard for ipsilateral invasive cancer peaked at 3 and 8 years, which was not seen for contralateral invasive cancer (Fig. 2c and d).
Cumulative incidence function of a ipsilateral invasive breast cancer and b contralateral invasive breast cancer, and smoothed hazard function of c ipsilateral invasive breast cancer and d contralateral invasive breast cancer. All presented with 95% confidence intervals during 10 years of follow-up after date of diagnosis of ductal carcinoma in situ
The risk estimates of subsequent malignancy vary by treatment of the DCIS (Table 2). For patients treated with mastectomy, the 10-year cumulative incidence of subsequent malignancy was 9.9%, and for patients treated with BCT the rate was 14.8%. Furthermore, the multivariate Cox regression analysis showed higher risk of subsequent ipsilateral invasive cancer in patients with unknown tumour size compared with tumours <20 mm (HR 1.9, 95% CI 1.1–3.3), higher risk in patients treated with BCT (all subgroups of XRT status) compared with mastectomy, and a tended lower risk in patients with screen detected compared with non-screen detected DCIS (HR 0.7, 95% CI 0.4–1.1) (Table 3). In particular, for the group of patients with a small (<20 mm) non-high-grade DCIS treated with BCT with no or unknown XRT status, the risk of subsequent malignancy was 11.0 (95% CI 5.5–18.6) within 10 years of follow-up. This was almost three times higher than for an analogous group of patients treated with mastectomy, which had a 10-year risk of 4.1 (95% CI 1.1–10.5). Amongst the patients treated with mastectomy who were subsequently diagnosed with an ipsilateral invasive cancer (n = 32), 15 had recurrence (either DCIS or invasive cancer) in the surgical scar, 12 had metastasis to the lymph nodes and 5 had both events.
The number of subsequent malignancies observed in this study cohort was higher than expected based on the incidence in a corresponding age- and period-matched female population in Norway (Table 4). For invasive cancer, the standardized incidence ratio (SIR) was 4.3 (95% CI 3.7–5.0); ipsilateral SIR 4.6 (3.7–5.6) and contralateral SIR 3.1 (2.5–4.0) and contralateral DCIS 13.3 (9.3–19.0). For all types of malignancies, the relative risk was higher in the first period (≤2.5 years) after diagnosis, but remained elevated in all time periods.
Amongst the 3163 patients, 210 deaths were recorded within 10 years after diagnosis during the period 1993–2007. The 10-year overall mortality (death from any cause) was 13.4% (Table 5). Forty-two DCIS patients died of breast cancer, whereas nine of these patients did not have any invasive cancer registered at the Cancer Registry. The 10-year probability of dying from breast cancer was 2.5%.
Compared with the general female population, there was no significant excess mortality amongst DCIS patients (SMR 1.04 (95% CI 0.9–1.2)), but the SMR of breast cancer was 4.3 (95% CI 3.2–5.8) (Table 6). The SMR was the highest in the first period (≤2.5 years) after diagnosis, but was significantly elevated in all time periods during follow-up.
Discussion
This population-based study showed that patients with a primary diagnosis of pure DCIS had an 11.2% risk of being diagnosed with subsequent breast malignancy within 10 years, which was five times higher than for women in the general population. The 10-year risk of dying from breast cancer amongst patients with DCIS was four times higher than the risk for women in the general population. These results were comparable with other studies [14, 15, 19, 26–28]. Interpreting and comparing results from studies may be difficult, as definitions and inclusion criteria for primary DCIS and subsequent event(s), as well as study design and conduct may have been imprecise or not uniformly defined.
The DCIS patients in our study had an increased risk of subsequent malignancies compared with the general population that were slightly higher than reported in other studies [13, 14, 22]. Most noteworthy was the rate for contralateral DCIS (SIR 13.3), which has previously been reported to be 4–7 times that of the general population [13, 14, 22]. The explanation for this might be the completeness of registry-based data and a more recent study period. In addition, intensive surveillance of the contralateral breast after diagnosis of DCIS (i.e. selective surveillance bias) may increase the detection of contralateral DCIS. The most pronounced increase in the first part of the follow-up period supports this, but since all the risk estimates for contralateral malignancies were elevated during the whole follow-up period, this may also indicate an increased susceptibility to the disease.
The risk of ipsilateral invasive cancer (both new primary and local recurrence) may be attributed to the patients’ individual susceptibility to invasive cancer and the increased risk due to diagnosis and treatment of DCIS. Assuming that the risk of contralateral invasive cancer reflects the susceptibility and that the risk of ipsilateral invasive cancer reflects the total risk, then the risk difference would reflect the patients’ excess risk due to the diagnosis and treatment of DCIS. As the hazard for ipsilateral invasive cancer was generally higher than for contralateral disease (Fig. 2c and d), this may express the risk during the 10-year follow-up period. Using our data, this excess was approximately 60% based on the 10-year risks (5.5 − 3.9 = 1.6). However, this calculation may underestimate the risk as patients treated with mastectomy contribute to enhanced person-time with reduced risk of ipsilateral invasive cancer. Moreover, the pathological discrimination between DCIS and atypical ductal hyperplasia on one side, and between DCIS and invasive cancer on the other side, may further complicate the interpretation of the results [5, 7, 29].
The 10-year risk estimates for subsequent malignancy were significantly lower in patients treated with mastectomy than in patients treated with BCT. Our estimates were in line with those reported by Bijker et al. [20], who presented 10-year invasive recurrence rates for BCT with XRT and without XRT of 8 and 13%, respectively . On the other hand, the risk of ipsilateral invasive cancer in patients treated with mastectomy was higher (3.8% within 10 years) than previously reported (1–2%) [10, 11]. Amongst the patients treated with mastectomy who were diagnosed with ipsilateral invasive cancer, about 60% had local recurrence in the surgical scar and about 50% metastasis to the lymph nodes. Excluding the cases with metastases to the lymph nodes as subsequent invasive cancer, our results would have been comparable with other studies. However, the increased risk could reflect foci of invasive cancer missed in the diagnosis or affected breast tissue remaining after mastectomy.
Patients in the group of not reported tumour size had a significant increased risk (HR 1.9) of ipsilateral invasive cancer compared with patients with small tumours (<20 mm). The explanation might be that unknown tumour size includes some large tumours with multifocal or multicentric disease which do not have any size reported. A previous study has shown that widely extended DCIS was often combined with the presence of occult invasion and multicentricity [30]. However, another study reported an increased risk of local (DCIS) recurrence in multifocal disease, but did not find any association with the development of invasive recurrence [31]. The ‘sick lobe theory’ by Tot explained the multifocal disease by the spread within the lobular system [32].
The lower risk of being diagnosed with a subsequent ipsilateral invasive cancer in screen detected compared with non-screen detected DCIS is probably because the majority of the non-screen detected is based on clinical symptoms. This finding is supported by a study showing that patients with palpably-detected DCIS had higher risk of subsequent malignancy than patients with mammographically detected DCIS [21]. However, since both studies have adjusted for tumour size, these findings might indicate different prognostic characteristics in screen detected versus non-screen detected DCIS.
The overall mortality in patients diagnosed with DCIS was 13.4% within 10 years of follow-up. This was not significantly higher than the mortality in the general female population. However, the probability of dying of breast cancer was 2.5%, yielding a fourfold risk compared with the female population. This was in line with a previous study presenting a 10-year breast cancer-specific mortality rate of 2.3% and an SMR of breast cancer between two and three times higher than in the general population [19], and somewhat lower than a study reporting results after diagnosis of DCIS or LCIS in combination [26]. The increased risk of dying from breast cancer may illustrate the harmful nature of the DCIS. And the potential for progression to invasive breast cancer can be exemplified by the 4% risk of subsequent malignancy in small non-high grade lesions treated with mastectomy.
The major strengths of our study were the population-based design, the large number of patients and the completeness of the registration and follow-up. However, registry-based material contains limited details on the patient’s risk factors, tumour characteristics and treatment procedures. For some records, the linkage of the Cancer Registry and the Cause of Death Register has been insufficient, as illustrated by the nine DCIS patients who were recorded to die of breast cancer with only a DCIS registered in the Registry. This was somewhat unexpected, since the completeness of invasive breast cancer registration has been shown to be 99.95% [23]. However, the general problem of declaring which women who died of breast cancer versus with breast cancer is an issue which remains a topic for discussion [33].
In summary, this study supports previous studies showing that patients with DCIS are at increased risk of subsequent malignancy either in the ipsilateral or the contralateral breast, and that patients with DCIS have an increased risk of breast cancer-specific death (even if the DCIS patient was treated) compared with that of the general population. This study also shows the heterogeneity of DCIS and demonstrates the need for unambiguous definitions of diagnosis and outcomes of the disease. However, progression of DCIS is by now not well understood, and continued investigation is needed to minimize under and overtreatment of patients with DCIS.
References
van Steenbergen LN, Voogd AC, Roukema JA, Louwman WJ, Duijm LE, Coebergh JW, van de Poll-Franse LV (2009) Screening caused rising incidence rates of ductal carcinoma in situ of the breast. Breast Cancer Res Treat 115:181–183
Virnig BA, Tuttle TM, Shamliyan T, Kane RL (2010) Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 102:170–178
Sørum R, Hofvind S, Skaane P, Haldorsen T (2010) Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast 19:499–505
Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM (2004) Ductal carcinoma in situ of the breast. N Engl J Med 350:1430–1441
Leonard GD, Swain SM (2004) Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst 96:906–920
Patani N, Cutuli B, Mokbel K (2008) Current management of DCIS: a review. Breast Cancer Res Treat 111:1–10
Pinder SE (2010) Ductal carcinoma in situ (DCIS): pathological features, differential diagnosis, prognostic factors and specimen evaluation. Mod Pathol 23(Suppl 2):S8–S13
Bombonati A, Sgroi DC (2011) The molecular pathology of breast cancer progression. J Pathol 223:307–317
Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N (2001) Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol 28:400–418
Romero L, Klein L, Ye W, Holmes D, Soni R, Silberman H, Lagios MD, Silverstein MJ (2004) Outcome after invasive recurrence in patients with ductal carcinoma in situ of the breast. Am J Surg 188:371–376
Godat LN, Horton JK, Shen P, Stewart JH, Wentworth S, Levine EA (2009) Recurrence after mastectomy for ductal carcinoma in situ. Am Surg 75:592–595
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, Peto R, Bijker N, Solin L, Darby S (2010) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr 2010:162–177
Innos K, Horn-Ross PL (2008) Risk of second primary breast cancers among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat 111:531–540
Habel LA, Moe RE, Daling JR, Holte S, Rossing MA, Weiss NS (1997) Risk of contralateral breast cancer among women with carcinoma in situ of the breast. Ann Surg 225:69–75
Claus EB, Stowe M, Carter D, Holford T (2003) The risk of a contralateral breast cancer among women diagnosed with ductal and lobular breast carcinoma in situ: data from the Connecticut Tumor Registry. Breast 12:451–456
Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, Pike MC, Reed SD, Saftlas AF, Scarvalone SA, Schwartz AM, Slomski C, Yothers G, Zon R (2009) NIH state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ (DCIS). NIH Consens State Sci Statements 26:1–27
Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, Wolmark N (1999) Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 86:429–438
Shamliyan T, Wang SY, Virnig BA, Tuttle TM, Kane RL (2010) Association between patient and tumor characteristics with clinical outcomes in women with ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010:121–129
Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R (2000) Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med 160:953–958
Bijker N, Meijnen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, Gennaro M, Rouanet P, Avril A, Fentiman IS, Bartelink H, Rutgers EJ (2006) Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 24:3381–3387
Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, Chew K, Moore DH, Waldman F (2003) Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst 95:1692–1702
Ji J, Hemminki K (2007) Risk for contralateral breast cancers in a population covered by mammography: effects of family history, age at diagnosis and histology. Breast Cancer Res Treat 105:229–236
Larsen IK, Smastuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, Moller B (2009) Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 45:1218–1231
Pintilie M (2006) Competing risks: a practical perspective. Wiley, Chichester
Pintilie M (2002) Dealing with competing risks: testing covariates and calculating sample size. Stat Med 21:3317–3324
Levi F, Randimbison L, Te VC, La Vecchia C (2005) Invasive breast cancer following ductal and lobular carcinoma in situ of the breast. Int J Cancer 116:820–823
Li CI, Malone KE, Saltzman BS, Daling JR (2006) Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer 106:2104–2112
Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, Land SR, Margolese RG, Swain SM, Costantino JP, Wolmark N (2011) Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 103:478–488
Provenzano E, Pinder SE (2009) Pre-operative diagnosis of breast cancer in screening: problems and pitfalls. Pathology (Phila) 41:3–17
Lagios MD, Westdahl PR, Margolin FR, Rose MR (1982) Duct carcinoma in situ. Relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer 50:1309–1314
Rakovitch E, Pignol JP, Hanna W, Narod S, Spayne J, Nofech-Mozes S, Chartier C, Paszat L (2007) Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast-conserving therapy. J Clin Oncol 25:5591–5596
Tot T (2005) DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch 447:1–8
Holmberg L, Duffy SW, Yen AM, Tabár L, Vitak B, Nyström L, Frisell J (2009) Differences in endpoints between the Swedish W-E (two county) trial of mammographic screening and the Swedish overview: methodological consequences. J Med Screen 16:73–80
Acknowledgments
The study was funded by South-Eastern Norway Regional Health Authority (Grant No. 3b-110), which has not had any further influence on the study.
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falk, R.S., Hofvind, S., Skaane, P. et al. Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast Cancer Res Treat 129, 929–938 (2011). https://doi.org/10.1007/s10549-011-1531-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1531-1