Abstract
Global gene expression profiles, consisting mainly of genes associated with proliferation, have been shown to subdivide histological grade 2 breast cancers into groups with different prognosis. We raised the question whether this subdivision could be done using a single proliferation marker, cyclin A. Furthermore, we combined cyclin A (CA), histological grade (G), and estrogen receptor—ER (E) into a new variable, CAGE. Our aim was to investigate not only the prognostic importance of cyclin A alone but also the value of the combination variable CAGE. In 219 premenopausal node-negative patients, cyclin A was assessed using immunohistochemistry on tissue microarrays. High cyclin A was defined as above the seventh decile of positive cells. Only 13% of the patients received adjuvant systemic therapy. Cox proportional hazards regression was used to model the impact of the factors on distant disease-free survival (DDFS). Cyclin A divided histological grade 2 tumors into two groups with significantly different DDFS (hazard ratio [HR]: 15, P < 0.001). When stratifying for ER status, cyclin A was a prognostic factor only in the ER positive subgroup. We found that CAGE was an independent prognostic factor for DDFS in multivariate analysis (HR: 4.1, P = 0.002), together with HER2. CAGE and HER2 identified 53% as low-risk patients with a 5-year DDFS of 95%. A new prognostic variable was created by combining cyclin A, histological grade, and ER (CAGE). CAGE together with HER2 identified a large low-risk group for whom adjuvant chemotherapy will have limited efficacy and may be avoided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a heterogeneous disease with large differences in prognosis. In spite of all efforts to individualize treatment, more than 80% of patients receive adjuvant treatment although the majority of patients would have been cured by surgery alone. An important improvement in standard clinical care would be to identify a larger low-risk group, that can be spared adjuvant systemic therapy, in particular chemotherapy.
High proliferation is a key feature in breast carcinogenesis and markers of proliferation, e.g. mitotic activity, thymidine-labeling index, S-phase fraction, Ki67, and cyclins have been shown to be associated to prognosis and to response to chemotherapy [1–7]. Ki67 and mitotic activity have recently been included in the St Gallen guidelines [8], but the role of other proliferation markers, such as cyclin A, is still debated. Cyclin A has been associated with a worse outcome in breast cancer patients in some studies [1, 9–11] but others were unable to confirm this finding [2, 12, 13]. Reasons for discrepant results include small numbers of patients (only two studies included more than 200 patients) and differences in lymph node status, cut-points, end-points and follow-up times, and adjuvant systemic treatment. Further studies in well-defined and homogeneous patient cohorts are therefore needed before the prognostic significance of cyclin A can be established. In this study, we have focused on premenopausal patients with lymph node-negative breast cancer. The majority of these patients (87%) had not received any adjuvant systemic therapy.
Histological grade is a well-established prognostic factor in breast cancer. However, a substantial percentage of tumors (30–60%) are classified as grade 2. This large group has an intermediate risk of developing recurrences, and grading is hence not always informative in treatment decision making. Using gene expression profiling, patients with histological grade 2 tumors could be subdivided into one group with good prognosis (similar to grade 1) and one group with poor prognosis (similar to grade 3) [14, 15]. The most important genes in these profiles were those associated with proliferation. In the St Gallen guidelines, it is suggested that multigene assays could add information in cases where the indication for adjuvant chemotherapy remains uncertain, for example, in lymph node-negative patients with estrogen receptor (ER) positive, human epidermal growth factor receptor 2 (HER2) normal, and histological grade 2 breast cancer [8]. We therefore raised the question whether this subdivision could be obtained using a single proliferation factor, specifically cyclin A.
Global gene expression analyses have also shown that the prognostic profiles of ER-positive and ER-negative breast cancers differ significantly. In the ER-positive subgroup, genes associated with proliferation seem to be the most important, whereas genes associated with immune response are more important among ER-negative breast cancers [16, 17]. In line with this, a recent study from our group has shown that the proliferation marker Ki67 was of prognostic importance only in ER-positive breast cancers [18]. In that study, we also showed that the prognostic importance of Ki67 was dependent on histological grade [18], again showing similarities with gene expression data [14, 15]. The latter finding was recently confirmed in a consecutive series consisting of more than 1,500 patients [19]. Consequently, it may be critical to consider the interaction between histological grade, Ki67 or some other marker of proliferation (e.g. cyclin A), and ER for treatment decisions in primary breast cancer. In line with this hypothesis, we combined cyclin A (CA), histological grade (G), and ER (E) into a new dichotomous variable, CAGE, resulting in a low-risk group constituting grade 1 or grade 2/ER+/low cyclin A tumors and a high-risk group of the remaining cases.
The aim of the study was to investigate not only the prognostic importance of cyclin A alone but also the value of combining proliferation, histological grade, and ER status (CAGE) in node-negative premenopausal breast cancer patients. The performance of this new variable was also compared to the transcriptionally based genetic grade in a subgroup (40%) for which this information was available.
Materials and methods
Patients
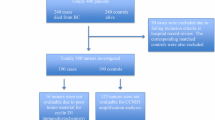
The initial patient population consisted of 237 premenopausal women with lymph node-negative breast cancer, included between 1991 and 1994 in a prospective study of the prognostic value of S-phase fraction [20]. In 14 cases, no paraffin-embedded material was retrieved from the pathology departments and of the remaining cases, three were not considered invasive and one case had insufficient number of cells (<200). Consequently, cyclin A was evaluated in 219 cases and all figures in the following are based on these patients (Fig. 1). Primary surgical treatment, postoperative radiation, and adjuvant systemic treatment have been described in detail earlier [20]. Adjuvant treatment was given to 29 of the patients (13%), of whom 21 received chemotherapy and 8 endocrine treatment. The median age was 47 years (range 30–57) and the median tumor size 15 mm (range 5–45). The median follow-up for distant metastasis was 10.3 years for patients alive and free from distant metastases at the latest review of the patients’ records, but because of nonproportional hazards for most of the factors studied, we restricted the analyses to the first 5 years. The study was approved by the ethics committee at Lund University (LU 240-01).
Patient and specimen selection according to the REMARK recommendations [26]
Methods
Tumor grading was performed according to Elston and Ellis, and ER, PgR, HER2, and Ki67 were analyzed as described earlier [18, 20, 21].
Preparation of tissue microarrays
Tissue microarrays (TMA) were prepared from paraffin-embedded blocks, using a manual arrayer (Beecher Instruments Inc., Sun Prairie, WI). Two 0.6 mm cores were taken from representative areas of each primary tumor block and transferred into a recipient paraffin block, constituting the TMA block.
Immunohistochemical staining
Sections (3–4 μm) were taken from each TMA block, transferred to glass slides, deparaffinized in xylene, and rehydrated in a ladder of graded ethanol (from absolute ethanol to distilled water). Antigen retrieval was done in Tris–EDTA buffer (pH 9) in a microwave oven for 10 min (750 W) + 15 min (350 W), prior to processing in an automatic immunohistochemistry staining machine according to standard procedures (Autostainer, Dako, Denmark). The cyclin A2 (NCL-Cyclin A, 1:100, Novocastra Laboratories) antibody was applied for 30 min at room temperature. Immunostainings were detected via Dako Cytomation envision/HRP kit K5007. Tonsil tissue was used as a positive control and the primary antibody was omitted as a negative control.
Evaluation of immunoreactivity
TMA slides stained for cyclin A were analyzed by two independent investigators (Cecilia Ahlin and Carina Strand). In the core with most positivity, 200 cells were counted manually in high-power fields, using the 40Xobjective of a light microscope with an ocular graticule consisting of a 10 × 10 grid. If there were not enough cells in the first core, additional cells in the second core were counted until 200 cells in total were counted.
The cut-off value was defined as above the seventh decile in the empirical cyclin A distribution [22], which in this series corresponded to ≥15% (Cecilia Ahlin) and ≥17% (Carina Strand) positive cells, respectively. The agreement between the investigators evaluations of cyclin A expression, above or below respective cut-off, was good (kappa-value 0.71). In this study, we chose to focus on the results from the more experienced investigator (Cecilia Ahlin).
Genetic grade
Gene expression analysis was performed as previously described [23]. Tumors were classified to a genetic grade signature as described elsewhere [15, 23]. In the original study, gene expression profiling was performed on 359 tumors. The overlap with the present study was 90 tumors.
Statistical analysis
The Kaplan–Meier method was used to estimate distant disease-free survival (DDFS), and the log-rank test to compare survival in different strata. The Cox proportional hazards model was used for estimation of univariate and multivariate hazard ratios. Proportional hazards assumptions were checked with Schoenfelds’s test [24].
All factors were used as dichotomous covariates in the statistical analysis with the exception of age, which was also analyzed as a continuous variable, and histological grade (three groups).
Because histological grade, Ki67, and cyclin A are highly correlated, they could not be evaluated in the same multivariate model. Therefore, we used a different approach, stepwise Cox regression with backward elimination, to select the best fitting model. In brief, the procedure starts with all variables and eliminates the least significant variable in each step until all the remaining variables have P-values <0.157, a stopping rule suggested by Royston and Sauerbrei [25]. The null hypothesis of identical prognostic effect of cyclin A in ER-positive versus ER-negative cases was evaluated using a Cox model with an interaction term between ER status and cyclin A. In the stepwise multivariate analysis, patients with missing values for one or more of the candidate variables were excluded. To minimize the information loss, the variables in the final multivariate model were evaluated in a separate Cox model.
All tests were two-sided and P-values <0.05 were considered significant. The statistical analysis software Stata 11.1, 2010 (StataCorp, College Station, TX) was used for statistical calculations. Agreement between investigators was measured using Cohen’s kappa. Whenever applicable, the REMARK recommendations for reporting of tumor marker studies were followed [26].
Results
Patient and tumor characteristics
During the first 5 years of follow-up, distant metastases were recorded in 34 patients, and at 5 years, the DDFS was 84% (95% confidence interval [CI] 79–89%). The median cyclin A value was 8.5% (interquartile range 4.0–19%). High cyclin A was associated with age <50 years, large tumors, histological grade 3, ER and PgR negativity, HER2 positivity, and high Ki67 (Table 1).
DDFS
Univariate analyses
We found a statistically significant association between cyclin A and DDFS in univariate analysis (hazard ratio [HR]: 3.6, 95% CI: 1.8–7.1, P < 0.001; Table 2). The corresponding HRs were 2.7 for Ki67 (95% CI: 1.4–5.5, P = 0.005), 2.7 for grade (95% CI: 1.4–5.2, P = 0.004), and 6.1 for HER2 (95% CI: 2.9–13, P < 0.001). Age, ER, and PgR were also significant factors, whereas tumor size was not (Table 2).
The prognostic value of cyclin A in different subgroups
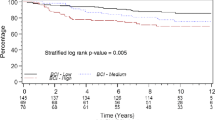
When subdividing according to histological grade, cyclin A could divide histological grade 2 tumors into two groups with significantly different DDFS (HR: 15, 95% CI: 4.3–52, P < 0.001; Fig. 2). In the grade 1 and 3 subgroups, on the other hand, cyclin A was not a significant prognostic factor. When stratifying for ER status, cyclin A was a prognostic factor in the ER-positive subgroup, but not in the ER-negative group (HR: 5.8, 95% CI: 2.2–16, P < 0.001 vs. HR: 1.5, 95% CI: 0.55–3.9, P = 0.44). The difference in prognostic importance of cyclin A between ER-positive and ER-negative cases was further analyzed, yielding a strong, but not significant, interaction (HR: 3.9, 95% CI: 0.98–16, P = 0.054). When stratifying histological grade 2 according to ER status, we found a statistically significant difference between ER-positive and ER-negative cases (HR: 3.5, 95% CI: 1.1–12, P = 0.038). In the histological grade 2 subgroup, ER-negative tumors, irrespective of cyclin A, had a 5-year DDFS of 71% (95% CI: 43–87%), thereby confirming these patients as belonging to the high-risk group.
The next step was to evaluate the prognostic importance of the predefined combination of cyclin A, histological grade, and ER according to the following: Low CAGE consisted of all histological grade 1 cases and histological grade 2/ER-positive/low cyclin A cases. The high CAGE group was defined as histological grade 3, histological grade 2/ER-negative, or histological grade 2/ER-positive/high cyclin A. We found a statistically significant association between CAGE and DDFS in univariate analysis (HR: 6.9 95% CI: 2.9–17, P < 0.001). Using Ki67 as the proliferation marker, the HR was 4.6 (95% CI: 1.9–11, P = 0.001). The results remained significant when the patients receiving systemic adjuvant therapy (n = 29) were excluded (data not shown).
Genetic grade
Overall, a strong correlation between genetic grade and cyclin A was identified (P < 0.001, chi2). In the histological grade 2 subgroup, more than two thirds (22/32) of the tumors were equally classified (high vs. low). For patients in this subgroup, there was a trend that genetic grade was associated with DDFS, but in this small material (n = 32), it did not reach significance (HR: 3.1, 95% CI: 0.7–13, P = 0.13).
Multivariate analyses
Stepwise regression identified age, HER2, and cyclin A as the most important prognostic variables (Table 2). Tumor size, histological grade, ER, PgR, and Ki67 were excluded from the model as nonsignificant (P > 0.157). Even when cyclin A was removed from the set of predictors defining the full model, Ki67 was not a significant factor in this material. If CAGE was used instead of cyclin A, histological grade, and ER, we found that CAGE was an independent prognostic factor for DDFS in multivariate analysis (HR: 3.6, 95% CI: 1.3–8.5, P = 0.010), together with HER2 and age (Table 3).
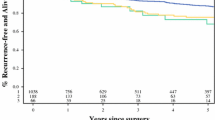
Finally, we created a prognostic index based on the independent prognostic factors in the multivariate analysis. However, because age is not used as a continuous variable in clinical routine, we tested the independent prognostic value of age by using one commonly applied cut-off (≤35 years vs. >35 years). Using this cut-off, age was no longer an independent prognostic factor in this material, which might be explained by the small size of the ≤35-years group (only eight patients), and age was therefore excluded in our final model, which included only CAGE and HER2. The low-risk group (low CAGE and HER2 normal) constituted 53% of the patients (107/201) and had a 5-year DDFS of 95% (95% CI: 89–98%). The DDFS for the remaining 47% of the patients was 73% (95% CI: 63–81%; HR: 6.6, 95% CI: 2.5–17, P < 0.001; Fig. 3).
Distant disease-free survival (DDFS) of 201 premenopausal women with lymph node-negative breast cancer. The low-risk group (low CAGE and HER2 normal) constituted 53% of the patients (107/201) and had a 5-year DDFS of 95% (95% CI: 89–98%). The DDFS for the remaining 47% of the patients was 73% (95% CI: 63–81%), (hazard ratio: 6.6, 95% CI: 2.5–17, P < 0.001)
Discussion
By combining the proliferation marker cyclin A, histological grade, and ER status into a new variable, CAGE, and also considering HER2 status, a prognostic index was defined. When using this index, 53% of the patients were classified into a low-risk group. These patients had a 5-year DDFS of 95% compared to 73% for the high-risk group. The underlying principle for combining cyclin A, histological grade, and ER status was based on previous findings indicating that the prognostic importance of proliferation seems to be restricted to the ER-positive subgroup and furthermore is most pronounced among histological grade 2 breast cancers. This has been shown with gene expression profiling [14–16] and also by using another single proliferation marker, Ki67 [18, 19]. Estrogen receptor negativity is associated with worse clinical outcome and high proliferation. However, proliferation alone seems not to give any additional prognostic information in the ER-negative subgroup.
A few (3 out of 68) breast cancers in the histological grade 1 subgroup have high proliferation. We did not find that these cases had worse prognosis compared to those with histological grade 1 and low proliferation. However, one cannot exclude that these patients should be included in the high-risk group. Unfortunately, this study lacked the power for such an analysis. In a recent study of 275 grade 1 tumors, a significant difference in metastasis-free survival was found between patients with low- and high-Ki67 tumors [19].
It should be stressed that the vast majority of the patients (87%) in this material did not receive any adjuvant systemic therapy. The proportion of systemically untreated patients was even higher in the low-risk group (95%, 102/107). The patients were diagnosed between 1991 and 1994, and today, most patients with ER-positive breast cancer would have been recommended adjuvant endocrine therapy. In the low-risk group, 91% (97/107) of the cases were ER-positive, and most likely adjuvant endocrine therapy would have increased the 5-year DDFS even further. The use of a proliferation marker for subdividing histological grade 2 tumors is also in line with the St Gallen guidelines, where it is suggested that multigene assays could add information in cases for whom indication for adjuvant chemotherapy remains uncertain, for example, in lymph node-negative breast cancer being ER-positive, HER2 normal, and histological grade 2 [8]. The generally most used prognostic gene profiles are the MammaPrint® and the Oncotype DX®. In both profiles, genes associated with proliferation are the most important. In this study, we have shown that among ER-positive cases, cyclin A can be used to divide histological grade 2 tumors into two groups with different prognosis, thereby identifying an additional quarter of the patients as low-risk patients (24%; 51/214). This additional group could thereby potentially be spared adjuvant chemotherapy.
The new array-based techniques will probably be of great value in the future for improving personalized therapy and also to identify new targets for treatment. They are however, not yet widely used, very expensive, and generally require frozen tissue, even though RNA extracted from paraffin-embedded tissue is used in Oncotype DX®. To this end, the identification of molecular signatures to select patients who could be spared chemotherapy was found to have the highest priority in an international web-based consultation of breast cancer professionals [27]. Our study suggests that by using immunohistochemistry, and combining ER, histological grade, proliferation (e.g. cyclin A or Ki67), and HER2, a low-risk group can be defined with good prognosis and not in need of adjuvant chemotherapy. Furthermore, in subgroup analysis, a strong association between cyclin A and genetic grade was obtained. The use of conventional factors thus challenges the use of gene profiles for this purpose. This has also been demonstrated in previous studies, showing that conventional factors seem to give similar prognostic information as MammaPrint® and Oncotype DX® [28, 29].
In the present study, cyclin A performs slightly better than Ki67 as a prognostic factor. However, the decision of which marker to use in the routine clinical management of breast cancer patients should be based on considerations of the prognostic strength of the factor and also of more practical issues, including the reproducibility and the costs of the analyses. In addition to cyclin A and Ki67, there are a number of other proliferation markers, e.g. mitotic activity and PPH3, associated with prognosis and which may also be useful for this purpose [7].
We chose to focus on the results from the evaluation of the more experienced investigator, but the kappa-value of 0.71 (good agreement) indicates that cyclin A can be reliably evaluated even if the investigator is less experienced. We believe that the similarities in HRs for the two readers (data not shown) also strengthen the robustness of the evaluation. When comparing with the reproducibility of other factors, a greater interobserver variability has been reported for the evaluation of histological grade [30]. In another study with a similar evaluation design as in this study, the reproducibility for Ki67 was, however, better (kappa-values >0.80). In this study, cyclin A was evaluated on sections obtained from TMA cores. If cyclin A is to be used in the clinic, the evaluation will be done on whole sections. Nevertheless, Aaltonen et al. [31] showed a good correlation between TMA cores and whole sections stained for cyclin A (kappa-value 0.62–0.75). In line with the results in an earlier publication, a predefined cut-point at the seventh decile (15%) was used for defining the high-risk group. This cut-point is within the range of cut-points from earlier published studies (8–30%), defined as optimized cut-points, median cut-points, or categorization in three groups, respectively [1, 2, 9–13, 22, 31].
In conclusion, by combining the proliferation marker cyclin A, histological grade, and ER status, a new risk variable (CAGE) was created. By adding HER2 status to this variable, a prognostic index was defined. When using this index, 53% of the patients in this study were classified as low-risk patients with a 5-year DDFS of 95%. For this low-risk group, adjuvant chemotherapy will have limited efficacy and may be avoided.
References
Bukholm IR, Bukholm G, Nesland JM (2001) Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Cancer 93(2):283–287. doi:10.1002/ijc.1311
Kuhling H, Alm P, Olsson H, Ferno M, Baldetorp B, Parwaresch R, Rudolph P (2003) Expression of cyclins e, a, and b, and prognosis in lymph node-negative breast cancer. J Pathol 199(4):424–431. doi:10.1002/path.1322
van Diest PJ, van der Wall E, Baak JP (2004) Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol 57(7):675–681. doi:10.1136/jcp.2003.010777 57/7/675 [pii]
Baldini E, Camerini A, Sgambato A, Prochilo T, Capodanno A, Pasqualetti F, Orlandini C, Resta L, Bevilacqua G, Collecchi P (2006) Cyclin a and e2f1 overexpression correlate with reduced disease-free survival in node-negative breast cancer patients. Anticancer Res 26(6B):4415–4421
de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12, 155 patients. Br J Cancer 96(10):1504–1513. doi:6603756[pii] 10.1038/sj.bjc.6603756
Stuart-Harris R, Caldas C, Pinder SE, Pharoah P (2008) Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32, 825 patients. Breast 17(4):323–334. doi:S0960-9776(08)00059-3[pii] 10.1016/j.breast.2008.02.002
Baak JP, Gudlaugsson E, Skaland I, Guo LH, Klos J, Lende TH, Soiland H, Janssen EA, Zur Hausen A (2009) Proliferation is the strongest prognosticator in node-negative breast cancer: Significance, error sources, alternatives and comparison with molecular prognostic markers. Breast Cancer Res Treat 115(2):241–254. doi:10.1007/s10549-008-0126-y
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329. doi:mdp322[pii] 10.1093/annonc/mdp322
Michalides R, van Tinteren H, Balkenende A, Vermorken JB, Benraadt J, Huldij J, van Diest P (2002) Cyclin a is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer 86(3):402–408. doi:10.1038/sj.bjc.6600072
Poikonen P, Sjostrom J, Amini RM, Villman K, Ahlgren J, Blomqvist C (2005) Cyclin a as a marker for prognosis and chemotherapy response in advanced breast cancer. Br J Cancer 93(5):515–519. doi:6602735[pii] 10.1038/sj.bjc.6602735
Ahlin C, Zhou W, Holmqvist M, Holmberg L, Nilsson C, Jirstrom K, Blomqvist C, Amini RM, Fjallskog ML (2009) Cyclin a is a proliferative marker with good prognostic value in node-negative breast cancer. Cancer Epidemiol Biomarkers Prev 18(9):2501–2506. doi:1055-9965.EPI-09-0169[pii] 10.1158/1055-9965.EPI-09-0169
Rudolph P, Kuhling H, Alm P, Ferno M, Baldetorp B, Olsson H, Parwaresch R (2003) Differential prognostic impact of the cyclins e and b in premenopausal and postmenopausal women with lymph node-negative breast cancer. Int J Cancer 105(5):674–680. doi:10.1002/ijc.11132
Konigsberg R, Rogelsperger O, Jager W, Thalhammer T, Klimpfinger M, De Santis M, Hudec M, Dittrich C (2008) Cell cycle dysregulation influences survival in high risk breast cancer patients. Cancer Invest 26(7):734–740. doi:795398532[pii] 10.1080/07357900801944864
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, Wong JE, Liu ET, Bergh J, Kuznetsov VA, Miller LD (2006) Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res 66(21):10292–10301. doi:66/21/10292[pii] 10.1158/0008-5472.CAN-05-4414
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C (2007) An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol 8(8):R157. doi:gb-2007-8-8-r157[pii] 10.1186/gb-2007-8-8-r157
Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M, Sotiriou C (2008) Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 14(16):5158–5165. doi:14/16/5158[pii] 10.1158/1078-0432.CCR-07-4756
Klintman M, Bendahl PO, Grabau D, Lovgren K, Malmstrom P, Ferno M (2010) The prognostic value of ki67 is dependent on estrogen receptor status and histological grade in premenopausal patients with node-negative breast cancer. Mod Pathol 23(2):251–259. doi:modpathol2009167[pii] 10.1038/modpathol.2009.167
Aleskandarany MA, Rakha EA, Macmillan RD, Powe DG, Ellis IO, Green AR (2010) Mib1/ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups. Breast Cancer Res Treat. doi:10.1007/s10549-010-1028-3
Malmstrom P, Bendahl PO, Boiesen P, Brunner N, Idvall I, Ferno M (2001) S-phase fraction and urokinase plasminogen activator are better markers for distant recurrences than Nottingham prognostic index and histologic grade in a prospective study of premenopausal lymph node-negative breast cancer. J Clin Oncol 19(7):2010–2019
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjallskog ML, Blomqvist C (2007) Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology 51(4):491–498. doi:HIS2798[pii] 10.1111/j.1365-2559.2007.02798.x
Jonsson G, Staaf J, Vallon-Christersson J, Ringner M, Holm K, Hegardt C, Gunnarsson H, Fagerholm R, Strand C, Agnarsson BA, Kilpivaara O, Luts L, Heikkila P, Aittomaki K, Blomqvist C, Loman N, Malmstrom P, Olsson H, Johannsson OT, Arason A, Nevanlinna H, Barkardottir RB, Borg A (2010) Genomic subtypes of breast cancer identified by array-comparative genomic hybridization display distinct molecular and clinical characteristics. Breast Cancer Res 12(3):R42. doi:bcr2596[pii] 10.1186/bcr2596
Schoenfeld DA (1983) Sample-size formula for the proportional-hazards regression model. Biometrics 39(2):499–503
Royston P, Sauerbrei W (2008) Multivariable model-building: a pragmatic approach to regression analysis based on fractional polynomials for continuous variables. John Wiley & Sons Ltd, Chichester
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor marker prognostic studies (remark). Breast Cancer Res Treat 100(2):229–235. doi:10.1007/s10549-006-9242-8
Dowsett M, Goldhirsch A, Hayes DF, Senn HJ, Wood W, Viale G (2007) International web-based consultation on priorities for translational breast cancer research. Breast Cancer Res 9(6):R81. doi:bcr1798[pii] 10.1186/bcr1798
Eden P, Ritz C, Rose C, Ferno M, Peterson C (2004) “Good old” clinical markers have similar power in breast cancer prognosis as microarray gene expression profilers. Eur J Cancer 40(12):1837–1841. doi:10.1016/j.ejca.2004.02.025 S0959804904002138 [pii]
Cuzick J, Dowsett M, Wale C, Salter J, Quinn E, Zabaglo L, Howell A, Buzdar A, Forbes J (2009) Prognostic value of a combined ER, PgR, ki67, HER2 immunohistochemical (IHC4) score and comparison with the GHI recurrence score: results from transATAC. Cancer Res 69(Suppl 24):503S–503S
Meyer JS, Alvarez C, Milikowski C, Olson N, Russo I, Russo J, Glass A, Zehnbauer BA, Lister K, Parwaresch R (2005) Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol 18(8):1067–1078. doi:3800388[pii] 10.1038/modpathol.3800388 [doi]
Aaltonen K, Ahlin C, Amini RM, Salonen L, Fjallskog ML, Heikkila P, Nevanlinna H, Blomqvist C (2006) Reliability of cyclin a assessment on tissue microarrays in breast cancer compared to conventional histological slides. Br J Cancer 94(11):1697–1702. doi:6603147[pii] 10.1038/sj.bjc.6603147
Acknowledgments
We are indebted to participating departments of the South Sweden Breast Cancer Group for providing samples and clinical follow-up. We thank Dorthe Grabau for collection of paraffin blocks, Kristina Lövgren for technical skills in creating the TMA blocks, and Markus Ringnér and Göran Jönsson for fruitful discussions about genetic grade. The study was supported by funds from the Swedish Cancer Society, the Swedish Research Council, the Gunnar Nilsson Cancer Foundation, the Mrs. Berta Kamprad Foundation, the Anna and Edwin Bergers Foundation, Skåne County Council’s Research and Development Foundation, and Governmental Funding of Clinical Research within the National Health Service.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strand, C., Ahlin, C., Bendahl, PO. et al. Combination of the proliferation marker cyclin A, histological grade, and estrogen receptor status in a new variable with high prognostic impact in breast cancer. Breast Cancer Res Treat 131, 33–40 (2012). https://doi.org/10.1007/s10549-011-1386-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1386-5