Abstract
Cytosolic glutathione S-transferase comprises multiple isoenzymes; studies have principally examined mu-1 (GSTM1: null/present), theta-1 (GSTT1: null/present) and pi-1 (GSTP1 Ile105Val) gene polymorphisms concerning breast cancer risk. Regarding GSTT1 and GSTP1 polymorphisms, studies remain controversial and no recent meta-analysis has appeared. This meta-analysis aims to examine whether GSTT1 and GSTP1 polymorphisms are associated with breast cancer risk. Separate analyses were performed on Chinese and non-Chinese populations, in an attempt to investigate race-specific effects. Eligible articles were identified by a search of MEDLINE bibliographic database for the period up to August 2009. Regarding GSTT1 null/present genotype, 41 case–control studies were eligible (16,589 breast cancer cases and 19,995 controls); 30 case–control studies were eligible for GSTP1 Ile105Val (16,908 cases and 20,016 controls). Pooled odds ratios (ORs) were appropriately derived from fixed-effects or random-effects models. At the overall analysis, the null GSTT1 genotype was associated with elevated breast cancer risk (pooled OR = 1.114, 95% CI: 1.035–1.199, random effects). However, the association seemed confined to non-Chinese populations (33 studies, pooled OR = 1.128, 95% CI: 1.042–1.221, random effects), given that the association was not significant in the subset of Chinese studies (eight studies, pooled OR = 1.061, 95% CI: 0.875–1.286, random effects). Regarding GSTP1 Ile105Val, no statistically significant associations were detected in non-Chinese populations (25 studies). On the other hand, the GG genotype was associated with increased breast cancer risk in Chinese populations (five studies, pooled OR = 1.297, 95% CI: 1.023–1.645, fixed effects); accordingly, the recessive model yielded statistically significant results (pooled OR = 1.273, 95% CI: 1.006–1.610, fixed effects). In conclusion, polymorphisms of both GSTT1 and GSTP1 genes seem associated with elevated breast cancer risk in a race-specific manner. Given the small number of Chinese studies, the finding on GSTP1 Ile105Val merits further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytosolic glutathione S-transferase (GST) comprises multiple isoenzymes; studies have principally examined mu-1 (GSTM1), theta-1 (GSTT1) and pi-1 (GSTP1) genes concerning breast cancer risk. GSTs catalyze reactions between glutathione and lipophilic compounds with electrophilic centers, leading to neutralization of toxic compounds [1].
GSTM1, GSTT1 and GSTP1 genes have been found polymorphic in the population, i.e. “present” or “null” genotypes may characterize an individual regarding GSTM1 and GSTT1, whereas a single-nucleotide polymorphism (A to G transition) has been identified in the exon 5 of the GSTP1 gene (Ile105Val) [2].
Attention has been drawn at a meta-analytical level upon those polymorphisms; a recent meta-analysis has demonstrated that GSTM1 null genotype is associated with elevated breast cancer risk [3]. Interestingly enough, the latest meta-analyses on the association between GSTT1, GSTP1 genotypes and breast cancer risk appeared in 2004 [4, 5]; these meta-analyses had been based upon a small number of studies available at that time period (at best 15 case–control studies) and had pointed to null associations. Since then, more than 20 case–control studies have been published regarding both GSTT1 [6–28] and GSTP1 [7, 8, 11–15, 17, 20–32] polymorphisms and breast cancer risk, with contradictory findings. Under the light of the substantial improvement in the power of the meta-analysis, the need for an up-to-date meta-analysis has become evident.
This meta-analysis aims to examine whether the status of GSTT1 and GSTP1 polymorphisms is associated with breast cancer risk. Separate analyses were performed on Chinese and non-Chinese populations, in an attempt to investigate race-specific effects.
Methods
Trial identification
Eligible articles were identified by a search of MEDLINE bibliographical database for the period up to August 2009 (last search: August 11, 2009) using combinations of the following keywords: “glutathione-S transferase”, “GSTT1”, “GSTP1”, “polymorphism”, “genotype”, “variant”, “breast cancer”. In addition, we checked all the references of relevant reviews and eligible articles that our search retrieved. Language restrictions were not used and two investigators (KPE and TNS), working independently, searched the literature and extracted data from each eligible case–control study.
Eligible studies and data abstraction
All case–control studies, irrespective of the sample size, examining the association between breast cancer and GSTT1 null genotype or GSTP1 Ile105Val were considered eligible for this meta-analysis. For each one of the eligible case–control studies, the following data were collected: journal name, year of publication, inclusion and exclusion criteria, demographic characteristics of the population being studied, menopausal status, frequencies of genotypes in cases and controls for each of the genotypes/polymorphisms.
Statistics
Based on the genotype frequencies in cases and controls, crude odds ratios (ORs) as well as their standard errors (SE) were calculated. Regarding GSTT1, the ORs pertained to null genotype carriers vs. present (positive) genotype carriers. Concerning GSTP1, four different ORs were calculated: (1) AG vs. AA (heterozygous carriers), (2) GG vs. AA (homozygous carriers), (3) G allele carriers (AG and GG grouped together) vs. AA (dominant model) and (4) GG genotype vs. A allele carriers (AG and AA grouped together) (recessive model). Separate analyses were performed in Chinese and non-Chinese populations.
The fixed-effects model (Mantel–Haenszel method), as well as the random-effects (DerSimonian Laird) model, was used to calculate the pooled OR. Between-study heterogeneity and between-study inconsistency were assessed by using Cochran Q statistic and by estimating I 2, respectively [33]. In case no significant heterogeneity was detected, the fixed-effects model was chosen. Evidence of publication bias was determined using Egger’s formal statistical test [34] and by visual inspection of the funnel plot. For the interpretation of Egger’s test, statistical significance was defined as P < 0.1. Meta-analysis was performed using the STATA “metan” command.
In addition, meta-regression was performed to assess whether Odds Ratio (OR) was associated with publication year. The exponentiated coefficient is provided, since the dependent variable in the meta-regression model is log(OR). Meta-regression was performed with the “metareg” STATA command. Analyses were conducted using STATA 10.0 (STATA Corp. College Station, TX, USA).
Results
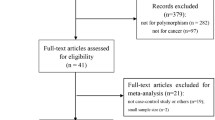
Figure 1 illustrates the trial flow chart. Out of the 161 abstracts retrieved through the search criteria, 94 were irrelevant, nine articles [35–43] were excluded because they were conducted on overlapping populations with other eligible studies [6, 8, 20, 25, 30, 44, 45] (these excluded articles represent smaller studies performed on subsets of larger eligible studies), five studies [46–50] were excluded because they have not included controls in their study design, two articles [4, 51] were reviews/meta-analyses and three studies [52–54] were excluded due to other reasons (two of them [52, 53] was excluded due to reporting reasons, i.e. no reporting of the relevant genotype frequencies, whereas the other [54] was excluded for examining the association between GSTA1 polymorphism and survival after breast cancer treatment). As a result, 48 case–control articles [5–32, 44, 45, 55–72] were included in this meta-analysis; 41 case–control studies [5–28, 44, 45, 55–59, 61–65, 67–71] were included in the meta-analysis on GSTT1 genotype (16,589 breast cancer cases and 19,995 controls) and 30 case–control studies [7, 8, 11–15, 17, 20–32, 60, 62, 64–68, 70, 72] on GSTP1 polymorphism (16,908 cases and 20,016 controls). Evidently, the sum of studies surpasses the number of eligible articles, as more than one study were presented per article. In addition, 33 case–control studies were included in the meta-analysis on GSTT1 genotype on non-Chinese subjects (14,139 cases and 16,465 controls) [7, 9, 12–15, 17, 19–28, 44, 45, 55–58, 61–65, 67–71] and eight case–control studies on Chinese subjects (2,450 cases and 3,530 controls) [5, 6, 8, 10, 11, 16, 18, 59]. Accordingly, 25 case–control studies [7, 12–15, 17, 20–28, 32, 60, 62, 64–70, 72] were included in the meta-analysis on GSTP1 genotype on non-Chinese subjects (12,652 cases and 14,843 controls) and five case–control studies on Chinese subjects (4,256 cases and 5,173 controls) [8, 11, 29–31].
At the overall analysis, the null GSTT1 genotype was associated with elevated breast cancer risk (pooled OR = 1.114, 95% CI: 1.035–1.199, random effects). Interestingly enough, stratification by race pointed to discrepancy between non-Chinese and Chinese studies. The association seemed confined to non-Chinese populations (pooled OR = 1.128, 95% CI: 1.042–1.221, random effects, Fig. 2a), given that the association was not significant in the subset of Chinese studies (pooled OR = 1.061, 95% CI: 0.875–1.286, random effects, Fig. 2b).
Forest plot for the overall association between null GSTT1 genotype and breast cancer risk for a non-Chinese and b Chinese subjects. Each study is shown by the point estimate of the Odds Ratio (OR) (the size of the square is proportional to the weight of each study) and 95% confidence interval for the OR (extending lines); the pooled OR and 95% confidence interval have been appropriately derived from random-effects model
Regarding GSTP1 Ile105Val, the results of the meta-analysis are presented in detail in Table 1. No statistically significant associations were detected in non-Chinese populations. On the other hand, the GG genotype was associated with increased breast cancer risk in Chinese populations; accordingly, the recessive model yielded statistically significant results (Fig. 3a, b).
Meta-regression with publication year did not point to any modifying effect of publication year in the case of GSTT1 polymorphism (exponentiated coefficient = 0.994, 95% CI: 0.970–1.019, P = 0.630). Accordingly, no modifying effects of publication year were observed at any approach regarding GSTP1 status (for heterozygous carriers, exponentiated coefficient = 0.993, 95% CI: 0.953–1.035, P = 0.738; for homozygous carriers, exponentiated coefficient = 0.993, 95% CI: 0.916–1.076, P = 0.864; for the dominant model, exponentiated coefficient = 0.995, 95% CI: 0.954–1.038, P = 0.809; for the recessive model, exponentiated coefficient = 0.999, 95% CI: 0.939–1.063, P = 0.970).
Concerning GSTT1 polymorphism, publication bias was significant in the overall meta-analysis (P = 0.048), mainly due to the publication bias in non-Chinese studies (P = 0.045); no significant publication bias was detected in Chinese studies (P = 0.742). On the contrary, no significant publication bias was detected in any meta-analysis performed on GSTP1 polymorphism status (P = 0.607 for heterozygous carriers, P = 0.557 for homozygous carriers, P = 0.787 for the dominant model, and P = 0.393 for the recessive model).
Discussion
The principal message of this meta-analysis is that polymorphisms of both GSTT1 and GSTP1 genes seem associated with elevated breast cancer risk in a race-specific manner. Specifically, GSTT1 null phenotype seems able to confer additional breast cancer risk in non-Chinese populations, whereas GSTP1 Ile105Val G allele seems associated with increased breast cancer risk in Chinese subjects following a recessive model.
Concerning GSTT1 null phenotype, it is worth mentioning that the present meta-analysis takes one step beyond the existing meta-analysis by Egan et al. [5], which had been published 5 years ago and had pointed to marginal associations, characterized by the authors as “null results”. In the present meta-analysis, under the light of a nearly threefold increase in the number of eligible studies, a clear association between GSTT1 null genotype and increased breast cancer risk became evident and seemed, indeed, confined to non-Chinese populations. Race-specific effects of GSTT1 polymorphism status may not seem surprising at a meta-analytical level, as race-specific associations have been described in meta-analyses examining lung [73] and gastric [74] cancer.
Similarly, the results concerning GSTP1 Ile105Val exhibited a race-specific pattern, but opposite to that demonstrated in GSTT1. The association between the G allele seemed confined in Chinese studies; nevertheless, given that the sample size of the study by Lee et al. [30] is considerably larger than that of the remaining studies, the latter emerges as particularly influential. As a result, the need for additional studies on Chinese populations seems warranted, so as to establish the present findings.
An issue that has been raised in previous meta-analyses examining GSTT1 and GSTP1 status vis-à-vis breast cancer risk is the putative interaction with menopausal status. In the present meta-analysis, such subanalyses have not been presented after carefully taking into account that solely 10 [6, 9, 15, 28, 44, 58, 65, 67, 69, 70] out of 41 studies on GSTT1 and 9 [15, 28–32, 65, 67, 70] out of 30 studies on GSTP1 status have presented subanalyses on pre- and post-menopausal women. Indeed, the power of these subsets of studies was so limited that the main associations were blurred (data not shown); as a result, proceeding to subanalyses on pre- and post-menopausal subjects did not seem to be of particular value.
In conclusion, this meta-analysis points to a positive association between GSTT1 null genotype and breast cancer risk in non-Chinese subjects; the positive association between GSTP1 G allele and elevated breast cancer risk in Chinese subjects is worth investigating further.
References
Hengstler JG, Arand M, Herrero ME, Oesch F (1998) Polymorphisms of N-acetyltransferases, glutathione S-transferases, microsomal epoxide hydrolase and sulfotransferases: influence on cancer susceptibility. Recent Results Cancer Res 154:47–85
Kano T, Sakai M, Muramatsu M (1987) Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res 47:5626–5630
Yu KD, Di GH, Fan L, Wu J, Hu Z, Shen ZZ, Huang W, Shao ZM (2009) A functional polymorphism in the promoter region of GSTM1 implies a complex role for GSTM1 in breast cancer. FASEB J 23:2274–2287
Vogl FD, Taioli E, Maugard C, Zheng W, Pinto LF, Ambrosone C, Parl FF, Nedelcheva-Kristensen V, Rebbeck TR, Brennan P et al (2004) Glutathione S-transferases M1, T1, and P1 and breast cancer: a pooled analysis. Cancer Epidemiol Biomarkers Prev 13:1473–1479
Egan KM, Cai Q, Shu XO, Jin F, Zhu TL, Dai Q, Gao YT, Zheng W (2004) Genetic polymorphisms in GSTM1, GSTP1, and GSTT1 and the risk for breast cancer: results from the Shanghai Breast Cancer Study and meta-analysis. Cancer Epidemiol Biomarkers Prev 13:197–204
Park SK, Yim DS, Yoon KS, Choi IM, Choi JY, Yoo KY, Noh DY, Choe KJ, Ahn SH, Hirvonen A et al (2004) Combined effect of GSTM1, GSTT1, and COMT genotypes in individual breast cancer risk. Breast Cancer Res Treat 88:55–62
Sarmanova J, Susova S, Gut I, Mrhalova M, Kodet R, Adamek J, Roth Z, Soucek P (2004) Breast cancer: role of polymorphisms in biotransformation enzymes. Eur J Hum Genet 12:848–854
Ceschi M, Sun CL, Van Den Berg D, Koh WP, Yu MC, Probst-Hensch N (2005) The effect of cyclin D1 (CCND1) G870A-polymorphism on breast cancer risk is modified by oxidative stress among Chinese women in Singapore. Carcinogenesis 26:1457–1464
Chacko P, Joseph T, Mathew BS, Rajan B, Pillai MR (2005) Role of xenobiotic metabolizing gene polymorphisms in breast cancer susceptibility and treatment outcome. Mutat Res 581:153–163
Cheng TC, Chen ST, Huang CS, Fu YP, Yu JC, Cheng CW, Wu PE, Shen CY (2005) Breast cancer risk associated with genotype polymorphism of the catechol estrogen-metabolizing genes: a multigenic study on cancer susceptibility. Int J Cancer 113:345–353
Chang TW, Wang SM, Guo YL, Tsai PC, Huang CJ, Huang W (2006) Glutathione S-transferase polymorphisms associated with risk of breast cancer in southern Taiwan. Breast 15:754–761
Edvardsen H, Kristensen VN, Grenaker Alnaes GI, Bohn M, Erikstein B, Helland A, Borresen-Dale AL, Fossa SD (2007) Germline glutathione S-transferase variants in breast cancer: relation to diagnosis and cutaneous long-term adverse effects after two fractionation patterns of radiotherapy. Int J Radiat Oncol Biol Phys 67:1163–1171
Nordgard SH, Ritchie MD, Jensrud SD, Motsinger AA, Alnaes GI, Lemmon G, Berg M, Geisler S, Moore JH, Lonning PE et al (2007) ABCB1 and GST polymorphisms associated with TP53 status in breast cancer. Pharmacogenet Genomics 17:127–136
Spurdle AB, Chang JH, Byrnes GB, Chen X, Dite GS, McCredie MR, Giles GG, Southey MC, Chenevix-Trench G, Hopper JL (2007) A systematic approach to analysing gene–gene interactions: polymorphisms at the microsomal epoxide hydrolase EPHX and glutathione S-transferase GSTM1, GSTT1, and GSTP1 loci and breast cancer risk. Cancer Epidemiol Biomarkers Prev 16:769–774
Steck SE, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Santella RM, Gammon MD (2007) Interactions among GSTM1, GSTT1 and GSTP1 polymorphisms, cruciferous vegetable intake and breast cancer risk. Carcinogenesis 28:1954–1959
Chang YL, Li J, Yao SQ, Hu WN, Jiang SF, Guo Z, Yang L, Li DD, Li YM, Liu Y (2008) A case–control study on serum organochlorines residues, genetic polymorphisms of glutathione S-transferase T1 and the risks of breast cancer. Zhonghua Liu Xing Bing Xue Za Zhi 29:763–766
Kadouri L, Kote-Jarai Z, Hubert A, Baras M, Abeliovich D, Hamburger T, Peretz T, Eeles RA (2008) Glutathione-S-transferase M1, T1 and P1 polymorphisms, and breast cancer risk, in BRCA1/2 mutation carriers. Br J Cancer 98:2006–2010
Li JY, Long QM, Tao P, Hu R, Li H, Lei FM, Zhou WD, Li SF (2008) Using MSR model to analyze the impact of gene–gene interaction with related to the genetic polymorphism of metabolism enzymes on the risk of breast cancer. Sichuan Da Xue Xue Bao Yi Xue Ban 39:780–783 See also 787
Morais LM, Cardoso Filho C, Lourenco GJ, Shinzato JY, Zeferino LC, Lima CS, Gurgel MS (2008) Polymorphisms GSTM1 and GSTT1 and sporadic breast cancer mammographic features. Rev Assoc Med Bras 54:61–66
Rajkumar T, Samson M, Rama R, Sridevi V, Mahji U, Swaminathan R, Nancy NK (2008) TGFbeta1 (Leu10Pro), p53 (Arg72Pro) can predict for increased risk for breast cancer in south Indian women and TGFbeta1 Pro (Leu10Pro) allele predicts response to neo-adjuvant chemo-radiotherapy. Breast Cancer Res Treat 112:81–87
Syamala VS, Sreeja L, Syamala V, Raveendran PB, Balakrishnan R, Kuttan R, Ankathil R (2008) Influence of germline polymorphisms of GSTT1, GSTM1, and GSTP1 in familial versus sporadic breast cancer susceptibility and survival. Fam Cancer 7:213–220
Torresan C, Oliveira MM, Torrezan GT, de Oliveira SF, Abuazar CS, Losi-Guembarovski R, Lima RS, Urban CA, Cavalli IJ, Ribeiro EM (2008) Genetic polymorphisms in oestrogen metabolic pathway and breast cancer: a positive association with combined CYP/GST genotypes. Clin Exp Med 8:65–71
Unlu A, Ates NA, Tamer L, Ates C (2008) Relation of glutathione S-transferase T1, M1 and P1 genotypes and breast cancer risk. Cell Biochem Funct 26:643–647
Van Emburgh BO, Hu JJ, Levine EA, Mosley LJ, Perrier ND, Freimanis RI, Allen GO, Rubin P, Sherrill GB, Shaw CS et al (2008) Polymorphisms in CYP1B1, GSTM1, GSTT1 and GSTP1, and susceptibility to breast cancer. Oncol Rep 19:1311–1321
The MARIE-GENICA Consortium (2009) Genetic polymorphisms in phase I and phase II enzymes and breast cancer risk associated with menopausal hormone therapy in postmenopausal women. Breast Cancer Res Treat. doi:10.1007/s10549-009-0407-0
McCarty KM, Santella RM, Steck SE, Cleveland RJ, Ahn J, Ambrosone CB, North K, Sagiv SK, Eng SM, Teitelbaum SL et al (2009) PAH-DNA adducts, cigarette smoking, GST polymorphisms, and breast cancer risk. Environ Health Perspect 117:552–558
Reding KW, Weiss NS, Chen C, Li CI, Carlson CS, Wilkerson HW, Farin FM, Thummel KE, Daling JR, Malone KE (2009) Genetic polymorphisms in the catechol estrogen metabolism pathway and breast cancer risk. Cancer Epidemiol Biomarkers Prev 18:1461–1467
Saxena A, Dhillon VS, Raish M, Asim M, Rehman S, Shukla NK, Deo SV, Ara A, Husain SA (2009) Detection and relevance of germline genetic polymorphisms in glutathione S-transferases (GSTs) in breast cancer patients from northern Indian population. Breast Cancer Res Treat 115:537–543
Kim SU, Lee KM, Park SK, Yoo KY, Noh DY, Choe KJ, Ahn SH, Hirvonen A, Kang D (2004) Genetic polymorphism of glutathione S-transferase P1 and breast cancer risk. J Biochem Mol Biol 37:582–585
Lee SA, Fowke JH, Lu W, Ye C, Zheng Y, Cai Q, Gu K, Gao YT, Shu XO, Zheng W (2008) Cruciferous vegetables, the GSTP1 Ile105Val genetic polymorphism, and breast cancer risk. Am J Clin Nutr 87:753–760
Sakoda LC, Blackston CR, Xue K, Doherty JA, Ray RM, Lin MG, Stalsberg H, Gao DL, Feng Z, Thomas DB et al (2008) Glutathione S-transferase M1 and P1 polymorphisms and risk of breast cancer and fibrocystic breast conditions in Chinese women. Breast Cancer Res Treat 109:143–155
Antognelli C, Del Buono C, Ludovini V, Gori S, Talesa VN, Crino L, Barberini F, Rulli A (2009) CYP17, GSTP1, PON1 and GLO1 gene polymorphisms as risk factors for breast cancer: an Italian case–control study. BMC Cancer 9:115
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Zheng T, Holford TR, Zahm SH, Owens PH, Boyle P, Zhang Y, Zhang B, Wise JP Sr, Stephenson LP, Ali-Osman F (2003) Glutathione S-transferase M1 and T1 genetic polymorphisms, alcohol consumption and breast cancer risk. Br J Cancer 88:58–62
Lee KM, Park SK, Kim SU, Doll MA, Yoo KY, Ahn SH, Noh DY, Hirvonen A, Hein DW, Kang D (2003) N-acetyltransferase (NAT1, NAT2) and glutathione S-transferase (GSTM1, GSTT1) polymorphisms in breast cancer. Cancer Lett 196:179–186
Park SK, Yoo KY, Lee SJ, Kim SU, Ahn SH, Noh DY, Choe KJ, Strickland PT, Hirvonen A, Kang D (2000) Alcohol consumption, glutathione S-transferase M1 and T1 genetic polymorphisms and breast cancer risk. Pharmacogenetics 10:301–309
Park SK, Kang D, Noh DY, Lee KM, Kim SU, Choi JY, Choi IM, Ahn SH, Choe KJ, Hirvonen A et al (2003) Reproductive factors, glutathione S-transferase M1 and T1 genetic polymorphism and breast cancer risk. Breast Cancer Res Treat 78:89–96
van der Hel OL, Peeters PH, Hein DW, Doll MA, Grobbee DE, Ocke M, Bueno de Mesquita HB (2004) GSTM1 null genotype, red meat consumption and breast cancer risk (The Netherlands). Cancer Causes Control 15:295–303
Gago-Dominguez M, Castelao JE, Sun CL, Van Den Berg D, Koh WP, Lee HP, Yu MC (2004) Marine n-3 fatty acid intake, glutathione S-transferase polymorphisms and breast cancer risk in post-menopausal Chinese women in Singapore. Carcinogenesis 25:2143–2147
Yang G, Shu XO, Ruan ZX, Cai QY, Jin F, Gao YT, Zheng W (2005) Genetic polymorphisms in glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer 103:52–58
Samson M, Swaminathan R, Rama R, Sridevi V, Nancy KN, Rajkumar T (2007) Role of GSTM1 (Null/Present), GSTP1 (Ile105Val) and P53 (Arg72Pro) genetic polymorphisms and the risk of breast cancer: a case control study from South India. Asian Pac J Cancer Prev 8:253–257
Justenhoven C, Hamann U, Schubert F, Zapatka M, Pierl CB, Rabstein S, Selinski S, Mueller T, Ickstadt K, Gilbert M et al (2008) Breast cancer: a candidate gene approach across the estrogen metabolic pathway. Breast Cancer Res Treat 108:137–149
Zheng T, Holford TR, Zahm SH, Owens PH, Boyle P, Zhang Y, Wise JP Sr, Stephenson LP, Ali-Osman F (2002) Cigarette smoking, glutathione-S-transferase M1 and t1 genetic polymorphisms, and breast cancer risk (United States). Cancer Causes Control 13:637–645
van der Hel OL, Peeters PH, Hein DW, Doll MA, Grobbee DE, Kromhout D, Bueno de Mesquita HB (2003) NAT2 slow acetylation and GSTM1 null genotypes may increase postmenopausal breast cancer risk in long-term smoking women. Pharmacogenetics 13:399–407
Nedelcheva Kristensen V, Andersen TI, Erikstein B, Geitvik G, Skovlund E, Nesland JM, Borresen-Dale AL (1998) Single tube multiplex polymerase chain reaction genotype analysis of GSTM1, GSTT1 and GSTP1: relation of genotypes to TP53 tumor status and clinicopathological variables in breast cancer patients. Pharmacogenetics 8:441–447
Sweeney C, McClure GY, Fares MY, Stone A, Coles BF, Thompson PA, Korourian S, Hutchins LF, Kadlubar FF, Ambrosone CB (2000) Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res 60:5621–5624
Ambrosone CB, Sweeney C, Coles BF, Thompson PA, McClure GY, Korourian S, Fares MY, Stone A, Kadlubar FF, Hutchins LF (2001) Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res 61:7130–7135
van der Hel OL, Bueno-de-Mesquita HB, van Gils CH, Roest M, Slothouber B, Grobbee DE, Peeters PH (2005) Cumulative genetic defects in carcinogen metabolism may increase breast cancer risk (The Netherlands). Cancer Causes Control 16:675–681
Van Emburgh BO, Hu JJ, Levine EA, Mosley LJ, Case LD, Lin HY, Knight SN, Perrier ND, Rubin P, Sherrill GB et al (2008) Polymorphisms in drug metabolism genes, smoking, and p53 mutations in breast cancer. Mol Carcinog 47:88–99
Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF (1999) A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 8:843–854
Sgambato A, Campisi B, Zupa A, Bochicchio A, Romano G, Tartarone A, Galasso R, Traficante A, Cittadini A (2002) Glutathione S-transferase (GST) polymorphisms as risk factors for cancer in a highly homogeneous population from southern Italy. Anticancer Res 22:3647–3652
Mitrunen K, Kataja V, Eskelinen M, Kosma VM, Kang D, Benhamou S, Vainio H, Uusitupa M, Hirvonen A (2002) Combined COMT and GST genotypes and hormone replacement therapy associated breast cancer risk. Pharmacogenetics 12:67–72
Sweeney C, Ambrosone CB, Joseph L, Stone A, Hutchins LF, Kadlubar FF, Coles BF (2003) Association between a glutathione S-transferase A1 promoter polymorphism and survival after breast cancer treatment. Int J Cancer 103:810–814
Khedhaier A, Remadi S, Corbex M, Ahmed SB, Bouaouina N, Mestiri S, Azaiez R, Helal AN, Chouchane L (2003) Glutathione S-transferases (GSTT1 and GSTM1) gene deletions in Tunisians: susceptibility and prognostic implications in breast carcinoma. Br J Cancer 89:1502–1507
de Amorim L, Rossini A, Mendonca G, Lotsch P, de Almeida Simao T, de Moura Gallo C, Pinto L (2002) CYP1A1, GSTM1, and GSTT1 polymorphisms and breast cancer risk in Brazilian women. Cancer Lett 181:179–186
Matheson MC, Stevenson T, Akbarzadeh S, Propert DN (2002) GSTT1 null genotype increases risk of premenopausal breast cancer. Cancer Lett 181:73–79
Zheng W, Wen WQ, Gustafson DR, Gross M, Cerhan JR, Folsom AR (2002) GSTM1 and GSTT1 polymorphisms and postmenopausal breast cancer risk. Breast Cancer Res Treat 74:9–16
Wu FY, Lee YJ, Chen DR, Kuo HW (2002) Association of DNA–protein crosslinks and breast cancer. Mutat Res 501:69–78
Zhao M, Lewis R, Gustafson DR, Wen WQ, Cerhan JR, Zheng W (2001) No apparent association of GSTP1 A(313)G polymorphism with breast cancer risk among postmenopausal Iowa women. Cancer Epidemiol Biomarkers Prev 10:1301–1302
Xiong P, Bondy ML, Li D, Shen H, Wang LE, Singletary SE, Spitz MR, Wei Q (2001) Sensitivity to benzo(a)pyrene diol-epoxide associated with risk of breast cancer in young women and modulation by glutathione S-transferase polymorphisms: a case–control study. Cancer Res 61:8465–8469
Gudmundsdottir K, Tryggvadottir L, Eyfjord JE (2001) GSTM1, GSTT1, and GSTP1 genotypes in relation to breast cancer risk and frequency of mutations in the p53 gene. Cancer Epidemiol Biomarkers Prev 10:1169–1173
Dialyna IA, Arvanitis DA, Spandidos DA (2001) Genetic polymorphisms and transcriptional pattern analysis of CYP1A1, AhR, GSTM1, GSTP1 and GSTT1 genes in breast cancer. Int J Mol Med 8:79–87
Krajinovic M, Ghadirian P, Richer C, Sinnett H, Gandini S, Perret C, Lacroix A, Labuda D, Sinnett D (2001) Genetic susceptibility to breast cancer in French-Canadians: role of carcinogen-metabolizing enzymes and gene-environment interactions. Int J Cancer 92:220–225
Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Vainio H, Uusitupa M, Hirvonen A (2001) Glutathione S-transferase M1, M3, P1, and T1 genetic polymorphisms and susceptibility to breast cancer. Cancer Epidemiol Biomarkers Prev 10:229–236
Maugard CM, Charrier J, Pitard A, Campion L, Akande O, Pleasants L, Ali-Osman F (2001) Genetic polymorphism at the glutathione S-transferase (GST) P1 locus is a breast cancer risk modifier. Int J Cancer 91:334–339
Millikan R, Pittman G, Tse CK, Savitz DA, Newman B, Bell D (2000) Glutathione S-transferases M1, T1, and P1 and breast cancer. Cancer Epidemiol Biomarkers Prev 9:567–573
Curran JE, Weinstein SR, Griffiths LR (2000) Polymorphisms of glutathione S-transferase genes (GSTM1, GSTP1 and GSTT1) and breast cancer susceptibility. Cancer Lett 153:113–120
Garcia-Closas M, Kelsey KT, Hankinson SE, Spiegelman D, Springer K, Willett WC, Speizer FE, Hunter DJ (1999) Glutathione S-transferase mu and theta polymorphisms and breast cancer susceptibility. J Natl Cancer Inst 91:1960–1964
Helzlsouer KJ, Selmin O, Huang HY, Strickland PT, Hoffman S, Alberg AJ, Watson M, Comstock GW, Bell D (1998) Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. J Natl Cancer Inst 90:512–518
Bailey LR, Roodi N, Verrier CS, Yee CJ, Dupont WD, Parl FF (1998) Breast cancer and CYPIA1, GSTM1, and GSTT1 polymorphisms: evidence of a lack of association in Caucasians and African Americans. Cancer Res 58:65–70
Harries LW, Stubbins MJ, Forman D, Howard GC, Wolf CR (1997) Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis 18:641–644
Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, Cote ML, Dialyna IA, Dolzan V, Filiberti R et al (2006) Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol 164:1027–1042
Saadat M (2006) Genetic polymorphisms of glutathione S-transferase T1 (GSTT1) and susceptibility to gastric cancer: a meta-analysis. Cancer Sci 97:505–509
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sergentanis, T.N., Economopoulos, K.P. GSTT1 and GSTP1 polymorphisms and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 121, 195–202 (2010). https://doi.org/10.1007/s10549-009-0520-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0520-0